Abstract
Following immunization of human subjects with the antigen bacteriophage phi X 174, concomitant with the rise in serum antibody, cells appear in the circulation which in vitro, without antigen stimulation, synthesize antibody of the same class as serum antibody in most subjects studied. This function is inhibited by puromycin or irradiation, is independent of T cells and occurs within the first 36-72 h of culture. Such cells are found only in recently immunized subjects. Peripheral blood mononuclear cells (PBM) from all immunized subjects synthesize more antibody to phi X 174 in vitro if antigen is present during cell culture; none was synthesized by antigen containing PBM cultures from unimmunized subjects. This antigen-induced antibody response is T cell and antigen dose-dependent and inhibited by puromycin or irradiation. Following primary immunization the antibody synthesized in vivo and in vitro is IgM. Following secondary immunization IgG antibody is immediately detected in vivo but in vitro antigen-induced antibody continues to be IgM for at least 3 months. IgG antibody then appears: once this class switch occurs, in vitro antigen-induced IgG antibody can be demonstrated in cultured PBM of subjects for many years, without further booster immunization.
Full text
PDF

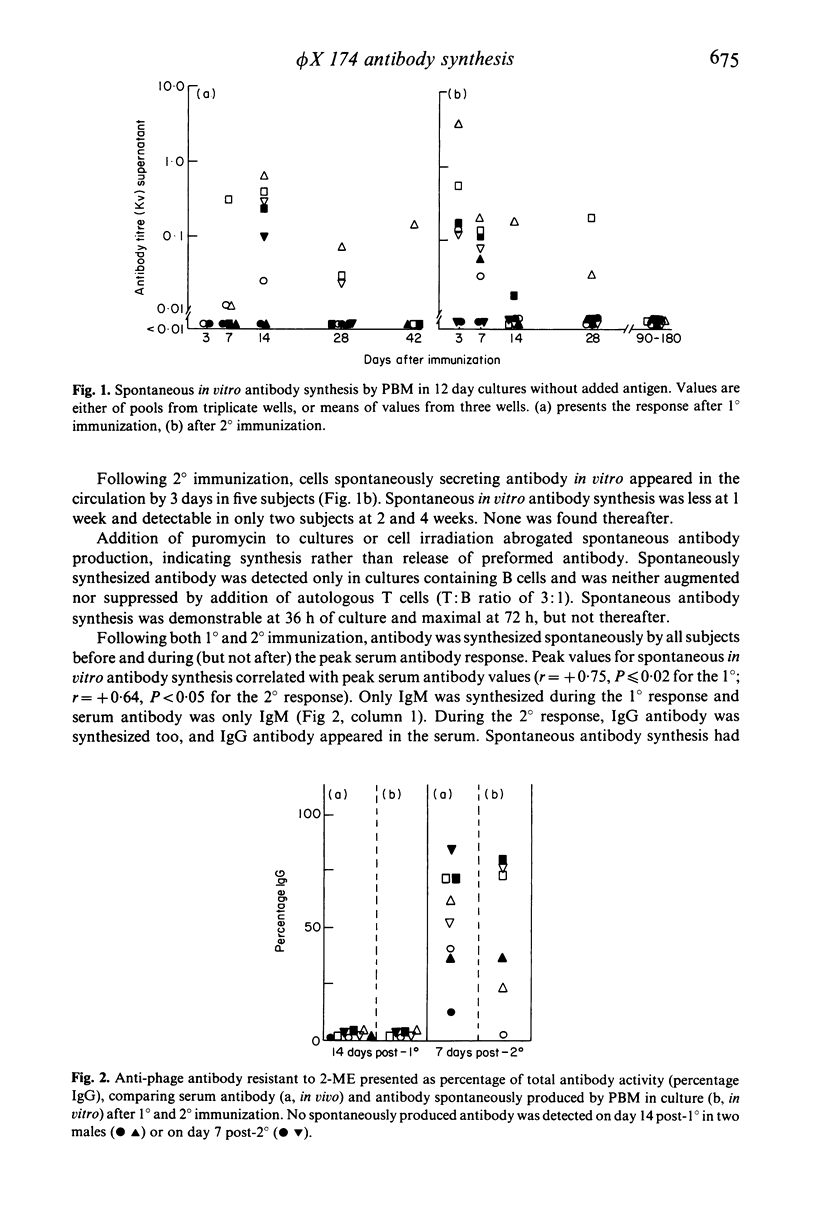
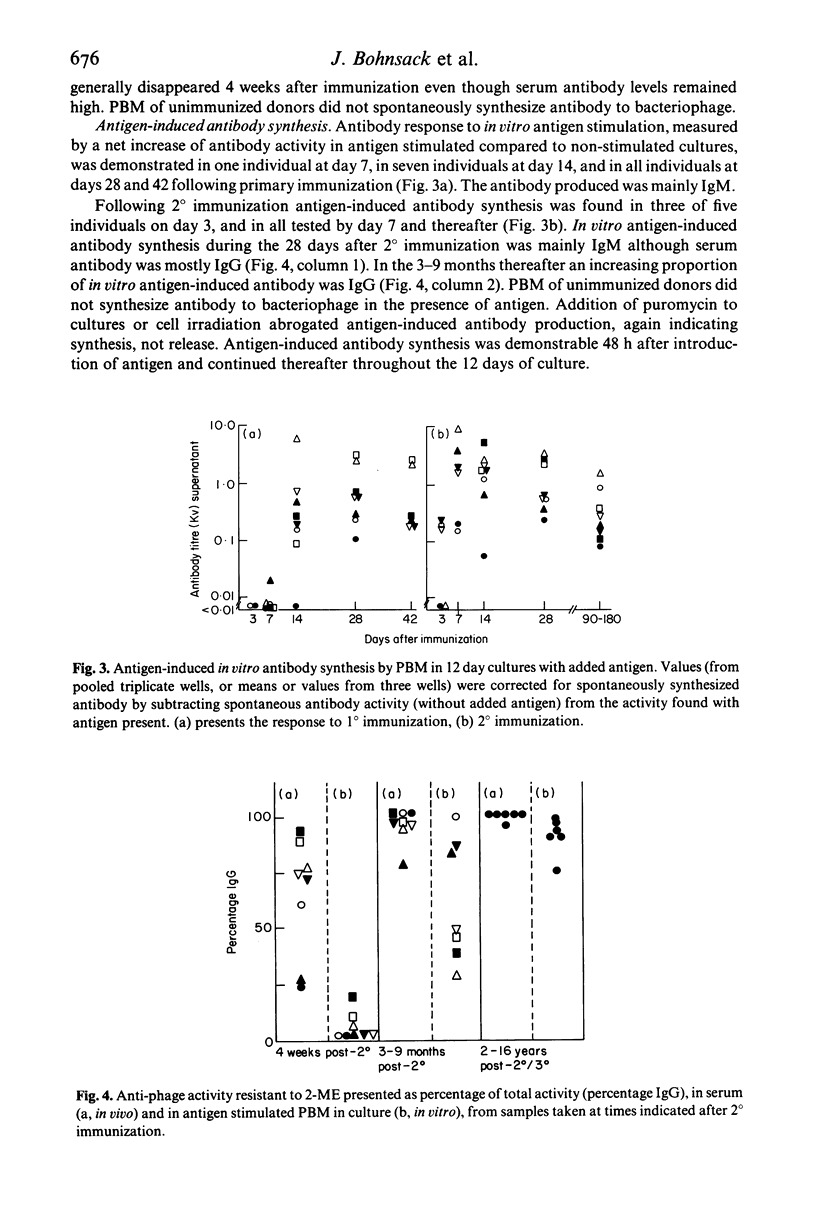


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner M. K., Munro A. J. Human anti-tetanus antibody response in vitro: autologous and allogeneic T cells provide help by different routes. Clin Exp Immunol. 1981 Oct;46(1):171–177. [PMC free article] [PubMed] [Google Scholar]
- Callard R. E. Specific in vitro antibody response to influenza virus by human blood lymphocytes. Nature. 1979 Dec 13;282(5740):734–736. doi: 10.1038/282734a0. [DOI] [PubMed] [Google Scholar]
- Ching Y. C., Davis S. D., Wedgwood R. J. Antibody studies in hypogammaglobulinemia. J Clin Invest. 1966 Oct;45(10):1593–1600. doi: 10.1172/JCI105465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froelich T. L., Lum L. G. Immunoglobulin secretion by human B lymphocytes exposed to herpes simplex type I antigen. Immunobiology. 1982;162(1):94–102. doi: 10.1016/S0171-2985(11)80021-5. [DOI] [PubMed] [Google Scholar]
- GRUBB R., SWAHN B. Destruction of some agglutinins but not of others by two sulfhydryl compounds. Acta Pathol Microbiol Scand. 1958;43(3):305–309. doi: 10.1111/j.1699-0463.1958.tb04899.x. [DOI] [PubMed] [Google Scholar]
- Ginsburg W. W., Finkelman F. D., Lipsky P. E. Circulating and mitogen-induced immunoglobulin-secreting cells in human peripheral blood: evaluation by a modified reverse hemolytic plaque assay. J Immunol. 1978 Jan;120(1):33–39. [PubMed] [Google Scholar]
- Lane H. C., Volkman D. J., Whalen G., Fauci A. S. In vitro antigen-induced, antigen-specific antibody production in man. Specific and polyclonal components, kinetics, and cellular requirements. J Exp Med. 1981 Oct 1;154(4):1043–1057. doi: 10.1084/jem.154.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudawwar F. B., Yunis E. J., Geha R. S. Antigen-specific helper factor in man. J Exp Med. 1978 Oct 1;148(4):1032–1043. doi: 10.1084/jem.148.4.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs H. D., Davis S. D., Wedgwood R. J. Immunologic responses to bacteriophage phi-X 174 in immunodeficiency diseases. J Clin Invest. 1971 Dec;50(12):2559–2568. doi: 10.1172/JCI106756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs H. D., Lum L. G., Johnson F. L., Schiffman G., Wedgwood R. J., Storb R. Bone marrow transplantation in the Wiskott-Aldrich syndrome. Complete hematological and immunological reconstitution. Transplantation. 1982 Nov;34(5):284–288. doi: 10.1097/00007890-198211000-00009. [DOI] [PubMed] [Google Scholar]
- Peacock D. B., Jones J. V., Gough M. The immune response to thetaX 174 in man. I. Primary and secondary antibody production in normal adults. Clin Exp Immunol. 1973 Apr;13(4):497–513. [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Dierich M. P., Reisfeld R. A. Enhancement of sheep red blood cell human lymphocyte rosette formation by the sulfhydryl compound 2-amino ethylisothiouronium bromide. Clin Immunol Immunopathol. 1975 Jan;3(3):324–333. doi: 10.1016/0090-1229(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Souhami R. L., Babbage J., Callard R. E. Specific in vitro antibody response to varicella zoster. Clin Exp Immunol. 1981 Oct;46(1):98–105. [PMC free article] [PubMed] [Google Scholar]
- Stevens R. H., Macy E., Morrow C., Saxon A. Characterization of a circulating subpopulation of spontaneous antitetanus toxoid antibody producing B cells following in vivo booster immunization. J Immunol. 1979 Jun;122(6):2498–2504. [PubMed] [Google Scholar]
- Stevens R. H., Saxon A. Immunoregulation in humans: control of antitetanus toxoid antibody production after booster immunization. J Clin Invest. 1978 Dec;62(6):1154–1160. doi: 10.1172/JCI109234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson P. D., Harris N. S. Detection of plaque-forming cells in the peripheral blood of actively immunized humans. J Immunol. 1977 Apr;118(4):1480–1482. [PubMed] [Google Scholar]
- UHR J. W., FINKELSTEIN M. S., BAUMANN J. B. Antibody formation. III. The primary and secondary antibody response to bacteriophage phi X 174 in guinea pigs. J Exp Med. 1962 Mar 1;115:655–670. doi: 10.1084/jem.115.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman D. J., Lane H. C., Fauci A. S. Antigen-induced in vitro antibody production in humans: a model for B cell activation and immunoregulation. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2528–2531. doi: 10.1073/pnas.78.4.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Wedgwood R. J., Ochs H. D., Davis S. D. The recognition and classification of immunodeficiency diseases with bacteriophage phiChi 174. Birth Defects Orig Artic Ser. 1975;11(1):331–338. [PubMed] [Google Scholar]


