Abstract
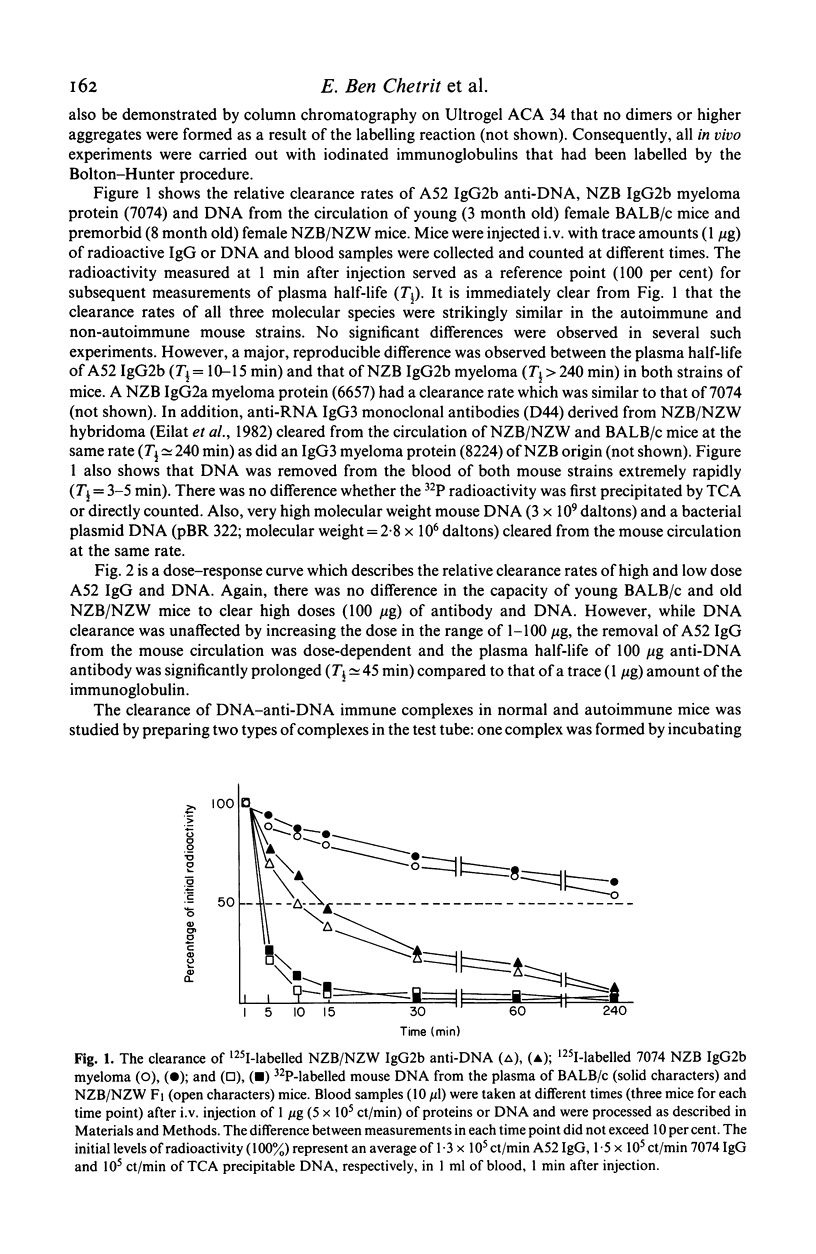
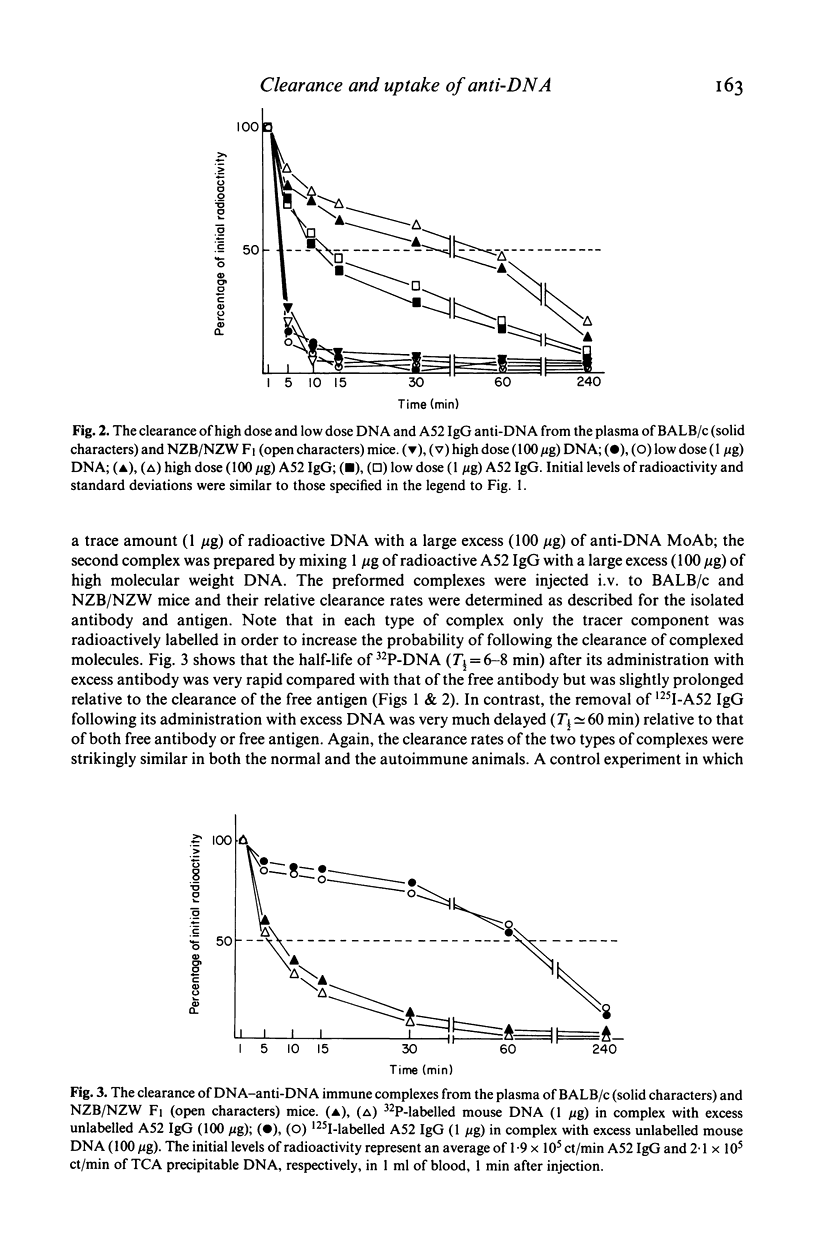
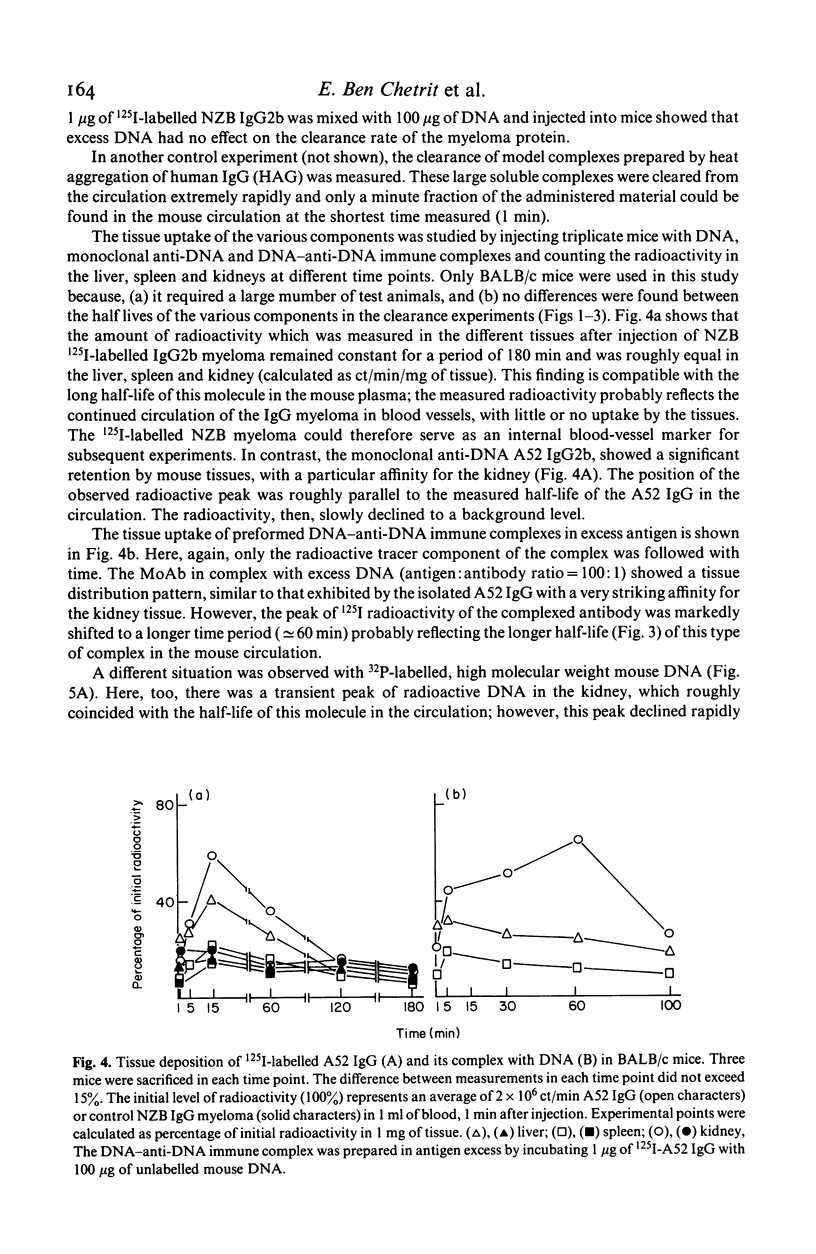
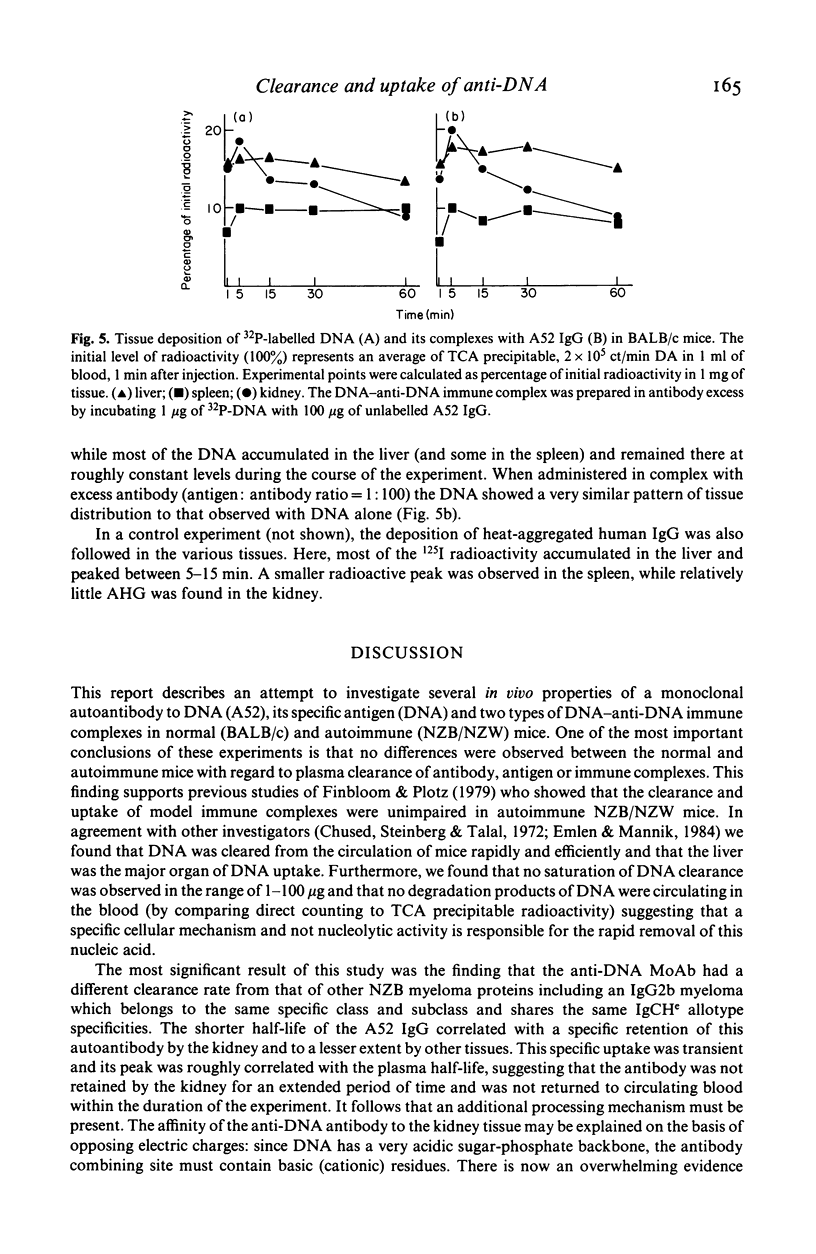
In vivo clearance and tissue localization of a purified mouse anti-DNA monoclonal antibody (MoAb) (A52 IgG2b) and its complexes with DNA were studied in normal BALB/c and autoimmune NZB/NZW mice. The plasma half-life of the autoantibody in both mouse strains was significantly shorter (T 1/2 = 10-15 min), compared with that of purified NZB myeloma proteins (T 1/2 greater than or equal to 180 min). DNA antigen and DNA-A52 IgG complexes in antibody excess were cleared very rapidly (T 1/2 = 4-8 min), while complexes formed in antigen excess persisted in the circulation much longer (T 1/2 = 60 min). Organ studies showed that the anti-DNA MoAb was transiently retained by the liver and the spleen but demonstrated a particular affinity for the kidney tissue. We suggest that tissue damage in SLE glomerulonephritis may be facilitated by direct interaction of anti-DNA antibodies with glomerular components.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnello V., Koffler D., Eisenberg J. W., Winchester R. J., Kundel H. G. C1g precipitins in the sera of patients with systemic lupus erythematosus and other hypocomplementemic states: characterization of high and low molecular weight types. J Exp Med. 1971 Sep 1;134(3 Pt 2):228s–241s. [PubMed] [Google Scholar]
- Ballard D. W., Voss E. W., Jr Monoclonal murine anti-nucleic acid antibody with double-stranded specificity. Mol Immunol. 1982 Jun;19(6):793–799. doi: 10.1016/0161-5890(82)90005-0. [DOI] [PubMed] [Google Scholar]
- Batsford S. R., Takamiya M., Vogt A. A model of in situ immune complex glomerulonephritis in the rat employing cationized ferritin. Clin Nephrol. 1980 Nov;14(5):211–216. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border W. A., Ward H. J., Kamil E. S., Cohen A. H. Induction of membranous nephropathy in rabbits by administration of an exogenous cationic antigen. J Clin Invest. 1982 Feb;69(2):451–461. doi: 10.1172/JCI110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau C., Benveniste J. Circulating DNA:anti-DNA complexes in systemic lupus erythematosus. Detection and characterization by ultracentrifugation. J Clin Invest. 1979 Jul;64(1):191–198. doi: 10.1172/JCI109439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Distribution of annionic sites in glomerular basement membranes: their possible role in filtration and attachment. Proc Natl Acad Sci U S A. 1976 May;73(5):1646–1650. doi: 10.1073/pnas.73.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chused T. M., Steinberg A. D., Talal N. The clearance and localization of nucleic acids by New Zealand and normal mice. Clin Exp Immunol. 1972 Dec;12(4):465–476. [PMC free article] [PubMed] [Google Scholar]
- Dang H., Harbeck R. J. The in vivo and in vitro glomerular deposition of isolated anti-double-stranded-DNA antibodies in NZB/W mice. Clin Immunol Immunopathol. 1984 Feb;30(2):265–278. doi: 10.1016/0090-1229(84)90061-8. [DOI] [PubMed] [Google Scholar]
- Eilat D., Hochberg M., Fischel R., Laskov R. Antibodies to RNA from autoimmune NZB/NZW mice recognize a similar antigenic determinant and show a large idiotypic diversity. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3818–3822. doi: 10.1073/pnas.79.12.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilat D., Hochberg M., Pumphrey J., Rudikoff S. Monoclonal antibodies to DNA and RNA from NZB/NZW F1 mice: antigenic specificities and NH2 terminal amino acid sequences. J Immunol. 1984 Jul;133(1):489–494. [PubMed] [Google Scholar]
- Emlen W., Mannik M. Clearance of circulating DNA-anti-DNA immune complexes in mice. J Exp Med. 1982 Apr 1;155(4):1210–1215. doi: 10.1084/jem.155.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen W., Mannik M. Effect of DNA size and strandedness on the in vivo clearance and organ localization of DNA. Clin Exp Immunol. 1984 Apr;56(1):185–192. [PMC free article] [PubMed] [Google Scholar]
- Faaber P., Capel P. J., Rijke G. P., Vierwinden G., van de Putte L. B., Koene R. A. Cross-reactivity of anti-DNA antibodies with proteoglycans. Clin Exp Immunol. 1984 Mar;55(3):502–508. [PMC free article] [PubMed] [Google Scholar]
- Finbloom D. S., Plotz P. H. Studies of reticuloendothelial function in the mouse with model immune complexes. II. Serum clearance, tissue uptake, and reticuloendothelial saturation in NZB/W mice. J Immunol. 1979 Oct;123(4):1600–1603. [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Frank M. M., Lawley T. J., Hamburger M. I., Brown E. J. NIH Conference: Immunoglobulin G Fc receptor-mediated clearance in autoimmune diseases. Ann Intern Med. 1983 Feb;98(2):206–218. doi: 10.7326/0003-4819-98-2-218. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hahn B. H. Characteristics of pathogenic subpopulations of antibodies to DNA. Arthritis Rheum. 1982 Jul;25(7):747–752. doi: 10.1002/art.1780250706. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Ebling F., Freeman S., Clevinger B., Davie J. Production of monoclonal murine antibodies to DNA by somatic cell hybrids. Arthritis Rheum. 1980 Aug;23(8):942–945. doi: 10.1002/art.1780230811. [DOI] [PubMed] [Google Scholar]
- Izui S., Lambert P. H., Miescher P. A. Failure to detect circulating DNA--anti-DNA complexes by four radioimmunological methods in patients with systemic lupus erythematosus. Clin Exp Immunol. 1977 Dec;30(3):384–392. [PMC free article] [PubMed] [Google Scholar]
- Izui S., Lambert P. H., Miescher P. A. In vitro demonstration of a particular affinity of glomerular basement membrane and collagen for DNA. A possible basis for a local formation of DNA-anti-DNA complexes in systemic lupus erythematosus. J Exp Med. 1976 Aug 1;144(2):428–443. doi: 10.1084/jem.144.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Isolation of glycosaminoglycans (heparan sulfate) from glomerular basement membranes. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4493–4497. doi: 10.1073/pnas.76.9.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Phillips M. L., Miller M. M., Teplitz R. L. Monoclonal autoantibody production by hybrid cell lines. Clin Immunol Immunopathol. 1981 Mar;18(3):368–374. doi: 10.1016/0090-1229(81)90130-6. [DOI] [PubMed] [Google Scholar]
- Koffler D., Carr R., Agnello V., Thoburn R., Kunkel H. G. Antibodies to polynucleotides in human sera: antigenic specificity and relation to disease. J Exp Med. 1971 Jul 1;134(1):294–312. doi: 10.1084/jem.134.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler D., Schur P. H., Kunkel H. G. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967 Oct 1;126(4):607–624. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T., Nagasawa R., Nagata N., Shirai T. Specificity of mouse hybridoma antibodies to DNA. Immunol Lett. 1982 Feb;4(2):93–97. doi: 10.1016/0165-2478(82)90006-2. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Lambert P. H., Dixon F. J. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968 Mar 1;127(3):507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskov R., Muller S., Hochberg M., Giloh H., Van Regenmortel M. H., Eilat D. Monoclonal autoantibodies to histones from autoimmune NZB/NZW F1 mice. Eur J Immunol. 1984 Jan;14(1):74–81. doi: 10.1002/eji.1830140114. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Lewis J. R., Morgan A. R., Mosmann T. R., Singh B. Monoclonal antibodies showing sequence specificity in their interaction with single-stranded DNAs. Nucleic Acids Res. 1981 Apr 10;9(7):1707–1721. doi: 10.1093/nar/9.7.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion T. N., Lawton A. R., 3rd, Kearney J. F., Briles D. E. Anti-DNA autoantibodies in (NZB X NZW)F1 mice are clonally heterogeneous, but the majority share a common idiotype. J Immunol. 1982 Feb;128(2):668–674. [PubMed] [Google Scholar]
- Pisetsky D. S., Caster S. A. Binding specificity of a monoclonal anti-DNA antibody. Mol Immunol. 1982 May;19(5):645–650. doi: 10.1016/0161-5890(82)90364-9. [DOI] [PubMed] [Google Scholar]
- Pisetsky D. S., Peters D. V. A simple enzyme-linked immunosorbent assay for antibodies to native DNA. J Immunol Methods. 1981;41(2):187–200. doi: 10.1016/0022-1759(81)90242-8. [DOI] [PubMed] [Google Scholar]
- Rennke H. G., Cotran R. S., Venkatachalam M. A. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J Cell Biol. 1975 Dec;67(3):638–646. doi: 10.1083/jcb.67.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Hsu-Lin S. C., Gabriels J. E., Silberstein L. E., Furie B. C., Furie B., Stollar B. D., Schwartz R. S. Production of autoantibodies by human-human hybridomas. J Clin Invest. 1982 Jul;70(1):205–208. doi: 10.1172/JCI110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman C. R. Circulating DNA in systemic lupus erythematosus. Isolation and characterization. J Clin Invest. 1984 Mar;73(3):832–841. doi: 10.1172/JCI111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tron F., Charron D., Bach J. F., Talal N. Establishment and characterization of a murine hybridoma secreting monoclonal anti-DNA autoantibody. J Immunol. 1980 Dec;125(6):2805–2809. [PubMed] [Google Scholar]
- Winfield J. B., Faiferman I., Koffler D. Avidity of anti-DNA antibodies in serum and IgG glomerular eluates from patients with systemic lupus erythematosus. Association of high avidity antinative DNA antibody with glomerulonephritis. J Clin Invest. 1977 Jan;59(1):90–96. doi: 10.1172/JCI108626. [DOI] [PMC free article] [PubMed] [Google Scholar]


