Abstract
Produced by dietary fiber, butyrate is a potential chemopreventive agent against colon cancer. It stimulates proliferation of normal colonic epithelial cells but induces growth inhibition, differentiation, apoptosis, or a combination of effects in colon carcinoma cells. In this study, we used cDNA membrane arrays and real-time reverse transcriptase– polymerase chain reaction to identify stress genes that were differentially regulated by sodium butyrate (NaB) in HT 29 human colon carcinoma cells. The results indicated that a group of heat shock protein (hsp) genes were upregulated by 3 mM NaB within the first 24 hours of exposure. Because the transcription of hsp genes is under the control of heat shock factors (HSFs), we measured the effects of overexpressed HSF-1 on the responses of HT 29 cells to NaB. Overexpression of HSF-1 inhibited NaB-induced differentiation as measured by alkaline phosphatase activity and carcinoembryonic antigen expression. These results suggest that increased expression of HSFs and Hsps might render colon carcinoma cells resistant to the chemopreventive effects of butyrate.
INTRODUCTION
The development of colon cancer often follows a defined pattern with a sequence of aberrant crypt cells, epithelial hyperplasia, adenomatous polyps, carcinoma, and metastasis (Fearon and Vogelstein 1990; Lengauer et al 1998). Multiple mutations, which either activate oncogenes or inactivate tumor suppressor genes, are required during both the initiation and promotion phases. This entire process often takes decades before a malignant tumor is finally formed. Because colon epithelia are directly exposed to dietary compounds, elimination of precancerous or cancerous cells by nutritional or chemopreventive interventions, or both, represents a promising approach to the lowering of the incidence of colon cancer (Lipkin et al 1999; Dove-Edwin and Thomas 2001).
The normal turnover rate of intestinal epithelia is quite high, and a complete renewal takes only 5 to 7 days (Cheng 1974; Barkla and Gibson 1999). New cells are generated in epithelial crypts and maturation occurs when newly generated cells move from the bottom of the crypt toward the top of the villus. Terminally differentiated intestinal epithelial cells have a life span of only a few days and then are sloughed into the lumen of the gut. This self-renewal process provides an efficient way to eliminate cells carrying genetic mutations that are caused by exposure to dietary carcinogens and other toxic compounds. However, cells carrying mutations that disrupt their intrinsic suicide program can escape from the defined life cycle and become potential sources of neoplasia. Certain dietary compounds, such as butyrate from fiber and vitamin D, have been shown in cultured cells to selectively promote and restore differentiation in neoplastic cells and thereby have the potential to eliminate aberrant cells at an early stage of tumor formation (Lipkin et al 1999; Dove-Edwin and Thomas 2001).
Intestinal microflora can ferment undigested dietary fiber and resistant starch to generate relatively high concentrations of butyric acid and other short-chain fatty acids (Cummings 1981). Thus, the fecal concentration of butyrate can range from 5 to 50 mM (Cummings 1981; Scheppach 1994). Butyrate provides an energy source to normal colonic epithelial cells (Scheppach 1994) and can stimulate their proliferation (Gibson et al 1992; Ichikawa and Sakata 1998). The latter contrasts with its effects on most cancer cell lines in vitro, in which butyrate has been shown to induce growth inhibition, differentiation, apoptosis, or a combination of effects at concentrations that can be readily achieved in vivo (Barnard and Warwick 1993; Velcich et al 1995; Kirlin et al 1999a). The mechanism(s) underlying differential effects on normal and neoplastic cells remains to be determined.
The differentiation process is often associated with an increased expression of heat shock proteins (Hsps; Singh and Yu 1984; Welsh and Gaestel 1998). Hsps constitute a family of molecular chaperone proteins that are highly conserved in all living organisms (Morimoto et al 1990; Kiang and Tsoko 1998). Induction of Hsps is one of the primary cellular defense mechanisms used by cells in response to a variety of stressful conditions. Hsps also function in normal physiological processes such as embryonic development and cellular differentiation (Pirkkala et al 2001). For example, hemin-induced differentiation of erythroid cells is associated with the accumulation of Hsp70 (Singh and Yu 1984). The level of Hsp27 is also increased when keratinocytes differentiate in hair follicles (Hashizume et al 1997). The functions of the upregulated Hsps in the differentiation process remain to be determined.
The transcriptional regulation of different heat shock protein (hsp) genes is under the control of heat shock factors (HSFs; Morimoto et al 1990; Pirkkala et al 2001). Upon stress, HSFs trimerize and bind to heat shock elements (HSEs) that are located within the promoter regions of the hsp genes. Four different HSFs have been identified thus far (Pirkkala et al 2001). Knock-out studies and overexpression of HSF-1 in transgenic mice have shown that HSF-1 has a critical role in heat shock response, as well as in spermatogenesis and embryonic development (Christians et al 2000; Nakai et al 2000).
In this report, we measured stress genes that were differentially regulated by sodium butyrate (NaB) in HT 29 human colon carcinoma cells. Results indicated that certain hsp genes were induced after exposure to butyrate. The finding led to our hypothesis that butyrate-induced heat shock–like response might regulate the differentiation, cell death processes, or both in HT 29 cells. To test the hypothesis, we stably overexpressed HSF-1 in HT 29 cells and measured the changes in response to NaB treatment. Overexpression of HSF-1 was found to inhibit butyrate-induced differentiation without affecting the cell growth and the cell death produced by this agent. These data suggest that HSF-1 can negatively affect the chemopreventive effects of butyrate by interfering with the maturation process of colonic epithelial cells.
MATERIALS AND METHODS
Cell lines and culture
HT 29 colon carcinoma cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in McCoy's 5A medium supplemented with 10% fetal bovine serum (Life Technologies, Grand Island, NY, USA). For butyrate treatments, cells were seeded into 60-mm culture dishes at 3 × 105 cells/plate and treated for up to 3 days. Phoenix amphotropic retroviral packaging cells (derived from 293 cells) were obtained from ATCC upon approval by Dr Gary Nolan (Stanford University, CA, USA) and were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum.
RNA differential display
After exposure to 3 mM NaB for 48 hours, total RNA was isolated from HT 29 cells with Trizol reagent (Life Technologies) and treated with RNase-free DNase (Promega, Madison, WI, USA) to remove any trace of genomic DNA contamination. Poly(A)+ RNA was enriched with the Oligotex mRNA purification system (Qiagen, Valencia, CA, USA), labeled with [32P]-dCTP, and hybridized to the Atlas human stress and toxicology array (Clontech, Palo Alto, CA, USA) according to the manufacture's guide. After stringent washes, the membranes were developed with the use of either a phosphoimager (Molecular Dynamics, Sunnyvale, CA, USA) or BioMax X-ray films (Eastman Kodak Company, Rochester, NY, USA) in the presence of an intensifying screen. Image QuaNT software (Molecular Dynamics) was used to draw a 24 × 16 grid to cover the entire array and quantify the intensity of the spots.
Real-time reverse transcriptase–polymerase chain reaction
HT 29 cells were treated with 3 or 5 mM NaB for 16 to 72 hours. RNA was isolated, treated with DNase I (Ambion, Austin, TX, USA), and reversely transcribed with random hexamer primers and M-MLV reverse transcriptase (Promega). Real-time polymerase chain reaction (PCR) was performed in an ABI 7300 system (Applied Biosystems, Forster City, CA, USA) with primer sets provided with the TaqMan® Gene Expression Assays. Average threshold cycle (Ct) values were used to determine the relative differences between control and treated groups and were normalized to the 18s ribosomal RNA (Cai et al 2004).
Dual luciferase reporter assay
The HT 29 cells were seeded into 6-well plates at 2.5 × 105 cells/well and transfected with 1.0 μg of a luciferase reporter plasmid containing the heat shock response element (pHSE-Luc; Clontech) with the use of the Fugene 6 Reagent (Roché; Branchburg, NJ, USA). To control for transfection efficiency, cells were cotransfected with 10 ng of the pRL-CMV vector (Promega), which contained the Renilla luciferase gene driven by the constitutive cytomegalovirus promoter. After 6 hours, the transfection reagents were removed and 3 mM NaB was added in fresh medium to treat the cells for additional time as indicated. By the end of the treatment, cells were lysed with 500 μL Passive Lysis Buffer (Promega). The luciferase activities were measured with the dual luciferase assay kit (Promega) in a Zylux FB12 luminometer (Pforzheim, Germany). The relative light units per second (RLU/s) were normalized to the R. luciferase activity and expressed as folds of control, the ratios between HSE-Luc activities obtained from cells with and without NaB treatment.
Transfection with retroviral vector
Human HSF-1 cDNA, kindly provided by Dr Pradip Roy-Burman (University of Southern California, Los Angeles, CA, USA), was subcloned into the pLXIN retroviral expression vector (Clontech). Viral stock preparation and infection of HT 29 cells were performed as described previously (Chen Y et al 2002). Briefly, the pLXIN vector alone or vector containing the HSF-1 insert were transfected into Phoenix Amphotropic cells with Fugene 6 reagent (Roche Biochem, Indianapolis, IN, USA). Two days after transfection, the culture medium containing replication-deficient virus was collected, filtered through a 0.45-μm filter, and used to infect HT 29 cells in the presence of 0.5 μg/mL polybrene (Sigma, St. Louis, MO, USA) for 6 hours. Infected cells were then selected in 800 μg/ mL of geneticin for 2 weeks, and individual clones were obtained by limiting dilution.
Measurement of carcinoembryonic antigen by flow cytometry
After treatment with 3 and 5 mM butyrate for 1 to 3 days, HT29 parental cells, cells transduced with HSF-1, or cells transduced with the viral vector that did not contain the HSF-1 cDNA were collected by gentle trypsinization and washed with a prechilled washing buffer (1× phosphate-buffered saline [PBS], 1% fetal bovine serum, 0.09% sodium azide). Cells were then incubated with 10 μg/mL of rabbit anti-human carcinoembryonic antigen (CEA) antibody (DAKO Corporation, Carpinteria, CA, USA) for 30 minutes on ice. After 2 washes, 10 μg/mL of a fluorescein isothiocyanate–conjugated goat anti-rabbit antibody (Jackson Immuno Research Laboratory, West Grove, PA, USA) was used for an additional 30 minutes of incubation at 4°C. Cells were then washed twice in PBS and kept on ice until analyzed by flow cytometry (BD Immunocytometry Systems, San Jose, CA, USA) as described previously (Chen Y et al 2002).
Measurement of alkaline phosphatase activity
HT 29 cells were treated with 3 and 5 mM NaB for 2 days. Cell extracts were prepared by 2 cycles of rapid freezing and thawing in a dry ice-ethanol bath. After centrifugation at 12 000 × g for 5 minutes, the supernatant was used for measurement of alkaline phosphatase (AP) activity, with p-nitrophenyl phosphate as the substrate (Kirlin et al 1999b).
Viability assay
After exposure to 3 and 5 mM butyrate for 3 days, floating cells were collected and pooled with attached cells released by trypsinization. Cells were centrifuged at 3000 × g for 5 minutes, washed once with PBS, and stained with LIVE/DEAD viability/cytotoxicity kit (Molecular Probes, Eugene, OR, USA) for 30 minutes at room temperature, followed by flow cytometry (Chen Y et al 2002).
Western blot analyses
Cells were disrupted in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1% NP-40, 0.25% Na-deoxycholate, 1 mM Na3VO4, 1 mM NaF, and supplemented with a cocktail of protease inhibitors [Roche Biochem]) and kept on ice for 10 minutes. After centrifugation at 12 000 × g for 15 minutes at 4°C, the supernatants were collected and Western blot analyses were performed as previously described (Samali et al 1999). Anti–HSF-1 antibody was purchased from Stressgen (Victoria, British Columbia, Canada). Anti-Hsp27, anti-Hsp70, and anti–heat shock cognate 70 protein (Hsc70) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Statistical analyses
Results from experiments that were repeated at least 3 times were averaged and expressed as mean ± standard error of the mean. Statistical analyses were performed with InStat3 (GraphPad Software Inc, San Diego, CA, USA) with 1-way analysis of variance (ANOVAl α = 0.05) and Dunnett post hoc multiple range tests.
RESULTS
Increased expressions of Hsps in response to butyrate
At physiologically achievable concentrations, butyrate has multiple anticancer effects in vitro. To gain information on the mechanisms by which butyrate exerts its effects on colon carcinoma cells, we used Clontech cDNA membrane arrays to measure genes that were differentially expressed after exposure of HT 29 cells to butyrate. The membrane array that we employed measured the expression of 234 genes that encode proteins with important functions during cellular stress. The data obtained from the array assays indicated that cells treated with 3 mM sodium butyrate for 2 days had increased expression of several hsps that included both the hsp70 family and small heat shock proteins such as hsp27 (data not shown).
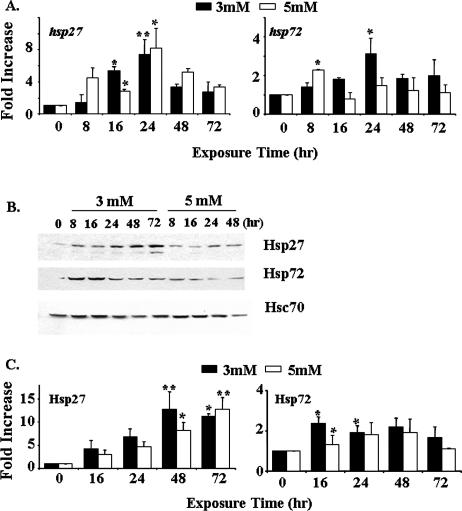
To confirm the findings from differential display, real-time reverse transcriptase (RT)–PCR analyses were performed to measure the changes in the expression of 2 genes—hsp70 and hsp27—after butyrate treatment. Results (Fig 1A) indicated that both hsps were induced by 3 mM NaB after 24 hours of exposure. Similar upregulation of hsp27 was observed in cells treated with 5 mM NaB. However, the hsp70 gene was only transiently induced by 5 mM NaB, and its mRNA level returned to near normal expression after 24 hours.
Fig 1.
NaB-induced upregulation of Hsp27 and Hsp70 in HT 29 colon carcinoma cells. (A) Real-time RT-PCR analyses. Cells were treated with 3 or 5 mM NaB for up to 72 hours. The mRNA levels of hsp27 and hsp70 were measured by TaqMan® Gene Expression Assays. The differences in the average threshold cycle (ΔCt) values were determined and normalized to the expression of 18s rRNA. (B) Western blot analyses of Hsp27 and Hsp70 expression after exposure to NaB. The anti-Hsc70 antibody, used as a loading control, does not recognize other Hsp70 family proteins. (C) Quantification of the data from panel B. The data presented in panels A and C are the averages of 3 separate experiments (mean ± SE). Statistical differences were determined by 1-way ANOVA. Once significance was reached, Dunnett's multiple comparison post hoc test was used to compare between 0 exposure time and other time points; * P < 0.05; ** P < 0.01
We next measured the changes in the protein levels of Hsp27 and Hsp70 in HT 29 cells treated with NaB. The results (Fig 1B,C) indicated that both 3 and 5 mM NaB caused a sustained increase in Hsp27. Consistent with the data from the real-time RT-PCR analyses, the upregulation of Hsp70 only occurred within the first 24 hours after exposure to either 3 or 5 mM NaB. Thus, exposure of cultured HT 29 colon carcinoma cells to butyrate is associated with stress responses and increased expression of Hsp27 and Hsp70. The induction of Hsp27 mRNA and protein was much more sustained than that of Hsp70.
Inhibition of butyrate-induced differentiation by overexpression of HSF-1
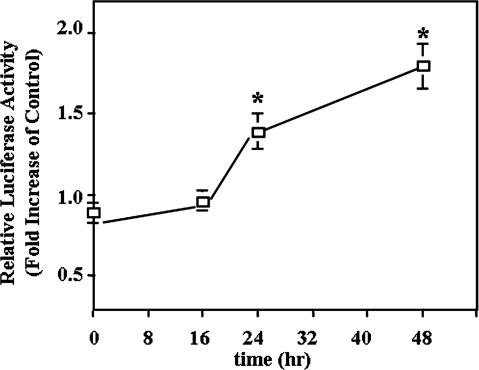
The heat shock response and the expression of the hsps are under the control of HSFs. By transfecting cells with the pHSE-Luc reporter plasmid, we measured the activities of HSFs after exposure to NaB. Results (Fig 2) showed that the luciferase activity driven by the HSE was increased by the 3 mM NaB treatment at 24 and 48 hours, indicating the involvement of HSF in the differentiation process induced by NaB.
Fig 2.
NaB-induced activation of HSF. HT 29 cells were transiently cotransfected with the pHSE-Luc reporter plasmid and the pRL-CMV plasmid for control of transfection efficiency. After transfection, cells were further treated with 3 mM NaB for the time indicated. The luciferase activities resulting from the HSE activation were normalized and calculated as a ratio with cells that did not received NaB. The data presented are the averages of 3 separate experiments (mean ± SE). Statistical differences were determined by 1-way ANOVA. Once significance was reached, Dunnett's multiple comparison post hoc test was used to compare between 0 exposure time and other time points; * P < 0.05
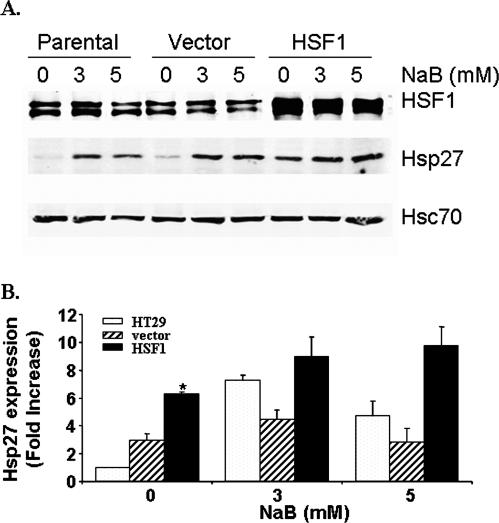
To test the hypothesis that the Hsps and the HSFs might participate in controlling the effects of butyrate on differentiation and cell death, we overexpressed HSF-1 in HT 29 cells by infection with a retroviral vector. Two bands were obtained for HSF-1 on the Western blots (Fig 3A), representing phosphorylated and nonphosphorylated forms of HSF-1, which correspond to the active and inactive forms, respectively (Cotto et al 1996; Chu et al 1998; Hoang et al 2000). NaB treatment did not increase the phosphorylation of HSF-1 (Fig 3A). Hsc70, which is a constitutively expressed member of the Hsp70 family of proteins and is not regulated by HSF-1, was used as a loading control. Cells with increased HSF-1 exhibited normal morphology and proliferation rates under nonstressed conditions (data not shown). Overexpression of HSF-1 resulted in increased expression of Hsp27 under nonstressed conditions (Fig 3B). However, after exposure to NaB for 24 hours, the protein levels of Hsp27 were not significantly different between cells with and without HSF-1 overexpression. Similar changes were observed for Hsp72 (data not shown). Thus, overexpression of HSF-1 increased the basal expression of the HSPs.
Fig 3.
Overexpression of HSF-1 increased the basal expression of Hsp27. (A) Western blot analyses were performed with HT 29 cells overexpressing HSF-1 and treated with NaB as indicated. (B) Quantification data showing the averages of 3 separate experiments (mean ± SE). Statistical differences were determined by 1-way ANOVA. Once significance was reached, Dunnett's multiple comparison post hoc test was used to compare between the parental HT 29 cells, cells transduced with the retroviral vector, and the cells overexpressing HSF-1; * P < 0.05
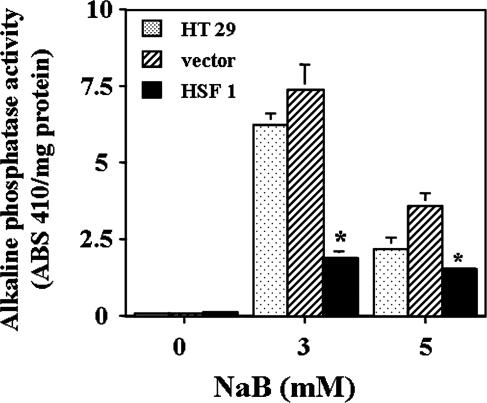
To test the hypothesis that increased HSF-1 might interfere with butyrate-induced cellular differentiation, we measured 2 independent markers of differentiation and compared the changes among HT 29 parental cells, HT 29 cells transduced with HSF-1, or the viral vector only that did not contain the HSF-1. First we measured the activities of AP, one of the brush border enzymes whose activities increase when HT 29 cells are differentiated by butyrate (Kim et al 1980). Compared with nontransduced parental cells and vector-expressing control cells, cells overexpressing HSF-1 had significantly lower AP activity, particularly after 3 mM butyrate treatment for 2 days (Fig 4). Cells treated with the higher concentration of 5 mM butyrate had lower AP activity, which could be artifactual because of the apoptotic or cytotoxic effects of this higher concentration of butyrate.
Fig 4.
Measurement of AP activity in butyrate-treated cells. Cell lysates were prepared with 2 cycles of rapid freezing and thawing, and AP activity was measured with the use of p-nitrophenyl phosphate as substrate after 2 days of exposure to 3 or 5 mM of NaB. The data presented are the averages of 3 separate experiments (mean ± SE). For 3 and 5 mM NaB-treated samples, statistical differences were determined by 1-way ANOVA. Once significance was reached, Dunnett's multiple comparisons test was used to compare between nontransduced HT 29 parental cells and cells transduced with either vector alone or with HSF1; * P < 0.05
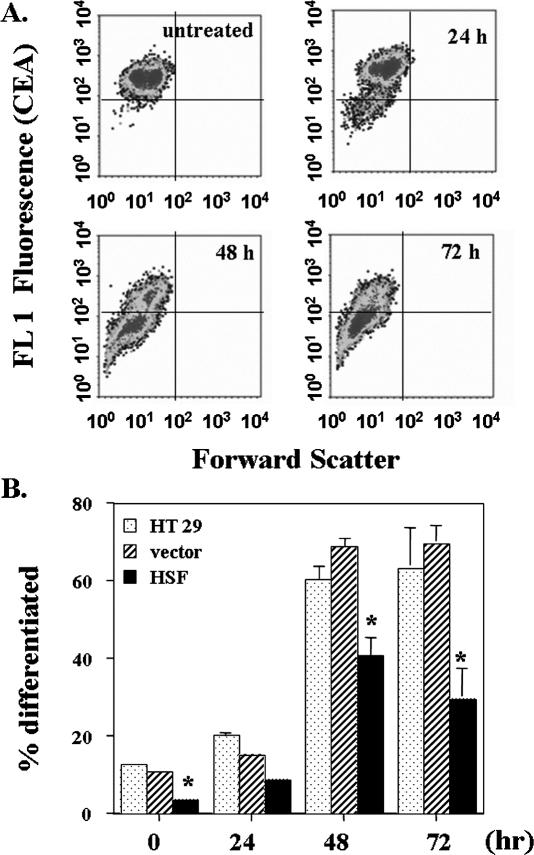
The differentiation of colon carcinoma cells is often associated with changes in the expression of CEA (Aquino et al 1998; Cai et al 2004). Flow cytometric analyses after labeling with anti-CEA antibody showed that butyrate downregulated CEA in HT 29 cells (Fig 5A). Cells with overexpressed HSF-1 had a lower percentage of differentiated cells as measured by CEA both before and after butyrate treatment; at 72 hours, only 32% of transfected cells were differentiated. In contrast, 75% of the parental HT 29 cells or the vector-transduced cells exposed to butyrate were differentiated (Fig 5B). Thus, results from measurement of AP activities and CEA expression both demonstrated that increased HSF-1 inhibited NaB-induced differentiation.
Fig 5.
Measurement of carcinoembryonic antigen (CEA) expression in response to exposure to 3 mM butyrate for various periods of time. A rabbit anti-human CEA antibody was used to label the CEA expression on the cell surface. Flow cytometry data showed a population of cells with lower CEA expression (lower left quadrant) after butyrate treatment (A) that were considered differentiated cells; (B) quantification data. The data presented are the average of 3 separate experiments (mean ± SE). Statistical differences were determined by 1-way ANOVA. Once significance was reached, Dunnett's multiple comparisons was used to compare between nontransduced HT 29 parental cells and cells transduced with either vector alone or with HSF1; * P < 0.05
Effects of overexpressing HSF-1 on butyrate-induced cell death
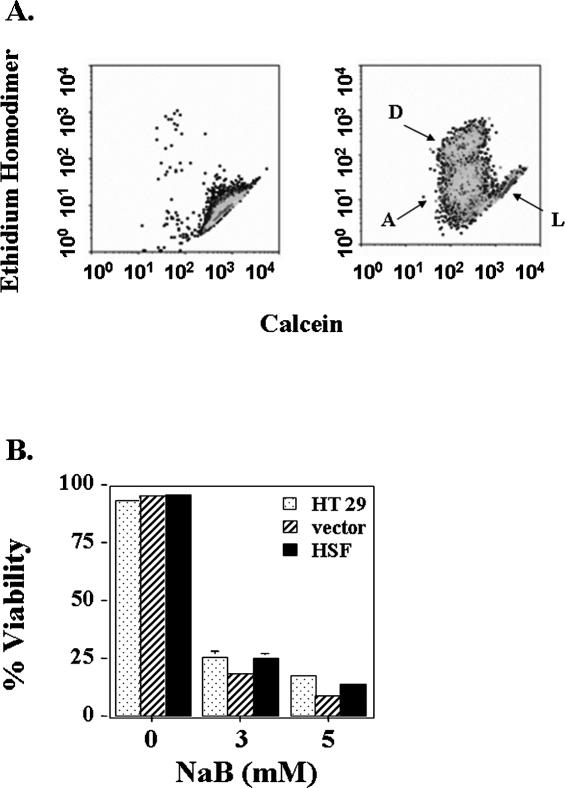
Terminally differentiated colon epithelial cells lose their potential to divide and eventually undergo a programmed cell death that involves activation of caspases (Hall et al 1994; Cai et al 2004). Because overexpression of HSF-1 inhibited differentiation, we hypothesized that it might also play a role in controlling butyrate-induced cell death. Therefore, we measured the percentage of dead cells after treatment with 3 or 5 mM butyrate in HT 29 cells with a technique involving staining cells with compromised plasma membranes with an ethidium homodimer (Chen Y et al 2002). Live cells have esterase activity and convert cell-permeable calcein AM to fluorescent calcein. Data (Fig 6) showed that butyrate caused about 80% cell death after 3 days of exposure, and this effect was not modified by overexpression of HSF-1. No difference was observed between nontransduced parental HT 29 cells, vector-transduced cells, or cells overexpressing HSF-1.
Fig 6.
Viability of HT 29 cells treated with butyrate for 3 days. (A) Flow cytometry measurement of cells double-stained with ethidium homodimer and calcein AM. Left panel represents control untreated cells, and right panel represents cells treated with 3 mM butyrate. The live cells (L) and dead cells (D) are indicated on the figure. Cells in between these 2 populations (A) are likely to be early apoptotic cells. (B) Quantification of the flow cytometry data. Data presented are the average of 3 separate experiments (mean ± SE). No statistically significant difference was determined by 1-way ANOVA between HT 29 parental cells, cells transduced with HSF1, or vector alone under conditions of either 3 or 5 mM NaB treatment
Effects of N-acetylcysteine on butyrate-induced upregulation of Hsps
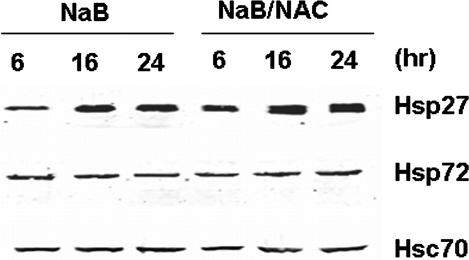
Our previous studies have shown that NaB-treated HT 29 cells exhibit a marked oxidation of the intracellular glutathione pool (Kirlin et al 1999b), and the oxidation occurred with a similar time course as the induction of hsps, as shown in Figure 1. Therefore, the observed upregulation of hsps may involve a mechanism that is subject to redox regulation. To test such a hypothesis, we treated cells with 5 mM N-acetylcysteine (NAC) at the same time as they were exposed to 3 mM NaB. Results (Fig 7) showed that the protein levels of Hsp27 and Hsp72 were not affected by NAC. We also found that NAC did not affect NaB-induced changes in cell proliferation and AP activity (data not shown). Thus, NAC did not suppress butyrate-induced differentiation and upregulation of Hsps in the HT 29 cells.
Fig 7.
Effects of NAC on NaB-induced changes in HSP expression. Cells were treated with 3 mM NaB and 5 mM NAC for the times indicated. Western blot analyses were performed to measure the expressions of Hsp27 and Hsp72. Hsc70 was used as a loading control. Data presented are representative of 3 separate experiments
DISCUSSION
Increased intake of dietary fiber has been shown to be associated with a decreased risk of developing colon cancer (Burkitt 1971; Howe et al 1992). One possible mechanism for the chemopreventive effects of fiber is through the action of its fermentation product, butyrate. Millimolar concentrations of butyrate exert a strong anticancer effect in vitro. However, in contrast to the promising results obtained from cultured colon carcinoma cell lines, the in vivo anticancer effects of fiber and butyrate are less consistent or effective (Lupton 1995; Hague et al 1997). Several reasons appear to account for these differences. Differentiation of colonocytes, either induced by butyrate or by other conditions, is reversible at early times of exposure. Thus, if a continuous in vivo exposure to high concentrations of butyrate is not attained, the irreversible commitment to differentiation might not occur and cells could return to their normal malignant phenotype and regain the capacity to proliferate. In addition, an upregulation of cellular proteins that protect the cell against differentiation and apoptosis could also contribute to resistance to the chemopreventive properties of butyrate and fiber.
In this study, we used cDNA membrane arrays to delineate differential gene expression patterns induced by butyrate treatment. In butyrate-treated HT 29 cells, the most distinctive change among the stress genes was the upregulation of the hsps. These results were confirmed by real-time RT-PCR analyses of 2 hsps: hsp70 and hsp27 (Fig 1). We also demonstrated the increased protein expressions of both Hsp27 and Hsp70 by Western blot analyses (Fig 1B,C). Thus, butyrate caused a stress response, which occurred in association with the differentiation process. Similar results indicating the NaB-induced upregulation of Hsps have been reported from other studies (Mariadason et al 2000; Yang et al 2001; Tan et al 2002), including the sustained increase in Hsp27 through 72 hours (Tan et al 2002). However, the magnitude and time course of the changes differed between Hsp27 and Hsp70. It is known that Hsp70 protein can serve as a negative regulator of the heat shock response (Morimoto 1998). Transient overexpression of Hsp70 was shown to inhibit the transcriptional activation function of HSF-1 (Shi et al 1998). In addition, the different genes encoding Hsp27 and Hsp70 might have unique regulatory mechanisms and consequently show different responses to butyrate.
Upregulation of the Hsps has been shown to occur during the differentiation of many other cell types (Singh and Yu 1984; Morimoto et al 1990; Hashizume et al 1997; Kiang and Tsoko 1998; Welsh and Gaestel 1998; Pirkkala et al 2001) and, therefore, could represent a generalized phenomenon. The roles of HSFs and Hsps in the differentiation process of colonic epithelial cells are largely unknown. In this study, instead of manipulating the Hsps, we stably overexpressed HSF-1 in HT 29 cells. The increased HSF-1 mimics the stress responses induced by NaB. By measuring 2 independent markers of differentiation, we observed that cells with higher levels of HSF-1 were less likely to undergo differentiation (Figs 4 and 5). Thus, cancer cells with aberrantly high expression of HSF-1 will be more resistant to NaB and consequently be less responsive to its chemopreventive effects in vivo.
Butyrate is an inhibitor of histone deacetylase (Sealy and Chalkley 1978). Histone hyperacetylation results in increased transcription of a variety of genes (Grunstein 1997) and has been reported to increase the expression of Hsps in various lower organisms (Chen T et al 2002; Ovakim and Heikkila 2003; Zhao et al 2005). Whether similar mechanisms could be applied to the colon cancer cells remains to be defined. The activation of HSFs might also be involved in the selective and ordered upregulation of hsps. It is well known that HSFs can respond to oxidative stress, a process also generated during heat shock (Kiang and Tsoko 1998). Although our data indicate that NAC was not effective in suppressing HSF activation (Fig 7), it does not exclude the possibility that other antioxidant compounds could exert distinct effects from NAC by interfering with different cellular signaling pathways. In addition, the levels of ubiquitin mRNA increased during HT 29 cell differentiation (data not shown), suggesting a possible effect on the protein degradation pathway. In support of this possibility, it has been shown that inhibition of proteosome function can result in activation of HSF-1 (Bush et al 1997; Pirkkala et al 2000).
Cancer cells often have increased Hsps (Fuqua et al 1994), which render them more resistant to apoptosis (Ozoren and El-Deiry 2002; Wang et al 2002; Rashmi et al 2003). A recent report by Cen et al (2004) showed that the mRNA levels of hsf1, hsp27, and hsp90 are increased in sporadic colorectal cancers. Increased expression of HSFs and Hsps might be a general mechanism employed by malignant cells to circumvent the terminal differentiation that is involved in prevention therapies. Therefore, the use of agents that interfere with the functions of the Hsps (Hostein et al 2001; Munster et al 2001) in combination with agents that induce differentiation, apoptosis, or both might result in enhanced therapeutic effects.
Acknowledgments
We thank Dr Pradip Roy-Burman (University of Southern California, Los Angeles, CA, USA) for providing the HSF-1 cDNA. This work was supported by US Public Health Service grants CA02817, ES09047, GM08248; Research to Prevent Blindness Inc, and a postdoctoral fellowship to J.C. from the American Institute for Cancer Research.
REFERENCES
- Aquino A, Prete SP, and Greiner JW. et al. 1998 Effect of the combined treatment with 5-fluorouracil, γ-interferon or folinic acid on carcinoembryonic antigen expression in colon cancer cells. Clin Cancer Res. 4:2473–2481. [PubMed] [Google Scholar]
- Barkla DH, Gibson PR. The fate of epithelial cells in the human large intestine. Pathology. 1999;31:230–238. doi: 10.1080/003130299105043.0031-3025(1999)031[0230:TFOECI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Barnard JA, Warwick G. Butyrate rapidly induces growth inhibition and differentiation in HT-29 cells. Cell Growth Differ. 1993;4:495–501.1044-9523(1993)004[0495:BRIGIA]2.0.CO;2 [PubMed] [Google Scholar]
- Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer. 1971;28:3–13. doi: 10.1002/1097-0142(197107)28:1<3::aid-cncr2820280104>3.0.co;2-n.0765-7846(1971)028[0003:EOCOTC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bush KT, Goldberg AL, Nigam SK. Proteosome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance. J Biol Chem. 1997;272:9086–9092. doi: 10.1074/jbc.272.14.9086.0021-9258(1997)272[9086:PILTAH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cai J, Chen Y, Murphy TJ, Jones DP. Role of caspase activation in butyrate-induced terminal differentiation of HT29 colon carcinoma cells. Arch Biochem Biophys. 2004;424:119–127. doi: 10.1016/j.abb.2004.02.012.0003-9861(2004)424[0119:ROCAIB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cen H, Zheng S, Fang YM, Tang XP, Dong Q. Induction of HSF 1 expression is associated with sporadic colorectal cancer. World J Gastroenterol. 2004;10:3122–3126. doi: 10.3748/wjg.v10.i21.3122.1007-9327(2004)010[3122:IOHEIA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Sun H, Lu J, Zhao Y, Tao D, Li X, Huang B. Histone acetylation is involved in hsp70 gene transcription regulation in Drosophila. Arch Biochem Biophys. 2002;408:171–176. doi: 10.1016/s0003-9861(02)00564-7.0003-9861(2002)408[0171:HAIIIH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chen Y, Cai J, Murphy TJ, Jones DP. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J Biol Chem. 2002;277:33242–33248. doi: 10.1074/jbc.M202026200.0021-9258(2002)277[33242:OHMTCR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cheng H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. Am J Anat. 1974;141:461–500. doi: 10.1002/aja.1001410403.0002-9106(1974)141[0461:ODAROT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Christians E, Davis AA, Thomas SD, Benjamin JJ. Maternal effects of HSF 1 on reproductive success. Nature. 2000;407:693–694. doi: 10.1038/35037669.1476-4687(2000)407[0693:MEOHOR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chu B, Zhong R, Soncin F, Stevenson MA, Calderwood SK. Transcriptional activity of heat shock factor 1 at 37°C is repressed through phosphorylation on two distinct serine residues by glycogen synthase kinase 3α and protein Cα and Cζ. J Biol Chem. 1998;273:18640–18646. doi: 10.1074/jbc.273.29.18640.0021-9258(1998)273[18640:TAOHSF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cotto JJ, Kline M, Morimoto RI. Activation of heat shock factor 1 DAN binding precedes stress-induced serine phosphorylation. J Biol Chem. 1996;271:3355–3358. doi: 10.1074/jbc.271.7.3355.0021-9258(1996)271[3355:AOHSFD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763–779. doi: 10.1136/gut.22.9.763.0017-5749(1981)022[0763:SCFAIT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove-Edwin I, Thomas HJ. The prevention of colorectal cancer. Aliment Pharmacol Ther. 2001;15:323–336. doi: 10.1046/j.1365-2036.2001.00934.x.0269-2813(2001)015[0323:TPOCC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i.0092-8674(1990)061[0759:AGMFCT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fuqua SA, Oesterreich S, Hilsenbeck SG, Von Hoff DD, Eckardt J, Osborne CK. Heat shock proteins and drug resistance. Breast Cancer Res Treat. 1994;32:67–71. doi: 10.1007/BF00666207.0167-6806(1994)032[0067:HSPADR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gibson PR, Moeller I, Kagelari O, Folino M, Young GP. Contrasting effects of butyrate on the expression of phenotypic markers of differentiation in neoplastic and non-neoplastic colonic epithelial cells in vitro. J Gastroenterol Hepatol. 1992;7:165–172. doi: 10.1111/j.1440-1746.1992.tb00956.x.0815-9319(1992)007[0165:CEOBOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–351. doi: 10.1038/38664.1476-4687(1997)389[0349:HAICSA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hague A, Singh B, Paraskeva C. Butyrate acts as a survival factor in colonic epithelial cells: further fuel of the in vivo versus in vitro debate. Gastroenterology. 1997;112:1036–1039. doi: 10.1053/gast.1997.v112.agast971036.0016-5085(1997)112[1036:BAAASF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: importance of apoptosis. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569.0021-9533(1994)107[3569:ROCNIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hashizume H, Tokura Y, Takigawa M, Paus R. Hair cycle-dependent expression of heat shock proteins in hair follicle epithelium. Int J Dermatol. 1997;36:587–592. doi: 10.1046/j.1365-4362.1997.00178.x.0011-9059(1997)036[0587:HCEOHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hoang AT, Huang J, Rudra-Ganguly N, Zheng J, Powell WC, Rabindran SK, Wu C, Roy-Burman P. A novel association between the human heat shock transcription factor 1 (HSF 1) and prostate adenocarcinoma. Am J Pathol. 2000;15:857–864. doi: 10.1016/S0002-9440(10)64954-1.0002-9440(2000)015[0857:ANABTH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostein I, Robertson D, Distefano F, Workman P, Clarke PA. Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis. Cancer Res. 2001;19:273–284.0008-5472(2001)019[0273:IOSTBT]2.0.CO;2 [PubMed] [Google Scholar]
- Howe GR, Benito E, and Castellato R. et al. 1992 Dietary intake of fiber and decreased risk of cancers of the colon and rectum: evidence from the combined analysis of 13 case-control studies. J Natl Cancer Inst. 84:1887–1896. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sakata T. Stimulation of epithelial cell proliferation of isolated distal colon of rats by continuous colonic infusion of ammonia or short-chain fatty acids is non additive. J Nutr. 1998;128:843–847. doi: 10.1093/jn/128.5.843.0022-3166(1998)128[0843:SOECPO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kiang JG, Tsoko GC. Heat shock protein 70 kDa: molecular biology, biochemistry and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x.0163-7258(1998)080[0183:HSPKMB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kim YS, Tsao D, Siddiqui B, Whitehead JS, Arnstein P, Bennett J, and Hicks J 1980 Effects of sodium butyrate and dimethylsulfoxide on biochemical properties of human colon cancer cells. Cancer. 45(5 Suppl). 1185–1182. [DOI] [PubMed] [Google Scholar]
- Kirlin WG, Cai J, DeLong MJ, Pattern EJ, Jones DP. Dietary compounds that induce cancer preventive phase 2 enzymes activate apoptosis at comparable doses in HT 29 colon carcinoma cells. J Nutr. 1999a;129:1827–1835. doi: 10.1093/jn/129.10.1827.0022-3166(1999)129[1827:DCTICP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kirlin WG, Cai J, Thompson SA, Diaz D, Kavanagh TJ, Jones DP. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med. 1999b;27:1208–1218. doi: 10.1016/s0891-5849(99)00145-8.0891-5849(1999)027[1208:GRPIRT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292.1476-4687(1998)396[0643:GIIHC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lipkin M, Reddy B, Newmark H, Lamprecht SA. Dietary factors in human colorectal cancer. Annu Rev Nutr. 1999;19:545–586. doi: 10.1146/annurev.nutr.19.1.545.0199-9885(1999)019[0545:DFIHCC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lupton JR. Butyrate and colonic cytokinetics: differences between in vitro and in vivo studies. Eur J Cancer Prev. 1995;4:373–378. doi: 10.1097/00008469-199510000-00007.0959-8278(1995)004[0373:BACCDB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mariadason JM, Corner GA, Augenlicht LH. Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res. 2000;60:4561–4572.0008-5472(2000)060[4561:GRIPOC]2.0.CO;2 [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Gene Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788.0890-9369(1998)012[3788:ROTHST]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Tissieres A, and Georgopoulos C 1990 The stress responses, function of the proteins, and perspectives. In: Stress proteins in biology and medicine, ed Morimoto RI, Tissieres A, Georgopoulos C. Cold Spring Harbor Laboratory Press, 1–36. [Google Scholar]
- Munster PN, Srethapakdi M, Moasser MM, Rosen N. Inhibition of heat shock protein 90 function by ansamycins causes the morphological and functional differentiation of breast cancer cells. Cancer Res. 2001;61:2945–2952.0008-5472(2001)061[2945:IOHSPF]2.0.CO;2 [PubMed] [Google Scholar]
- Nakai A, Suzuki M, Tanabe M. Arrest of spermatogenesis in mice expressing an active heat shock transcription factor 1. EMBO J. 2000;19:1545–1554. doi: 10.1093/emboj/19.7.1545.1460-2075(2000)019[1545:AOSIME]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovakim DH, Heikkila JJ. Effect of histone deacetylase inhibitors on heat shock protein gene expression during Xenopus development. Genesis. 2003;36:88–96. doi: 10.1002/gene.10202.1061-2289(2003)036[0088:EOHDIO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ozoren N, El-Deiry W. Heat shock protects HCT116 and H460 cells from TRAIL-induced apoptosis. Exp Cell Res. 2002;281:175–181. doi: 10.1006/excr.2002.5660.0014-4827(2002)281[0175:HSPHAH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pirkkala L, Nykanen P, Sistonen L. Roles of the heat shock transcription factor in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–1131. doi: 10.1096/fj00-0294rev.0892-6638(2001)015[1118:ROTHST]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pirkkala L, Alastalo TP, Zuo X, Benjamin IJ, Sistonen L. Disruption of heat shock factor 1 reveals an essential role in the ubiquitin proteolytic pathway. Mol Cell Biol. 2000;20:2670–2675. doi: 10.1128/mcb.20.8.2670-2675.2000.0270-7306(2000)020[2670:DOHSFR]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashmi R, Santhosh K, Kumar TR, Karunagaran D. Human colon cancer cells differ in their sensitivity to curcumin-induced apoptosis and heat shock protects them by inhibiting the release of apoptosis-inducing factor and capsases. GEBS Lett. 2003;538:19–24. doi: 10.1016/s0014-5793(03)00099-1.0014-5793(2003)538[0019:HCCCDI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase 3, Hsp 60 and Hsp 10 in the mitochondrial fraction of Jurkat cells. EMBO J. 1999;18:2040–2048. doi: 10.1093/emboj/18.8.2040.1460-2075(1999)018[2040:POAPCO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut. 1994;35:S35–S38. doi: 10.1136/gut.35.1_suppl.s35.0017-5749(1994)035[S35:EOSCFA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978;14:115–121. doi: 10.1016/0092-8674(78)90306-9.0092-8674(1978)014[0115:TEOSBO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shi Y, Mosser DD, Morimoto RI. Molecular chaperones as HSF1-specific transcriptional repressors. Gene Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654.0890-9369(1998)012[0654:MCAHTR]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Yu J. Accumulation of a heat shock-like protein during differentiation of human erythroid cell line K562. Nature. 1984;309:631–633. doi: 10.1038/309631a0.1476-4687(1984)309[0631:AOAHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tan S, Seow TK, Liang RC, Koh S, Lee CP, Chung MC, Hooi SC. Proteome analysis of butyrate-treated human colon cancer cells (HT-29) Int J Cancer. 2002;98:523–531. doi: 10.1002/ijc.10236.0020-7136(2002)098[0523:PAOBHC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Velcich A, Palumbo L, Jarry A, Laboisse C, Racevskis J, Augenlicht L. Patterns of expression of lineage-specific markers during the in vitro–induced differentiation of HT 29 colon carcinoma cells. Cell Growth Differ. 1995;6:749–757.1044-9523(1995)006[0749:POEOLM]2.0.CO;2 [PubMed] [Google Scholar]
- Wang HP, Hanlon JG, Rainbow AJ, Espiritu M, Singh G. Up-regulation of Hsp27 plays a role in the resistance of human colon carcinoma HT29 cells to photooxidative stress. Photochem Photobiol. 2002;76:98–104. doi: 10.1562/0031-8655(2002)076<0098:urohpa>2.0.co;2.0031-8655(2002)076[0098:UOHPAR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Gaestel M. Small heat-shock protein family: function in health and disease. Ann NY Acad Sci. 1998;851:28–35. doi: 10.1111/j.1749-6632.1998.tb08973.x.0077-8923(1998)851[0028:SHPFFI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yang W, Velcich A, and Mariadason J. et al. 2001 p21Waf1/cip1 is an important determinant of intestinal cell response to sulindac in vitro and in vivo. Cancer Res. 61:6297–6302. [PubMed] [Google Scholar]
- Zhao Y, Sun H, Lu J, Li X, Chen X, Tao D, Huang W, Huang B. Lifespan extension and elevated hsp gene expression in Drosophila caused by histone deacetylase inhibitors. J Exp Biol. 2005;208:697–705. doi: 10.1242/jeb.01439.0022-0949(2005)208[0697:LEAEHG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]