Abstract
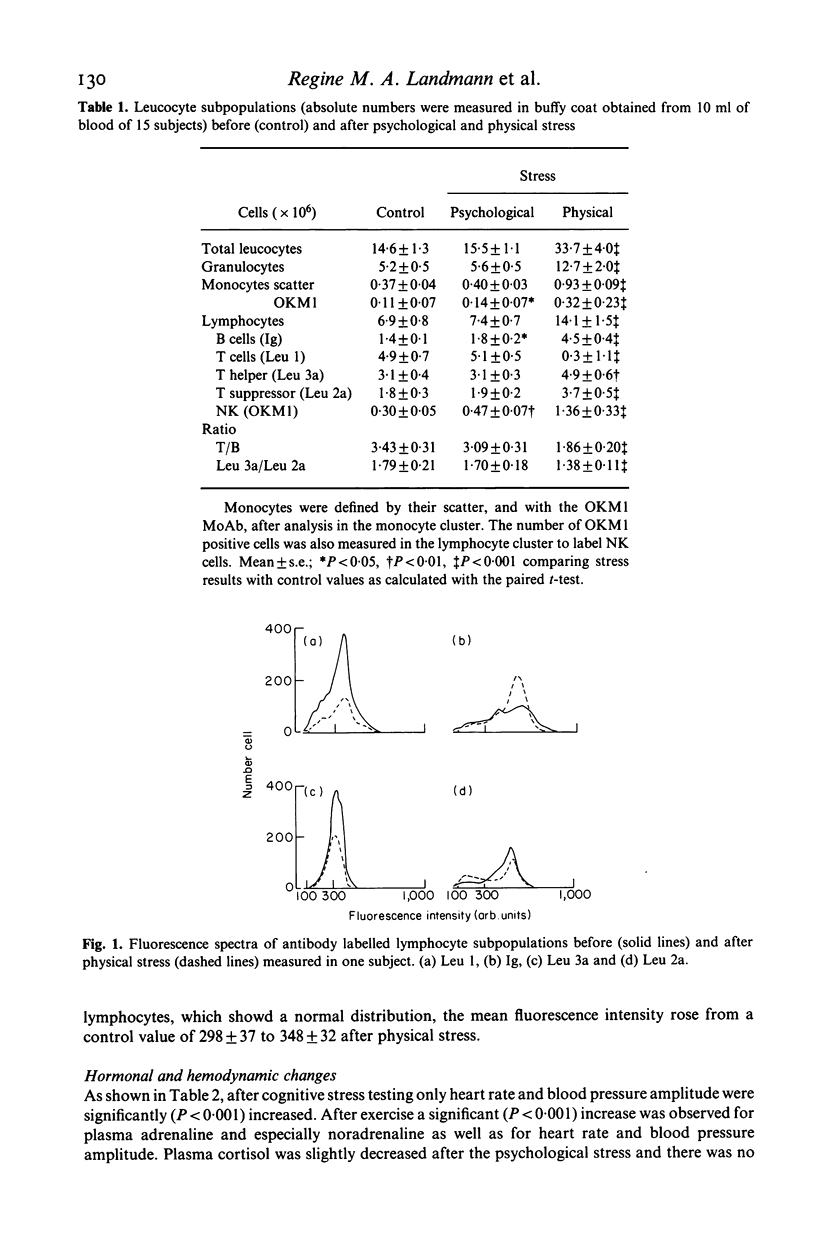
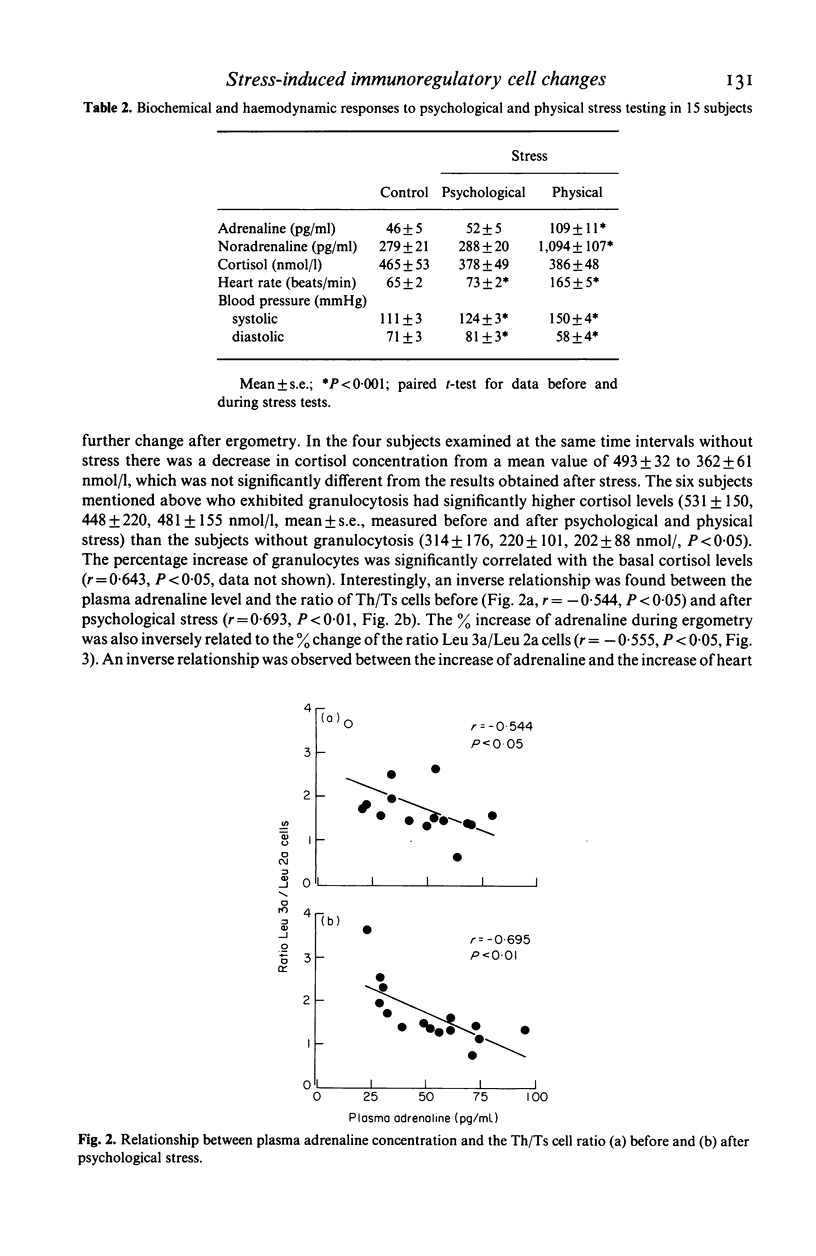
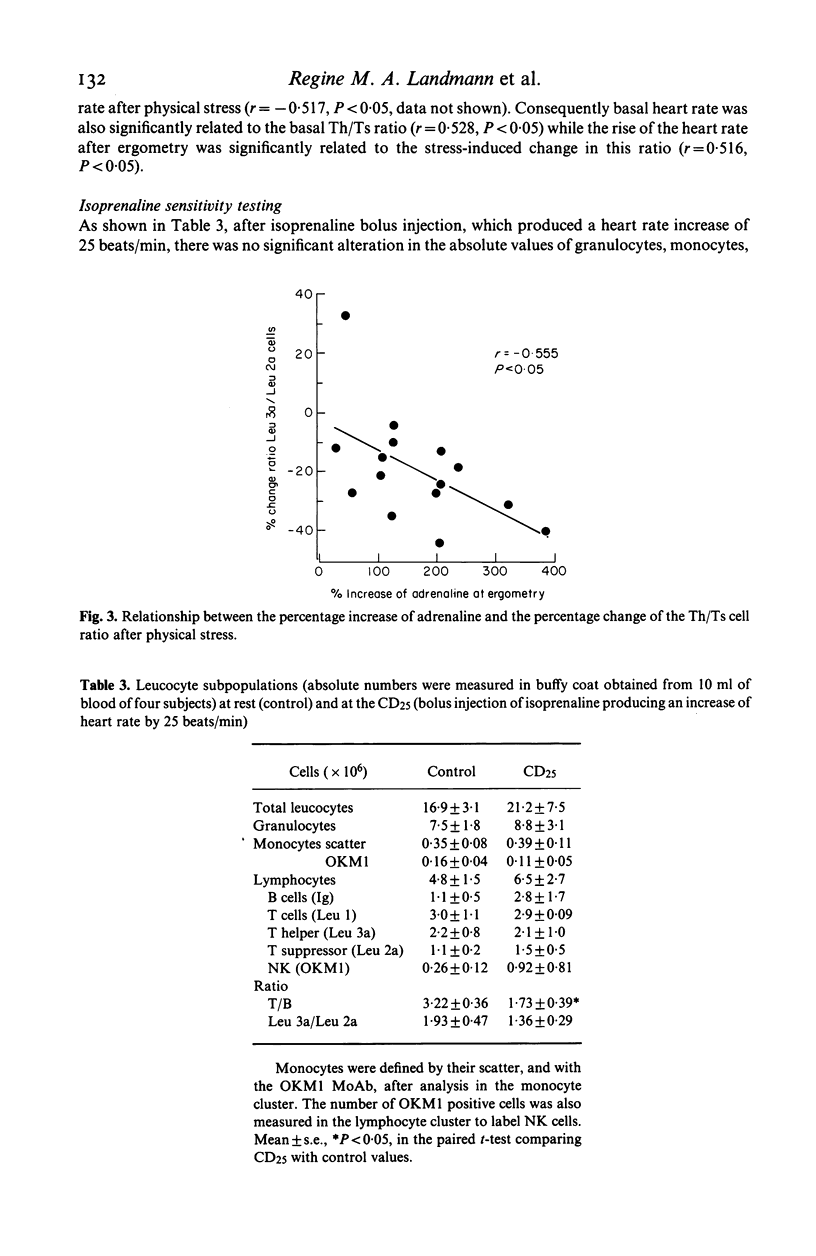
Lymphocyte subpopulations were measured before and after physical and psychological stress in 15 healthy subjects and correlated with plasma catecholamine and cortisol levels. During psychological stress monocytes (P less than 0.05), NK (P less than 0.01), B cells (P less than 0.05) and heart rate (P less than 0.001) increased, while catecholamines remained unchanged. With physical stress granulocytes, monocytes and all lymphocyte subsets increased significantly, although B cells rose more than T cells and T (suppressor) cells more than T (helper) cells. Thus the ratio of T/B cells and of Th/Ts cells decreased (P less than 0.001 and P less than 0.01). Adrenaline and noradrenaline concentrations increased (P less than 0.001), while cortisol remained unchanged. There was a negative relationship between adrenaline and the Th/Ts cell ratio before and after stress (P less than 0.05). Lymphocyte subpopulations from a different group of 4 healthy subjects were analysed before and after isoproterenol infusion. There was a small increase in Ts and B cells only (P less than 0.1) and a decrease of the T/B cell ratio (P less than 0.05). The predominant enrichment of circulating B, Ts and NK cells during short lasting adrenergic activation, as well as the relationship of the T cell changes to plasma adrenaline, suggest an immunoregulatory effect of the sympathetic nervous system in stress.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartrop R. W., Luckhurst E., Lazarus L., Kiloh L. G., Penny R. Depressed lymphocyte function after bereavement. Lancet. 1977 Apr 16;1(8016):834–836. doi: 10.1016/s0140-6736(77)92780-5. [DOI] [PubMed] [Google Scholar]
- Bertel O., Bühler F. R., Kiowski W., Lütold B. E. Decreased Beta-adrenoreceptor responsiveness as related to age, blood pressure, and plasma catecholamines in patients with essential hypertension. Hypertension. 1980 Mar-Apr;2(2):130–138. doi: 10.1161/01.hyp.2.2.130. [DOI] [PubMed] [Google Scholar]
- Bishop C. R., Athens J. W., Boggs D. R., Warner H. R., Cartwright G. E., Wintrobe M. M. Leukokinetic studies. 13. A non-steady-state kinetic evaluation of the mechanism of cortisone-induced granulocytosis. J Clin Invest. 1968 Feb;47(2):249–260. doi: 10.1172/JCI105721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary B., Borysenko M., Sutherland D. C., Kutz I., Borysenko J. Z., Benson H. Decrease in mitogen responsiveness of mononuclear cells from peripheral blood after epinephrine administration in humans. J Immunol. 1983 Feb;130(2):694–697. [PubMed] [Google Scholar]
- Da Prada M., Zürcher Simultaneous radioenzymatic determination of plasma and tissue adrenaline, noradrenaline and dopamine within the femtomole range. Life Sci. 1976 Oct 15;19(8):1161–1174. doi: 10.1016/0024-3205(76)90251-4. [DOI] [PubMed] [Google Scholar]
- Depelchin A., Letesson J. J. Adrenaline influence on the immune response. I. Accelerating or suppressor effects according to the time of application. Immunol Lett. 1981 Oct;3(4):199–205. doi: 10.1016/0165-2478(81)90075-4. [DOI] [PubMed] [Google Scholar]
- Eriksson B., Hedfors E. The effect of adrenaline, insulin and hydrocortisone on human peripheral blood lymphocytes studied by cell surface markers. Scand J Haematol. 1977 Feb;18(2):121–128. doi: 10.1111/j.1600-0609.1977.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Ernström U., Sandberg G. Effects of adrenergic alpha- and beta-receptor stimulation on the release of lymphocytes and granulocytes from the spleen. Scand J Haematol. 1973;11(4):275–286. doi: 10.1111/j.1600-0609.1973.tb00130.x. [DOI] [PubMed] [Google Scholar]
- Eskola J., Ruuskanen O., Soppi E., Viljanen M. K., Järvinen M., Toivonen H., Kouvalainen K. Effect of sport stress on lymphocyte transformation and antibody formation. Clin Exp Immunol. 1978 May;32(2):339–345. [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974 Jan;53(1):240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. Mechanisms of corticosteroid action on lymphocyte subpopulations. I. Redistribution of circulating T and b lymphocytes to the bone marrow. Immunology. 1975 Apr;28(4):669–680. [PMC free article] [PubMed] [Google Scholar]
- Gader A. M., Cash J. D. The effect of adrenaline, noradrenaline, isoprenaline and salbutamol on the resting levels of white blood cells in man. Scand J Haematol. 1975 Mar;14(1):5–10. doi: 10.1111/j.1600-0609.1975.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Gisler R. H. Stress and the hormonal regulation of the immune response in mice. Psychother Psychosom. 1974;23(1-6):197–208. doi: 10.1159/000286643. [DOI] [PubMed] [Google Scholar]
- Hedfors E., Holm G., Ivansen M., Wahren J. Physiological variation of blood lymphocyte reactivity: T-cell subsets, immunoglobulin production, and mixed-lymphocyte reactivity. Clin Immunol Immunopathol. 1983 Apr;27(1):9–14. doi: 10.1016/0090-1229(83)90051-x. [DOI] [PubMed] [Google Scholar]
- Hedfors E., Holm G., Ohnell B. Variations of blood lymphocytes during work studied by cell surface markers, DNA synthesis and cytotoxicity. Clin Exp Immunol. 1976 May;24(2):328–335. [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. A., Kung P. C., Hansen W. P., Goldstein G. Simple and rapid measurement of human T lymphocytes and their subclasses in peripheral blood. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4914–4917. doi: 10.1073/pnas.77.8.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indiveri F., Scudeletti M., Pende D., Barabino A., Russo C., Pellegrino M. A., Ferrone S. Inhibitory effect of a low dose of prednisone on PHA-induced Ia antigen expression by human T cells and on proliferation of T cells stimulated with autologous PHA-T cells. Cell Immunol. 1983 Sep;80(2):320–328. doi: 10.1016/0008-8749(83)90120-x. [DOI] [PubMed] [Google Scholar]
- Keller S. E., Weiss J. M., Schleifer S. J., Miller N. E., Stein M. Stress-induced suppression of immunity in adrenalectomized rats. Science. 1983 Sep 23;221(4617):1301–1304. doi: 10.1126/science.6612346. [DOI] [PubMed] [Google Scholar]
- Laudenslager M. L., Ryan S. M., Drugan R. C., Hyson R. L., Maier S. F. Coping and immunosuppression: inescapable but not escapable shock suppresses lymphocyte proliferation. Science. 1983 Aug 5;221(4610):568–570. doi: 10.1126/science.6603018. [DOI] [PubMed] [Google Scholar]
- Monjan A. A., Collector M. I. Stress-induced modulation of the immune response. Science. 1977 Apr 15;196(4287):307–308. doi: 10.1126/science.557841. [DOI] [PubMed] [Google Scholar]
- Moorthy A. V., Zimmerman S. W. Human leukocyte response to an endurance race. Eur J Appl Physiol Occup Physiol. 1978 May 30;38(4):271–276. doi: 10.1007/BF00423109. [DOI] [PubMed] [Google Scholar]
- Reite M., Harbeck R., Hoffman A. Altered cellular immune response following peer separation. Life Sci. 1981 Sep 14;29(11):1133–1136. doi: 10.1016/0024-3205(81)90201-0. [DOI] [PubMed] [Google Scholar]
- Riley V. Psychoneuroendocrine influences on immunocompetence and neoplasia. Science. 1981 Jun 5;212(4499):1100–1109. doi: 10.1126/science.7233204. [DOI] [PubMed] [Google Scholar]
- Slade J. D., Hepburn B. Prednisone-induced alterations of circulating human lymphocyte subsets. J Lab Clin Med. 1983 Mar;101(3):479–487. [PubMed] [Google Scholar]
- Steel C. M., French E. B., Aitchison W. R. Studies on adrenaline-induced leucocytosis in normal man. I. The role of the spleen and of the thoracic duct. Br J Haematol. 1971 Oct;21(4):413–421. doi: 10.1111/j.1365-2141.1971.tb02701.x. [DOI] [PubMed] [Google Scholar]
- Yu D. T., Clements P. J. Human lymphocyte subpopulations effect of epinephrine. Clin Exp Immunol. 1976 Sep;25(3):472–479. [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Clouse K. A., Biddison W. E., Kung P. C. Phenotypes of human natural killer cell populations detected with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2575–2580. [PubMed] [Google Scholar]


