Abstract
Age-dependent changes in heat shock response (HSR) were studied in mononuclear cells (monocytes and lymphocytes) collected from young (mean age = 22.6 ± 1.7 years) and middle-aged (mean age = 56.3 ± 4.7 years) subjects after 1 hour of heat shock at 42°C. Genotype-specific HSR was measured by genotyping the subjects for 3 single nucleotide polymorphisms, HSPA1A(A-110C), HSPA1B(A1267G), and HSPA1L(T2437C), 1 each in the 3 HSP70 genes. A significant age-related decrease in the induction of Hsp70 occurred after heat shock in both monocytes and lymphocytes. The noninducible and inducible forms of Hsp70 decreased 1.3-fold (P < 0.001) and 1.4-fold (P < 0.001), respectively, in the monocytes with age. In the young subjects, a positive association was found between HSPA1L(T2437C) polymorphism and HSR. CC carriers had a significantly lower induction than TT carriers in both monocytes (P = 0.015) and lymphocytes (P = 0.044). This polymorphism, which is present in the coding region of HSPA1L gene, can affect the chaperoning function of Hsp70. These data consolidate our other observations that the CC genotype is unfavorable for human longevity and provide a functional explanation in terms of variations in HSR.
INTRODUCTION
The heat shock response (HSR) is a cellular stress response that acts as a defense against protein misfolding and denaturation and its lethal consequences (Lindquist 1986; Verbeke et al 2001). Furthermore, HSR is strongly associated with cellular and organismic survival, aging, and longevity (Rattan and Clark 2005). However, at present, it is not clear whether individual differences in HSR and its effects on aging and longevity have a genetic basis in terms of polymorphisms. Recently, we and others have studied single nucleotide polymorphisms (SNPs) in one of the HSR genes, heat shock protein 70 (HSP70), in a human population with different parameters of health, longevity, and survival (Altomare et al 2003; Ross et al 2003; Singh et al 2004, 2006).
In humans, 11 isoforms of HSP70 are encoded by different genes located at disperse loci. Three of the HSP70 genes are mapped within major histocompatibility complex class III region and are present in tandem on chromosome 6p21.3 (Goate et al 1987). These are intronless HSP70-1(HSPA1A), HSP70-2(HSPA1B), and HSP70-Hom(HSPA1L) (Milner and Campbell 1990) genes with homologous sequences that differ in their regulation.
Here, we hypothesize that variants in the HSP70 genes might be associated with differences in the cellular response to stressful conditions such as heat shock (HS). Therefore, we have performed a series of functional tests to evaluate whether peripheral blood mononuclear cells collected from individuals of 2 age groups have different abilities to respond to stress and whether this difference is correlated with the genotypes generated from the 3 SNPs present in the 3 HSP70 genes.
The 3 SNPs that we studied are A-110C (marker rs1008438), present in the promoter region of HSPA1A; the synonymous A1267G (marker rs1061581), present in the coding region of HSPA1B; and the nonsynonymous T2437C (Marker rs2227956), present in the coding region of HSPA1L. The latter SNP leads to a change in Hsp70 amino acid at position 493 from a nonpolar hydrophobic acid methionine (Met) to a polar neutral threonine (Thr) and could have molecular functional significance with respect to the stability and activity of this chaperoning protein.
MATERIALS AND METHODS
Sampling
Venous blood samples were collected from healthy male and female donors who visited the blood bank of the Aarhus University Hospital. Blood samples from 52 middle-aged individuals (30 males, mean age 57.7 ± 4.4 years; 22 females, mean age 54.4 ± 4.4 years) and 59 young individuals (25 males, 22.6 ± 1.7 years; 34 females, 22.7 ± 1.7 years) were collected. Permission for collecting the blood samples was taken from the Danish ethical committee. Written consent was also taken from the donors before retrieving a 20-mL blood sample in collection tubes containing ethylenediaminetetraacetic acid (EDTA).
Cell preparation
Peripheral mononuclear cells were isolated by the Ficoll– Hypaque gradient method. EDTA-blood (10 mL) mixed with an equal volume of phosphate-buffered saline (PBS) was gently poured over 5 mL of Ficoll–Paque Plus solution (Amersham Biosciences, Hillerød, Denmark), taking care that the solutions did not mix with each other. After centrifugation for 40 minutes at 500 × g, a clear layer containing mononuclear cells (monocytes and lymphocytes) was sandwiched between the layers containing plasma, Ficoll–Paque solution, and erythrocytes. Mononuclear cells were removed from the interface and transferred to another tube and centrifuged for 10 minutes at 100 × g. Cells were then washed twice in PBS containing 1% bovine serum albumin (PBS-BSA) by centrifuging for 3 minutes at 900 × g. After washing, the cells were either resuspended in cell culture medium RPMI1640 (10% fetal calf serum, 2 mM N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid buffer, 2 mM glutamine, penicillin, streptomycin) in a 35-mm-diameter petri dish (Nunclon) or were directly frozen at −135°C so that the DNA could be isolated at later stage.
Aliquots of cell suspension (1 mL) were treated immediately at room temperature for intracellular immune staining of Hsp70. This gave the measure of the basal (non-HS) level of the Hsp70. For measuring HSR, cells were plated at a concentration of approximately 3 million cells/mL in 35-mm-diameter petri dishes and incubated in a 5% CO2 incubator at 37°C for 10–15 hours.
Heat shock and cell staining
The cells were given HS for 1 hour at 42°C in a water bath. The cells were then trypsinized and stained for Hsp70-specific antibodies after 5 hours of HS. Kinetics of Hsp70 synthesis and accumulation were studied by staining the cells after 0, 2, 4, 6, 8, 12, and 24 hours. After specific recovery times, the cell content from the petri dish was trypsinized and transferred into a 15-mL tube. Cells were then washed in PBS-BSA. The cell suspension (100 μL) was then transferred to an eppendorf tube for staining.
Intracellular staining
To the 100-μL cell suspension (containing around 1 million cells), 10 μL each of R-phycoerythrin (R-PE)–conjugated anti-CD14 antibody and allophycocyanin (APC)-conjugated anti-CD45 antibody (BD Biosciences, Brødby, Denmark) was added. CD14 is a cell surface marker on monocytes, whereas CD45 is a cell surface marker both on monocytes and lymphocytes. Cells were washed in 1 mL of PBS-BSA and centrifuged at 900 × g for 3 minutes and then fixed for 15 minutes at room temperature in 75 μL of a solution containing formaldehyde (reagent A; DAKO Cytomation, Glostrup, Denmark). Cells were again washed in 1 mL of PBS-BSA and then permeablized with solution containing reagent B (DAKO Cytomation) and at the same time incubated with Hsp70-specific monoclonal antibody (diluted in PBS-BSA at 1:10) conjugated with fluorescein isothiocyanate (FITC, SPA-810FI; Biosite, Taby, Sweden). The cells were then incubated at room temperature for 30 minutes in the dark followed by a wash in PBS-BSA. After washing, cells were suspended in 500 μL of PBS and were then analyzed by flow cytometry.
Quantification by fluorescence-activated cell sorting
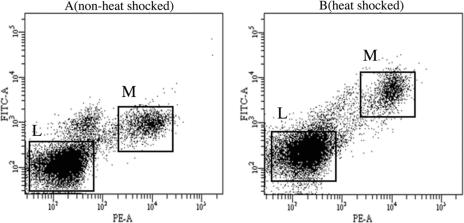
After staining with intracellular antibodies, cells were analyzed by fluorescence-activated cell sorting (FACS; BD FACS Aria). This allowed us to analyze the level of Hsp70 in a cell type–specific manner. Specific cell populations were gated with the use of fluorescence measured on the CD14-PE and CD45-APC parameters. Because the Hsp70-specific antibody was conjugated with FITC, the mean fluorescence index (MFI) of FITC from a particular cell type was measured. This corresponded to the amount of protein present in a specific cell population. FITC was measured on a 530/30 filter. Furthermore, a control sample from the same individual was included with every run to minimize day-to-day variations in the measurement of FITC and to adjust the voltage of the photomultiplier. MFI of FITC was calculated for 30 000 cells from each cell type in every sample (Fig 1).
Fig 1.
Scatterplot of distribution of monocyte (M) and lymphocyte (L) cell populations analyzed by FACS before (A) and after (B) heat shock. Y-axis shows the measure of FITC dye, which corresponds to the amount of Hsp70 synthesized. X-axis shows the measure of PE dye corresponding to the CD14 antigen, a cell surface marker specific for monocytes
DNA preparation
DNA was isolated from the mononuclear cells in pellet form at −135°C. Cells were thawed at room temperature, and 500 μL of lysis buffer (Tris 10 mM, EDTA 1 mM, NaCl 150 mM, SDS 0.5%, pH 10.5, autoclaved) was added. At the same time, 10–15 μL of proteinase K solution was added to the cells (stock concentration 20 μg/μL). The tubes were then kept for 1 hour at 37°C on a heating block/incubator. After 1 hour, the contents in the tubes were mixed gently. NaCl (160 μL, 6 M, 20°C) was added, and tubes were shaken for 15 seconds after centrifugation for 5 minutes at high speed at 4°C.
Supernatant was then transferred to a fresh 1.5-mL eppendorf tube with a plastic pipette and centrifuged. Supernatant was then transferred to a 2-mL eppendorf tube. Three volumes of absolute alcohol kept at −20°C was added, and the tubes were turned gently. DNA was eluted at this stage. Tubes were then centrifuged, and the supernatant was drained. The DNA in the pellet was then washed with 70% ethanol. The tubes were dried of alcohol, and DNA was dissolved in 20 μL of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.5, autoclaved) or double-distilled water.
Genotyping
Three SNPs, HSPA1A(A-110C) (marker rs1008438), HSPA1B(A1267G) (marker rs1061581), and HSPA1L(T2437C) (marker rs2227956), in the 3 HSP70 genes were genotyped by real-time PCR on the LightCycler system (Roche Applied Sciences, Hvidovre, Denmark) with fluorescently labeled, sequence-specific oligonucleotide probes. The LightCycler reaction was performed on 1 μL of DNA in the 10-μL reaction mix containing the following reagents: 10X Titanium Taq PCR buffer (1 μL), ADV Taq 50X (0.25 μL), 10 mM dNTPs (0.13 μL), forward primer (5 pmol/μL; 1.0 μL), reverse primer (5 pmol/μL; 1.0 μL), anchor probe (5 pmol/μL; 0.2 μL), sensor probe (5 pmol/μL; 0.2 μL), dimethyl sulfoxide (0.5 μL), and double-distilled water (4.72 μL). The sequence of the primers and probes used is given in Table 1.
Table 1.
Primers and probes used for genotyping using lightcycler
Statistical analysis
To test differences in Hsp70 synthesis between the young and middle-aged populations, an independent-sample t-test was performed. Cell-specific differences in the ability to synthesize Hsp70 were tested with a paired-sample t-test. The linear correlation coefficient (Pearson r) was used to measure the correlation between Hsp70 and age. Analysis of variance was performed to see whether the genotypes in the 3 HSP70 genes are associated with Hsp70 synthesis, independent of the age. Significance was set at the P < 0.05 level for all comparisons. Statistical analyses were performed with SPSS, Version 13.0.
RESULTS
Kinetics of Hsp70 induction
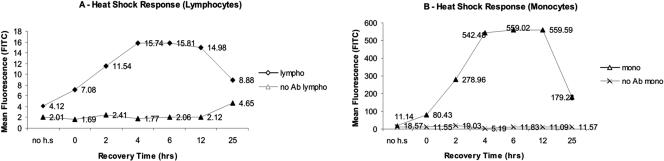
Kinetics of HSR in terms of the induction of synthesis of Hsp70 and accumulation over time were determined in monocytes and lymphocytes isolated from blood samples taken from a young (age 29 years) healthy male. Figure 2 shows that Hsp70 levels had already increased significantly during the 1-hour period of HS at 42°C; continued to increase until 4 hours after HS, after a stable period of about 6–8 hours; and finally declined toward the basal level after 24 hours.
Fig 2.
Kinetics of heat shock response in lymphocytes (A) and monocytes (B). The values on the y-axis are arbitrary and show the amount of fluorescence emitted by FITC, which corresponds to the amount of Hsp70 present inside the cells. no Ab, measure of the control when no Hsp70-specific antibody was used
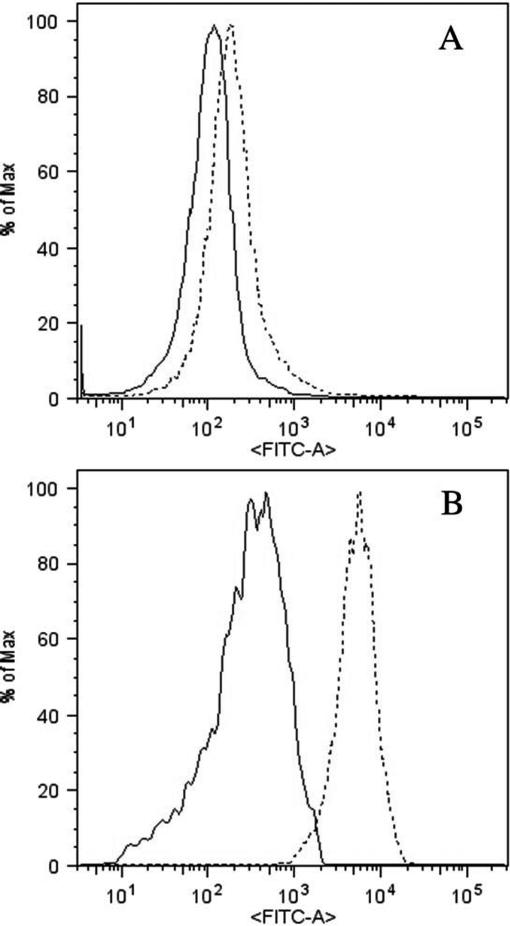
This time-dependent trend in the increase and decrease in Hsp70 levels was observed both for lymphocytes (Fig 2A) and monocytes (Fig 2B). On the basis of these observations, the condition at 5 hours, after 1 hour of HS at 42°C, was chosen as a time point for all the following studies and comparisons. Cell-specific induction of Hsp70 showed that the basal and induced levels of Hsp70 in monocytes were several-fold higher than in lymphocytes (Fig 3).
Fig 3.
Histograms of the cell-specific induction of Hsp70 in lymphocytes (A) and monocytes (B) by FACS. The solid peak corresponds to the non–heat-shocked (basal) level, whereas the dashed peak corresponds to the heat-shocked (induced) level of Hsp70, depicted by the FITC signal. Both the basal and heat-shocked levels of Hsp70 are higher in monocytes than in lymphocytes. Also, more Hsp70 is induced in monocytes than in lymphocytes
Basal and induced levels of Hsp70 with age
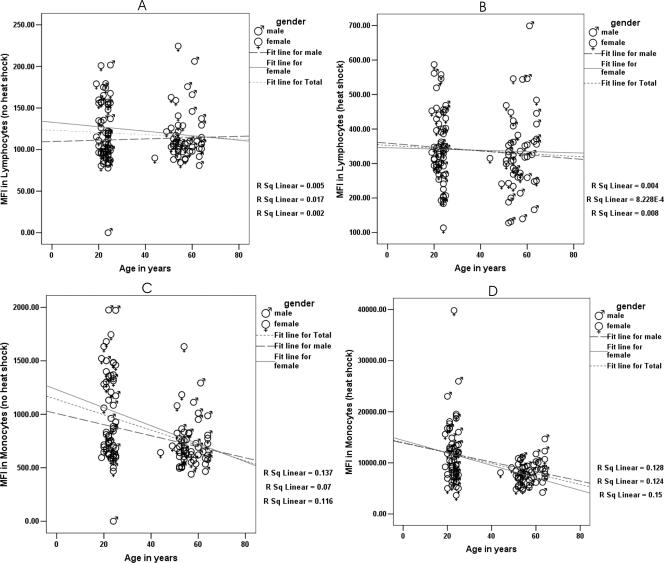
An age-dependent decrease in the ability of monocytes and lymphocytes to synthesize Hsp70 was observed, both before (basal) and after (induced) HS. Figure 4 shows the basal levels at 37°C and induced levels (5 hours after 1 hour of HS at 42°C) of Hsp70 in lymphocytes and monocytes from young and middle-aged individuals.
Fig 4.
Correlation before and after exposure to heat shock between mean fluorescence index (MFI), which corresponds to the amount of Hsp70 synthesized, and age in lymphocytes (A, 37°C), lymphocytes (B, 42°C), monocytes (C, 37°C), and monocytes (D, 42°C). Hsp70 synthesis in monocytes, both before (C; r = −0.341, P < 0.001) and after (D; r = −0.357, P < 0.001) heat shock was significantly correlated with age
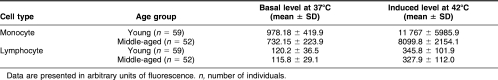
Table 2 summarizes the data, showing that basal levels of Hsp70 significantly differed between monocytes and lymphocytes in both young and middle-aged individuals. The induced level of Hsp70 was also significantly different between monocytes and lymphocytes in both young and middle-aged individuals. Upon HS, monocytes from both young and middle-aged individual showed around a 12-fold increase in the synthesis of Hsp70, whereas lymphocytes from both age groups showed an approximately 3-fold increase in Hsp70 synthesis. A paired-sample t-test showed a significant increase in the HS-induced synthesis of Hsp70 in both cell types in young and middle-aged individuals (P < 0.001; Table 2).
Table 2.
Cell-specific induction of Hsp70 in young and middle-aged individuals
With respect to age-related response, the basal level of Hsp70 in lymphocytes showed no significant difference. However, induced levels decreased with age only in monocytes. The t-test for independent samples was done to compare means in the 2 age groups. In monocytes, the amount of basal and inducible forms of Hsp70 decreased 1.3-fold (P < 0.001) and 1.4-fold (P < 0.001), respectively, with age. In lymphocytes, too, the amount of both basal and inducible Hsp70 decreased with age. This decrease in the synthesis of Hsp70 was maintained when gender-specific analysis was done (data not shown).
A negative correlation between Hsp70 synthesis and age was also observed for monocytes. Hsp70 synthesis in monocytes, both before (r = −0.341; P < 0.001) and after (r = −0.357; P < 0.001) HS, was significantly correlated with age. However, the correlation between Hsp70 synthesis and age in lymphocytes was not significant either before (r = −0.069; P = 0.474) or after (r = −0.065; P = 0.501) HS (Fig 4).
Association of HSP70 gene variations and Hsp70 synthesis
Three polymorphisms in the 3 HSP70 genes were found to be in Hardy–Weinberg equilibrium in the young and middle-aged populations.
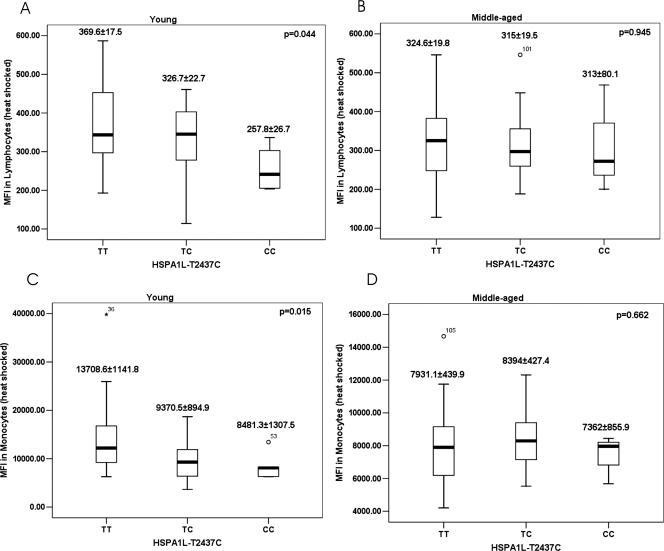
The association of genotypes with Hsp70 induction is shown in Figure 5 in terms of MFI, in arbitrary units, in young and middle-aged individuals. An age-independent association of genotypes, generated by 3 SNPs, with Hsp70 induction showed that genotype HSPA1L-CC had a significantly lower induction than HSPA1L-TT in both monocytes (P = 0.015) and lymphocytes (P = 0.044) in the young population (Fig 5A,C), but not in the middle-aged group (Fig 5B,D). However, there was no association between genotypes and Hsp70 induction in the nonshocked cells (data not shown).
Fig 5.
Mean fluorescence index (MFI) corresponding to the induced Hsp70 represented in the 3 genotypes for the 2 cell types. Comparing the mean by analysis of variance shows a significant decrease in the induction of Hsp70 in both the lymphocytes (A; P = 0.044) and monocytes (C; P = 0.015) among the young carriers of the CC genotype. There was no differential induction among the middle-aged individuals (B, D)
DISCUSSION
In this study, we reported an age-dependent decrease in the ability of human mononuclear cells to respond to HS in terms of induced synthesis of Hsp70. This confirms the result from previous studies (Njemini et al 2002; Visala et al 2003; Jin et al 2004). Because we did not see any gender effects on the ability to respond to stress, we grouped males and females for our further analyses.
The significant novel aspect of our studies is that we have observed that the young carriers of HSPA1L-CC genotype had significantly decreased induction of Hsp70 compared with HSPA1L-TC and HSPA1L-TT genotypes in both cell types. In a population-based genetic association study, we (in preparation) and others (Ross et al 2003) observed that the frequency of HSPA1L-CC genotype carriers decreased with age. This polymorphism is present in the coding region of HSPA1L and leads to an amino acid change at position 493 from a nonpolar hydrophobic Met to a polar neutral Thr. Amino acid 493 is present in the 18-kDa peptide-binding domain on the beta sheet that forms the floor of the peptide binding groove (Pociot et al 1993). Hence, an amino acid change at this position could be associated with the peptide-binding specificity of Hsp70. A change to a polar neutral Thr (allele C) might affect the chaperone activity and the functional efficiency of HSPA1L by lowering the strength of the hydrophobic interactions between chaperones and the target protein (Ross et al 2003). The result from this study reiterates this fact. With this observation, we have been able to give a functional explanation for the earlier findings from gene association studies that this polymorphism affects human longevity. In another study associating human survival with HSP70 genes, we observed that the carriers of HSPA1L-CC genotype had worse survival than the noncarriers (in preparation).
Though HSR has been shown to decrease with age in several studies, Marini et al (2004) did not see any significant difference in the HSR between centenarians and young controls, suggesting that, unlike most elderly subjects, centenarians had kept constant the important element of stress response. They also observed that the carriers of HSPA1A-AA genotype had less HSR than the noncarriers. This A-110C polymorphism is present in the 5′ flanking region, 3 base pairs upstream, of the HS element (HSE) of the promoter region of HSPA1A (Milner and Campbell 1992). Favatier et al (1999) had earlier shown that this polymorphism does not affect the binding of HS factor to the HSE and, hence, the synthesis of Hsp70. Accordingly, in our study, we did not observe any association of this polymorphism with HSR.
In this study, we observed that the HSR difference between different genotypes of HSPA1L(T2437C) was only present at a young age and this genotypic difference diminished in middle-aged individuals. However, in our other studies, we had observed that the decreased mortality associated with CC genotype and haplotypes carrying allele C (A-A-C) is more notable in old age (data not shown). A possible explanation for this could be that, because T to C substitution causes decreased HSR in the young, it can in turn lead to increased stress-related cell death of specific cell types in the young carriers of A-A-C. This in turn might lead to high turnover of the cells, leading to the synthesis of progenitor cells with shorter telomeres. Hence, these cells might go into premature replicative senescence, which could, in turn, lead to decreased survival of the C-carrying haplotype at a later age.
One of the major limitations that all population-based longevity association studies face is that possible genetic association is rarely evaluated and tested through functional assays. Hence, although one gets some information on a possible difference in the allele, genotype, or haplotype frequencies between young and old individuals or knows the survival advantage of the carriers of one type of genetic variant over the other, one never gets to know how these genetic differences express themselves at the cellular level. In this study, we have successfully substantiated our observations from the genetic study with a series of functional analyses, showing that those genetic variants that are positively associated with human longevity and with survival advantage are also associated with an increased ability to respond to stress.
Acknowledgments
We thank the personnel of the blood bank, Aarhus University hospital, for their help in collecting samples. Special thanks to Dr Marianne Hokland, Department of Microbiology, Aarhus University, for discussions on flow cytometry. Thanks also to Hanne Jacobsen for her help with flow cytometer. These studies received financial support from the Danish Center for Molecular Gerontology, and the Danish Research Councils, FNU and FSS.
REFERENCES
- Altomare K, Greco V, and Bellizzi D. et al. 2003 The allele (A)(-110) in the promoter region of the HSP70-1 gene is unfavorable to longevity in women. Biogerontology. 4:215–220. [DOI] [PubMed] [Google Scholar]
- Favatier F, Jacquier-Sarlin MR, Swierczewski E, Polla BS. Polymorphism in the regulatory sequence of the human hsp70-1 gene does not affect heat shock factor binding or heat shock protein synthesis. Cell Mol Life Sci. 1999;56:701–708. doi: 10.1007/s000180050463.1420-682X(1999)056[0701:PITRSO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goate AM, Cooper DN, Hall C, Leung TKC, Solomon E, Lim L. Localization of a human heat-shock Hsp-70 gene sequence to chromosome-6 and detection of 2 other loci by somatic-cell hybrid and restriction-fragment-length-polymorphism analysis. Hum Genet. 1987;75:123–128. doi: 10.1007/BF00591072.0340-6717(1987)075[0123:LOAHHH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jin X, Wang R, and Xiao C. et al. 2004 Serum and lymphocyte levels of heat shock protein 70 in aging: a study in the normal Chinese population. Cell Stress Chaperones. 9:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Ann Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443.0066-4154(1986)055[1151:THR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Marini M, Lapalombella R, and Canaider S. et al. 2004 Heat shock response by EBV-immortalized B-lymphocytes from centenarians and control subjects: a model to study the relevance of stress response in longevity. Exp Gerontol. 39:83–90. [DOI] [PubMed] [Google Scholar]
- Milner CM, Campbell RD. Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics. 1990;32:242–251. doi: 10.1007/BF00187095.0093-7711(1990)032[0242:SAEOTT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Milner CM, Campbell RD. Polymorphic analysis of the three MHC-linked HSP70 genes. Immunogenetics. 1992;36:357–362. doi: 10.1007/BF00218042.0093-7711(1992)036[0357:PAOTTM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Njemini R, Vanden Abeele M, Demanet C, Lambert M, Vandebosch S, Mets T. Age-related decrease in the inducibility of heat-shock protein 70 in human peripheral blood mononuclear cells. J Clin Immunol. 2002;22:195–205. doi: 10.1023/a:1016036724386.0271-9142(2002)022[0195:ADITIO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pociot F, Ronningen KS, Nerup J. Polymorphic analysis of the human MHC-linked heat shock protein 70 (HSP70-2) and HSP70-Hom genes in insulin-dependent diabetes mellitus (IDDM) Scand J Immunol. 1993;38:491–495. doi: 10.1111/j.1365-3083.1993.tb02593.x.0300-9475(1993)038[0491:PAOTHM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rattan SI, Clark BF. Understanding and modulating ageing. IUBMB Life. 2005;57:297–304. doi: 10.1080/15216540500092195.1521-6543(2005)057[0297:UAMA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ross OA, Curran MD, Crum KA, Rea IM, Barnett YA, Middleton D. Increased frequency of the 2437T allele of the heat shock protein 70-Hom gene in an aged Irish population. Exp Gerontol. 2003;38:561–565. doi: 10.1016/s0531-5565(03)00006-8.0531-5565(2003)038[0561:IFOTTA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Singh R, Kølvraa S, Bross P, Gregersen N, Nexo BA, Frederiksen H, Christensen K, Rattan SIS. Association between low self-rated health and heterozygosity for −110A>C polymorphism in the promoter region of HSP70-1 in aged Danish twins. Biogerontology. 2004;5:169–176. doi: 10.1023/B:BGEN.0000031154.57176.4f.1389-5729(2004)005[0169:ABLSHA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Singh R, Kølvraa S, and Bross P. et al. 2006 Heat shock protein 70 genes and human longevity: a view from Denmark. Ann NY Acad Sci. 1067:303–310. [DOI] [PubMed] [Google Scholar]
- Verbeke P, Fonager J, Clark BFC, Rattan SIS. Heat shock response and ageing: mechanisms and applications. Cell Biol Int. 2001;25:845–857. doi: 10.1006/cbir.2001.0789.1065-6995(2001)025[0845:HSRAAM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Visala RD, Boyle GM, Parsons PG, Watson K, Jones GL. Influence of ageing, heat shock treatment and in vivo total antioxidant status on gene-expression profile and protein synthesis in human peripheral lymphocytes. Mech Ageing Dev. 2003;124:55–69. doi: 10.1016/s0047-6374(02)00170-7.0047-6374(2003)124[0055:IOAHST]2.0.CO;2 [DOI] [PubMed] [Google Scholar]