Abstract
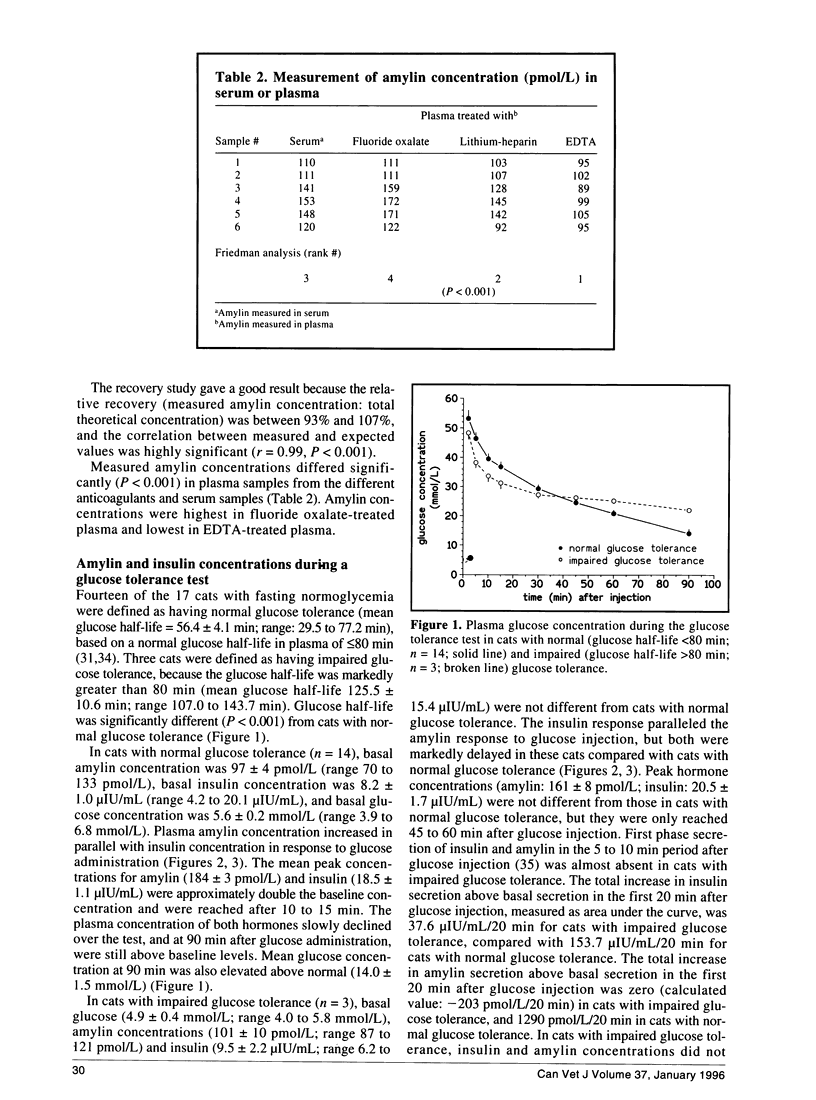
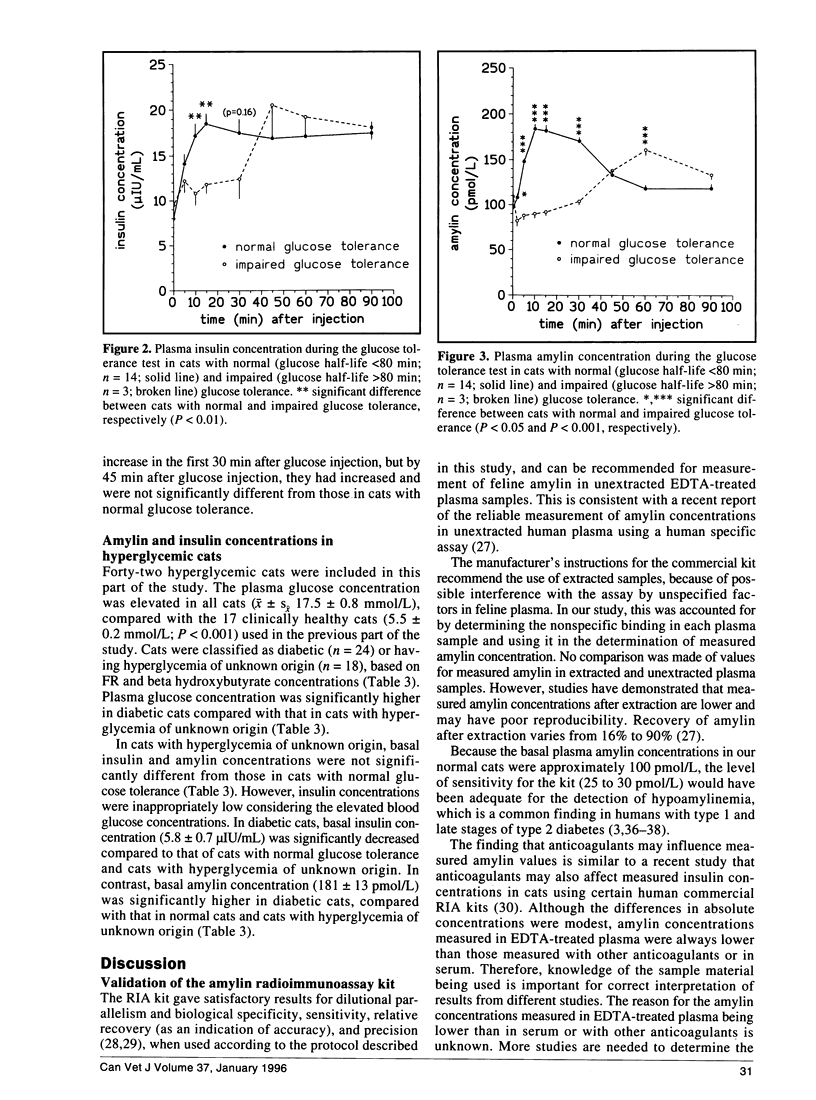
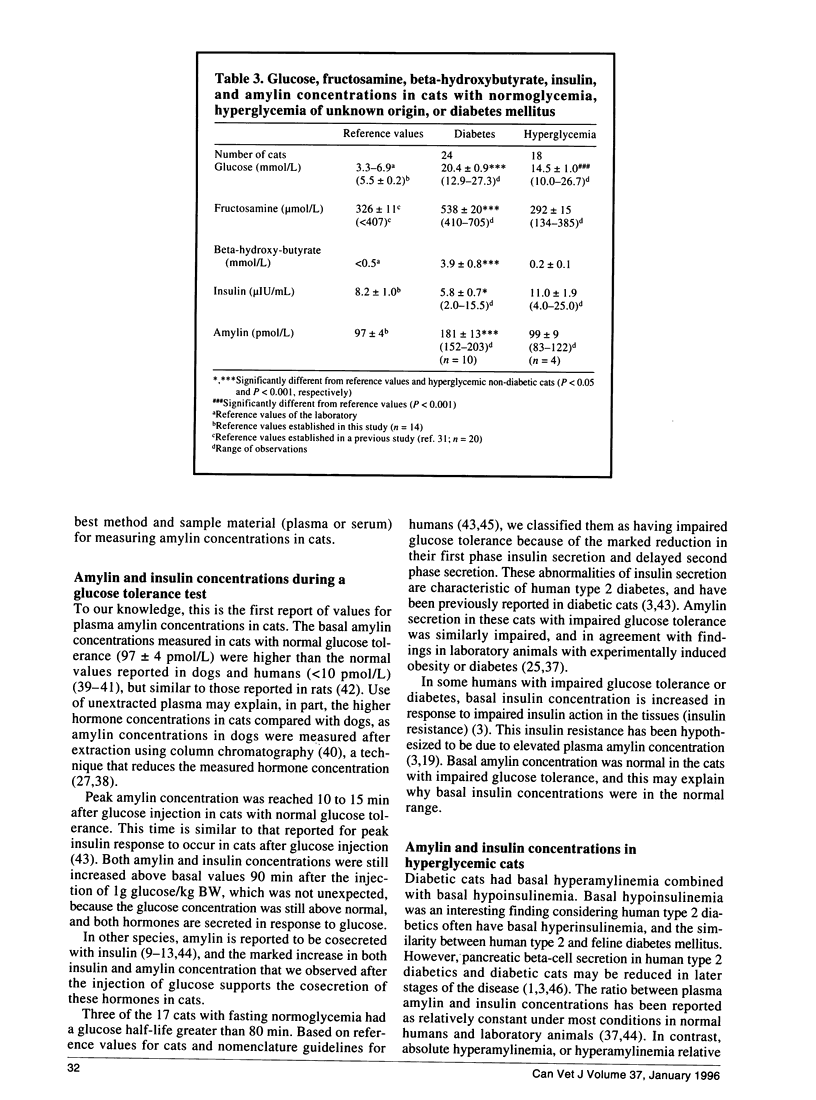
The recently discovered pancreatic peptide amylin is postulated to be involved in the pathogenesis of feline diabetes mellitus. However, plasma amylin concentrations in normal and diabetic cats have not yet been published. The aim of the present study was to validate a commercial amylin radioimmunoassay kit for the measurement of feline amylin in unextracted plasma, and to measure plasma amylin concentrations in normal and diabetic cats. The kit had satisfactory specificity, sensitivity, accuracy, and precision, and can be recommended for measurement of feline amylin in unextracted EDTA plasma, when nonspecific binding of plasma samples is used in the calculation of measured amylin concentration. Fasting amylin concentration in cats with normal glucose tolerance was 97 +/- 4 pmol/L. Plasma amylin increased in parallel with insulin after glucose administration in cats with normal and impaired glucose tolerance. In contrast to cats with normal glucose tolerance, cats with impaired glucose tolerance had markedly delayed amylin and insulin secretion. Diabetic cats had basal hypoinsulinemia combined with hyperamylinemia. Hyperamylinemia may lead to reduced insulin secretion and insulin resistance, and contribute to the development of feline diabetes. In conclusion, feline amylin can be measured in unextracted EDTA plasma. Fasting amylin concentrations are approximately 100 pmol/L, and amylin and insulin are cosecreted in cats with normal and impaired glucose tolerance. Increased amylin concentrations may contribute to the development of feline diabetes mellitus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackard W. G., Clore J. N., Kellum J. M. Amylin/insulin secretory ratios in morbidly obese man: inverse relationship with glucose disappearance rate. J Clin Endocrinol Metab. 1994 May;78(5):1257–1260. doi: 10.1210/jcem.78.5.8175987. [DOI] [PubMed] [Google Scholar]
- Butler P. C., Chou J., Carter W. B., Wang Y. N., Bu B. H., Chang D., Chang J. K., Rizza R. A. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes. 1990 Jun;39(6):752–756. doi: 10.2337/diab.39.6.752. [DOI] [PubMed] [Google Scholar]
- Cooper G. J. Amylin compared with calcitonin gene-related peptide: structure, biology, and relevance to metabolic disease. Endocr Rev. 1994 Apr;15(2):163–201. doi: 10.1210/edrv-15-2-163. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Day A. J., Willis A. C., Roberts A. N., Reid K. B., Leighton B. Amylin and the amylin gene: structure, function and relationship to islet amyloid and to diabetes mellitus. Biochim Biophys Acta. 1989 Dec 14;1014(3):247–258. doi: 10.1016/0167-4889(89)90220-6. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Leighton B., Dimitriadis G. D., Parry-Billings M., Kowalchuk J. M., Howland K., Rothbard J. B., Willis A. C., Reid K. B. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7763–7766. doi: 10.1073/pnas.85.20.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dégano P., Silvestre R. A., Salas M., Peiró E., Marco J. Amylin inhibits glucose-induced insulin secretion in a dose-dependent manner. Study in the perfused rat pancreas. Regul Pept. 1993 Jan 22;43(1-2):91–96. doi: 10.1016/0167-0115(93)90411-z. [DOI] [PubMed] [Google Scholar]
- Enoki S., Mitsukawa T., Takemura J., Nakazato M., Aburaya J., Toshimori H., Matsukara S. Plasma islet amyloid polypeptide levels in obesity, impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1992 Jan;15(1):97–102. doi: 10.1016/0168-8227(92)90074-2. [DOI] [PubMed] [Google Scholar]
- Gedulin B., Cooper G. J., Young A. A. Amylin secretion from the perfused pancreas: dissociation from insulin and abnormal elevation in insulin-resistant diabetic rats. Biochem Biophys Res Commun. 1991 Oct 31;180(2):782–789. doi: 10.1016/s0006-291x(05)81133-7. [DOI] [PubMed] [Google Scholar]
- Gill A. M., Yen T. T. Effects of ciglitazone on endogenous plasma islet amyloid polypeptide and insulin sensitivity in obese-diabetic viable yellow mice. Life Sci. 1991;48(7):703–710. doi: 10.1016/0024-3205(91)90546-n. [DOI] [PubMed] [Google Scholar]
- Hartter E., Svoboda T., Ludvik B., Schuller M., Lell B., Kuenburg E., Brunnbauer M., Woloszczuk W., Prager R. Basal and stimulated plasma levels of pancreatic amylin indicate its co-secretion with insulin in humans. Diabetologia. 1991 Jan;34(1):52–54. doi: 10.1007/BF00404025. [DOI] [PubMed] [Google Scholar]
- Inoue K., Hiramatsu S., Hisatomi A., Umeda F., Nawata H. Hypersecretion of amylin from the perfused pancreas of genetically obese (fa/fa) rats and its alteration with aging. Metabolism. 1993 May;42(5):654–658. doi: 10.1016/0026-0495(93)90227-f. [DOI] [PubMed] [Google Scholar]
- Inoue K., Hisatomi A., Umeda F., Nawata H. Effects of exogenous somatostatin and insulin on islet amyloid polypeptide (amylin) release from perfused rat pancreas. Horm Metab Res. 1992 Jun;24(6):251–253. doi: 10.1055/s-2007-1003306. [DOI] [PubMed] [Google Scholar]
- Inoue K., Hisatomi A., Umeda F., Nawata H. Release of amylin from perfused rat pancreas in response to glucose, arginine, beta-hydroxybutyrate, and gliclazide. Diabetes. 1991 Aug;40(8):1005–1009. doi: 10.2337/diab.40.8.1005. [DOI] [PubMed] [Google Scholar]
- Jansson L. Influence of adrenaline on blood perfusion and vascular conductance of the whole pancreas and the islets of Langerhans in the rat. Arch Int Pharmacodyn Ther. 1991 Sep-Oct;313:90–97. [PubMed] [Google Scholar]
- Johnson K. H., Hayden D. W., O'Brien T. D., Westermark P. Spontaneous diabetes mellitus-islet amyloid complex in adult cats. Am J Pathol. 1986 Nov;125(2):416–419. [PMC free article] [PubMed] [Google Scholar]
- Johnson K. H., O'Brien T. D., Betsholtz C., Westermark P. Islet amyloid polypeptide: mechanisms of amyloidogenesis in the pancreatic islets and potential roles in diabetes mellitus. Lab Invest. 1992 May;66(5):522–535. [PubMed] [Google Scholar]
- Johnson K. H., O'Brien T. D., Jordan K., Betsholtz C., Westermark P. The putative hormone islet amyloid polypeptide (IAPP) induces impaired glucose tolerance in cats. Biochem Biophys Res Commun. 1990 Mar 16;167(2):507–513. doi: 10.1016/0006-291x(90)92053-3. [DOI] [PubMed] [Google Scholar]
- Johnson K. H., O'Brien T. D., Jordan K., Westermark P. Impaired glucose tolerance is associated with increased islet amyloid polypeptide (IAPP) immunoreactivity in pancreatic beta cells. Am J Pathol. 1989 Aug;135(2):245–250. [PMC free article] [PubMed] [Google Scholar]
- Kanatsuka A., Makino H., Ohsawa H., Tokuyama Y., Yamaguchi T., Yoshida S., Adachi M. Secretion of islet amyloid polypeptide in response to glucose. FEBS Lett. 1989 Dec 18;259(1):199–201. doi: 10.1016/0014-5793(89)81527-3. [DOI] [PubMed] [Google Scholar]
- Kassir A. A., Upadhyay A. K., Lim T. J., Moossa A. R., Olefsky J. M. Lack of effect of islet amyloid polypeptide in causing insulin resistance in conscious dogs during euglycemic clamp studies. Diabetes. 1991 Aug;40(8):998–1004. doi: 10.2337/diab.40.8.998. [DOI] [PubMed] [Google Scholar]
- Kirk C. A., Feldman E. C., Nelson R. W. Diagnosis of naturally acquired type-I and type-II diabetes mellitus in cats. Am J Vet Res. 1993 Mar;54(3):463–467. [PubMed] [Google Scholar]
- Koopmans S. J., van Mansfeld A. D., Jansz H. S., Krans H. M., Radder J. K., Frölich M., de Boer S. F., Kreutter D. K., Andrews G. C., Maassen J. A. Amylin-induced in vivo insulin resistance in conscious rats: the liver is more sensitive to amylin than peripheral tissues. Diabetologia. 1991 Apr;34(4):218–224. doi: 10.1007/BF00405079. [DOI] [PubMed] [Google Scholar]
- Ludvik B., Lell B., Hartter E., Schnack C., Prager R. Decrease of stimulated amylin release precedes impairment of insulin secretion in type II diabetes. Diabetes. 1991 Dec;40(12):1615–1619. doi: 10.2337/diab.40.12.1615. [DOI] [PubMed] [Google Scholar]
- Lukinius A., Wilander E., Westermark G. T., Engström U., Westermark P. Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia. 1989 Apr;32(4):240–244. doi: 10.1007/BF00285291. [DOI] [PubMed] [Google Scholar]
- Lutz T. A., Rand J. S. A review of new developments in type 2 diabetes in human beings and cats. Br Vet J. 1993 Nov-Dec;149(6):527–536. doi: 10.1016/S0007-1935(05)80037-5. [DOI] [PubMed] [Google Scholar]
- Lutz T. A., Rand J. S. Comparison of five commercial radioimmunoassay kits for the measurement of feline insulin. Res Vet Sci. 1993 Jul;55(1):64–69. doi: 10.1016/0034-5288(93)90036-f. [DOI] [PubMed] [Google Scholar]
- Lutz T. A., Rand J. S., Ryan E. Fructosamine concentrations in hyperglycemic cats. Can Vet J. 1995 Mar;36(3):155–159. [PMC free article] [PubMed] [Google Scholar]
- Moore C. X., Cooper G. J. Co-secretion of amylin and insulin from cultured islet beta-cells: modulation by nutrient secretagogues, islet hormones and hypoglycemic agents. Biochem Biophys Res Commun. 1991 Aug 30;179(1):1–9. doi: 10.1016/0006-291x(91)91325-7. [DOI] [PubMed] [Google Scholar]
- Nakazato M., Asai J., Kangawa K., Matsukura S., Matsuo H. Establishment of radioimmunoassay for human islet amyloid polypeptide and its tissue content and plasma concentration. Biochem Biophys Res Commun. 1989 Oct 16;164(1):394–399. doi: 10.1016/0006-291x(89)91732-4. [DOI] [PubMed] [Google Scholar]
- Nelson R. W., Himsel C. A., Feldman E. C., Bottoms G. D. Glucose tolerance and insulin response in normal-weight and obese cats. Am J Vet Res. 1990 Sep;51(9):1357–1362. [PubMed] [Google Scholar]
- O'Brien T. D., Butler P. C., Westermark P., Johnson K. H. Islet amyloid polypeptide: a review of its biology and potential roles in the pathogenesis of diabetes mellitus. Vet Pathol. 1993 Jul;30(4):317–332. doi: 10.1177/030098589303000401. [DOI] [PubMed] [Google Scholar]
- O'Brien T. D., Hayden D. W., Johnson K. H., Stevens J. B. High dose intravenous glucose tolerance test and serum insulin and glucagon levels in diabetic and non-diabetic cats: relationships to insular amyloidosis. Vet Pathol. 1985 May;22(3):250–261. doi: 10.1177/030098588502200308. [DOI] [PubMed] [Google Scholar]
- O'Brien T. D., Westermark P., Johnson K. H. Islet amyloid polypeptide and insulin secretion from isolated perfused pancreas of fed, fasted, glucose-treated, and dexamethasone-treated rats. Diabetes. 1991 Dec;40(12):1701–1706. doi: 10.2337/diab.40.12.1701. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes. 1991 Feb;40(2):166–180. doi: 10.2337/diab.40.2.166. [DOI] [PubMed] [Google Scholar]
- Reimers T. J., Cowan R. G., McCann J. P., Ross M. W. Validation of a rapid solid-phase radioimmunoassay for canine, bovine, and equine insulin. Am J Vet Res. 1982 Jul;43(7):1274–1278. [PubMed] [Google Scholar]
- Rink T. J., Beaumont K., Koda J., Young A. Structure and biology of amylin. Trends Pharmacol Sci. 1993 Apr;14(4):113–118. doi: 10.1016/0165-6147(93)90081-t. [DOI] [PubMed] [Google Scholar]
- Silvestre R. A., Salas M., Dégano P., Peiró E., Marco J. Reversal of the inhibitory effects of calcitonin gene-related peptide (CGRP) and amylin on insulin secretion by the 8-37 fragment of human CGRP. Biochem Pharmacol. 1993 Jun 9;45(11):2343–2347. doi: 10.1016/0006-2952(93)90209-f. [DOI] [PubMed] [Google Scholar]
- Silvestre R. A., Salas M., García-Hermida O., Fontela T., Dégano P., Marco J. Amylin (islet amyloid polypeptide) inhibition of insulin release in the perfused rat pancreas: implication of the adenylate cyclase/cAMP system. Regul Pept. 1994 Feb 24;50(2):193–199. doi: 10.1016/0167-0115(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Sowa R., Sanke T., Hirayama J., Tabata H., Furuta H., Nishimura S., Nanjo K. Islet amyloid polypeptide amide causes peripheral insulin resistance in vivo in dogs. Diabetologia. 1990 Feb;33(2):118–120. doi: 10.1007/BF00401051. [DOI] [PubMed] [Google Scholar]
- Stockham S. L., Nachreiner R. F., Krehbiel J. D. Canine immunoreactive insulin quantitation using, five commercial radioimmunoassay kits. Am J Vet Res. 1983 Nov;44(11):2179–2183. [PubMed] [Google Scholar]
- Stridsberg M., Wilander E., Oberg K., Lundqvist G., Eriksson B. Islet amyloid polypeptide-producing pancreatic islet cell tumor. A clinical and biochemical characterization. Scand J Gastroenterol. 1992 May;27(5):381–387. doi: 10.3109/00365529209000092. [DOI] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Hayden D. W., O'Brien T. D., Johnson K. H. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986 Nov 14;140(3):827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- Young A. A., Wang M. W., Cooper G. J. Amylin injection causes elevated plasma lactate and glucose in the rat. FEBS Lett. 1991 Oct 7;291(1):101–104. doi: 10.1016/0014-5793(91)81113-m. [DOI] [PubMed] [Google Scholar]
- van Hulst K. L., Hackeng W. H., Höppener J. W., van Jaarsveld B. C., Nieuwenhuis M. G., Blankenstein M. A., Lips C. J. An improved method for the determination of islet amyloid polypeptide levels in plasma. Ann Clin Biochem. 1994 Mar;31(Pt 2):165–170. doi: 10.1177/000456329403100209. [DOI] [PubMed] [Google Scholar]


