Abstract
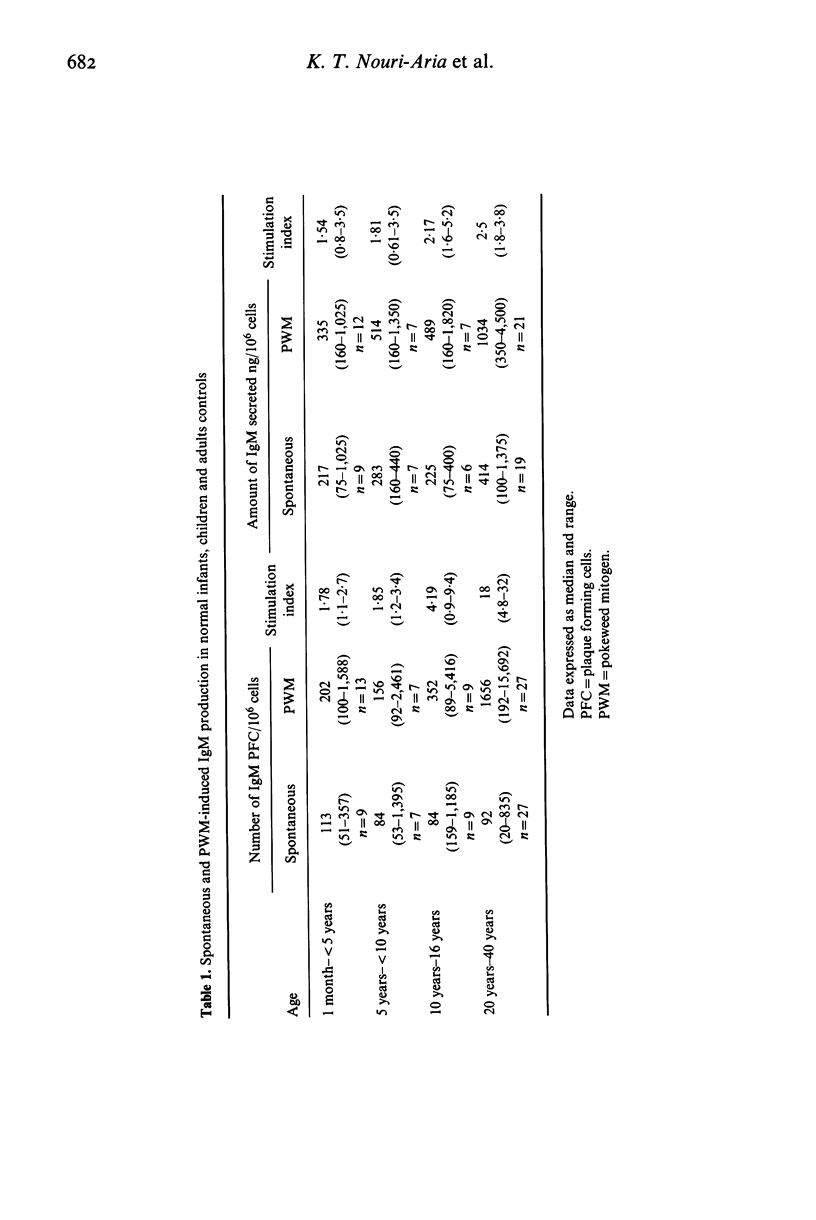
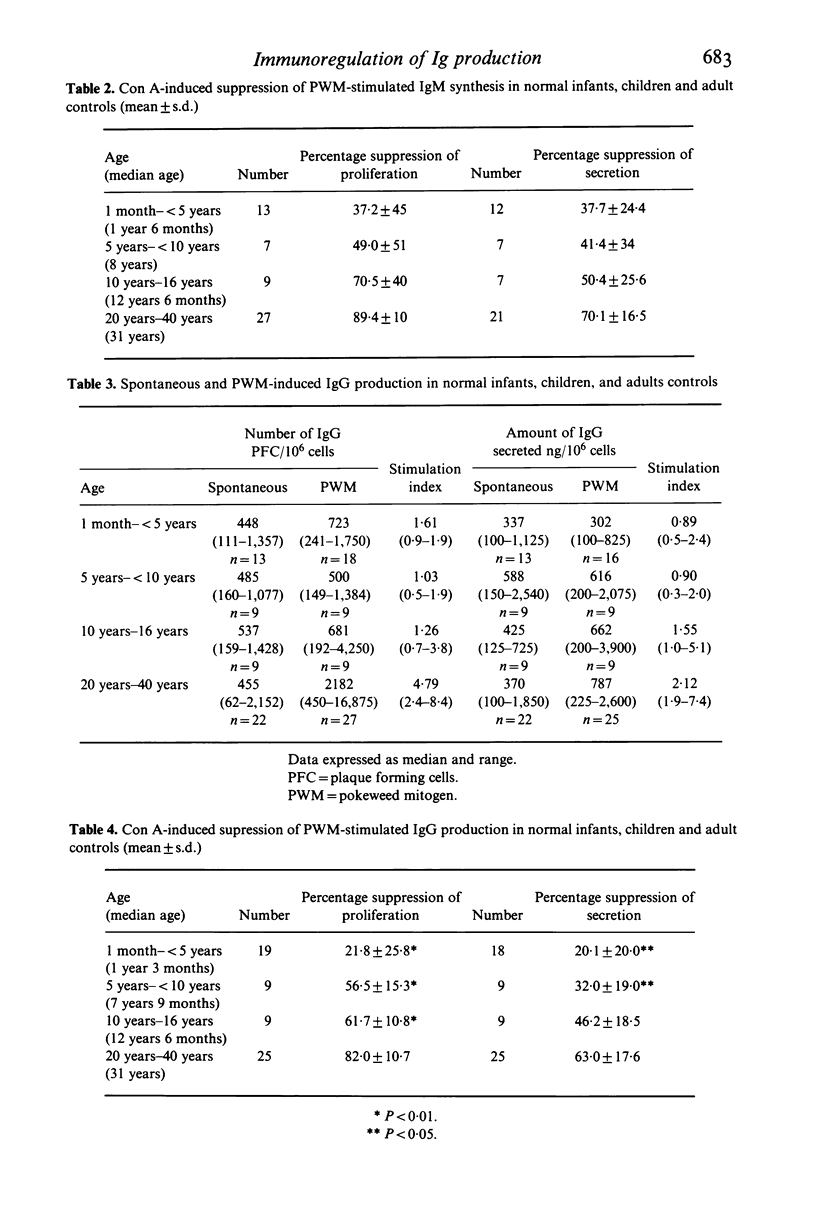
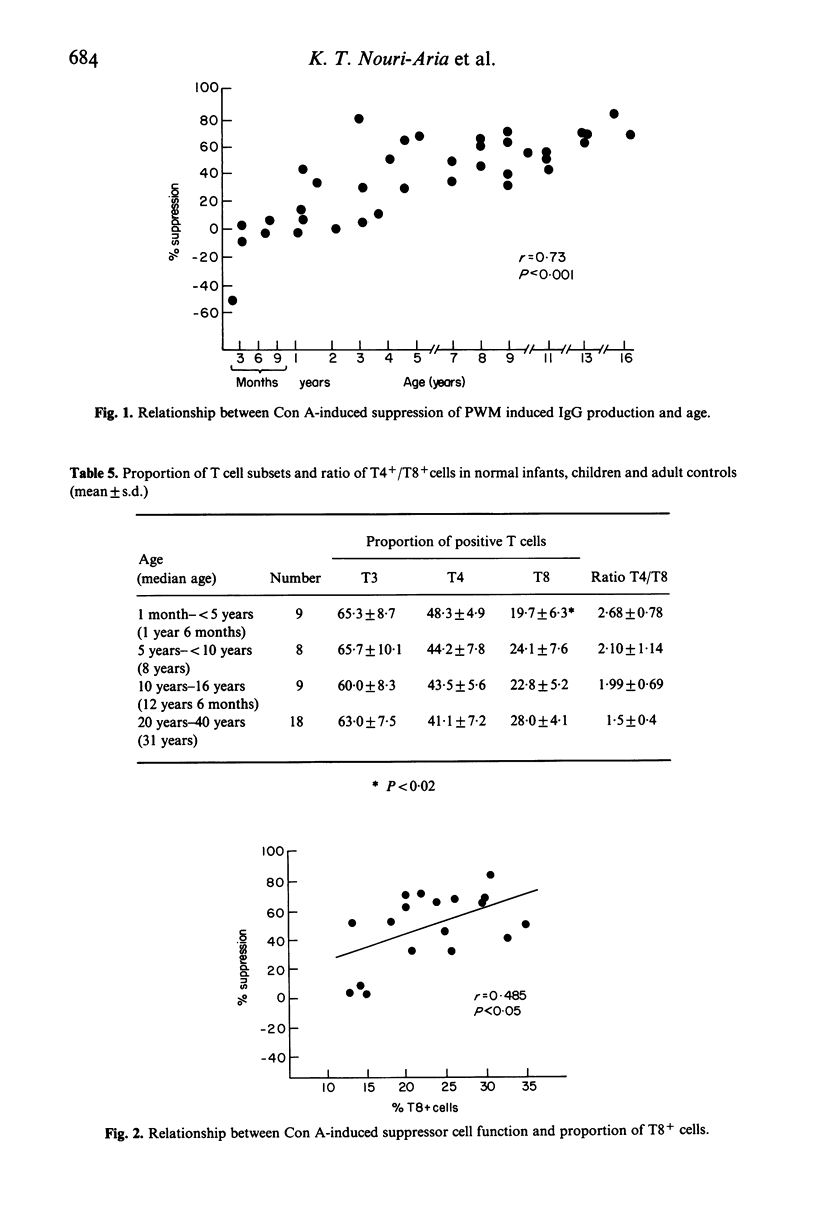
Proportion of T cell subsets, spontaneous and PWM stimulated immunoglobulin production by peripheral blood lymphocytes and concanavalin A- (Con A) stimulated suppressor cell activity on immunoglobulin production by B cells was studied in 37 infants and children, to investigate changes of these parameters with age. Proportion of suppressor/cytotoxic (T8+) T lymphocytes was significantly lower in children below the age of 5 years, while there was no difference in proportion of total T lymphocytes (T3+) and helper/inducer (T4+) T cells. Spontaneous production and secretion of IgG and IgM by lymphocytes from children of all age groups was similar to that found in adults, but lymphocytes of children below the age of 10 years showed a low response to PWM stimulation. The activity of Con A-induced suppressor cells in inhibiting B cells producing immunoglobulins was almost absent in infancy, gradually increased during childhood and reached adult levels in teenagers. A significant association between the proportion of T8+ cells and Con A-induced suppression of B cell proliferation and a relationship between T4+ cells and spontaneous Ig production indicated the increasing maturity with respect to both number and function of peripheral blood lymphocyte subsets with advancing age.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson U., Britton S., De Ley M., Bird G. Evidence for the ontogenic precedence of suppressor T cell functions in the human neonate. Eur J Immunol. 1983 Jan;13(1):6–13. doi: 10.1002/eji.1830130104. [DOI] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Griscelli C. Active suppression of B lymphocyte maturation by two different newborn T lymphocyte subsets. J Immunol. 1979 Dec;123(6):2644–2650. [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Hammarström L., Bird A. G., Britton S., Smith C. I. Pokeweed mitogen induced differentiation of human B cells: evaluation by a protein A haemolytic plaque assay. Immunology. 1979 Sep;38(1):181–189. [PMC free article] [PubMed] [Google Scholar]
- Hayward A. R., Merrill D. Requirement for OKT8+ suppressor cell proliferation for suppression by human newborn T cells. Clin Exp Immunol. 1981 Sep;45(3):468–474. [PMC free article] [PubMed] [Google Scholar]
- Jacoby D. R., Oldstone M. B. Delineation of suppressor and helper activity within the OKT4-defined T lymphocyte subset in human newborns. J Immunol. 1983 Oct;131(4):1765–1770. [PubMed] [Google Scholar]
- Miyawaki T., Kubo M., Nagaoki T., Moriya N., Yokoi T., Mukai M., Taniguchi N. Developmental changes in humoral suppressor activity released from concanavalin A-stimulated human lymphocytes on B cell differentiation. J Immunol. 1981 May;126(5):1720–1723. [PubMed] [Google Scholar]
- Preud'homme J. L., Flandrin G. Identification by peroxidase staining of monocytes in surface immunofluorescence tests. J Immunol. 1974 Nov;113(5):1650–1653. [PubMed] [Google Scholar]
- Schwartz S. A. Heavy chain-specific suppression of immunoglobulin synthesis and secretion by lymphocytes from patients with selective IgA deficiency. J Immunol. 1980 Apr;124(4):2034–2041. [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato G., Magrath I. T., Koski I. R., Dooley N. J., Blaese R. M. B cell differentiation and immunoregulatory T cell function in human cord blood lymphocytes. J Clin Invest. 1980 Aug;66(2):383–388. doi: 10.1172/JCI109867. [DOI] [PMC free article] [PubMed] [Google Scholar]


