Abstract
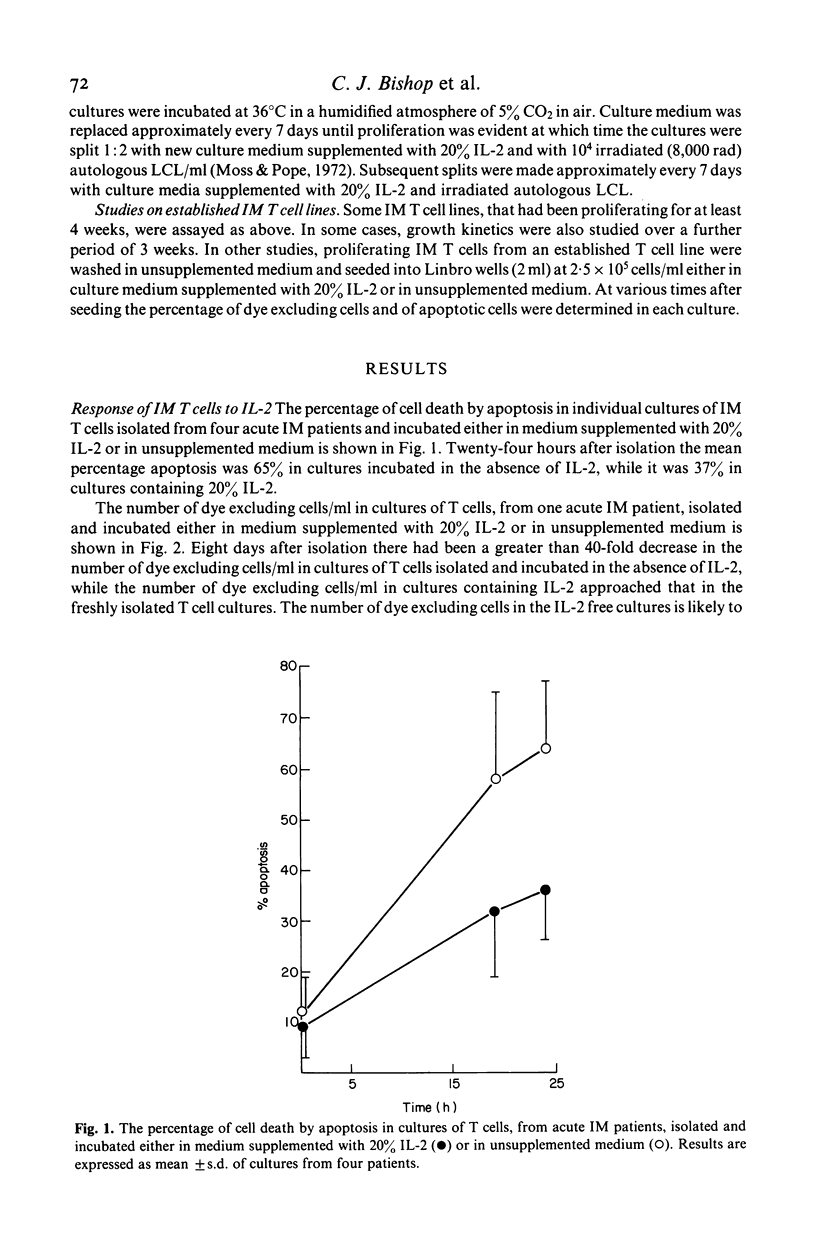
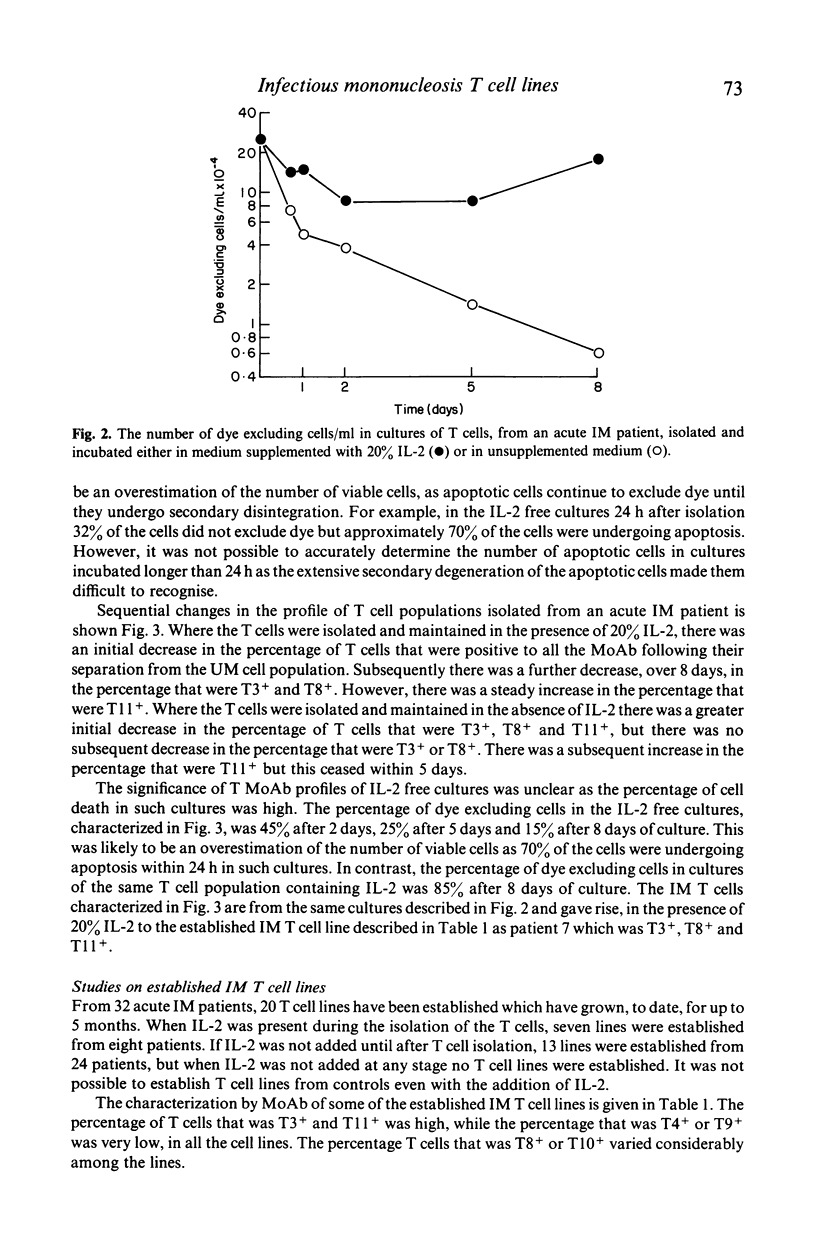
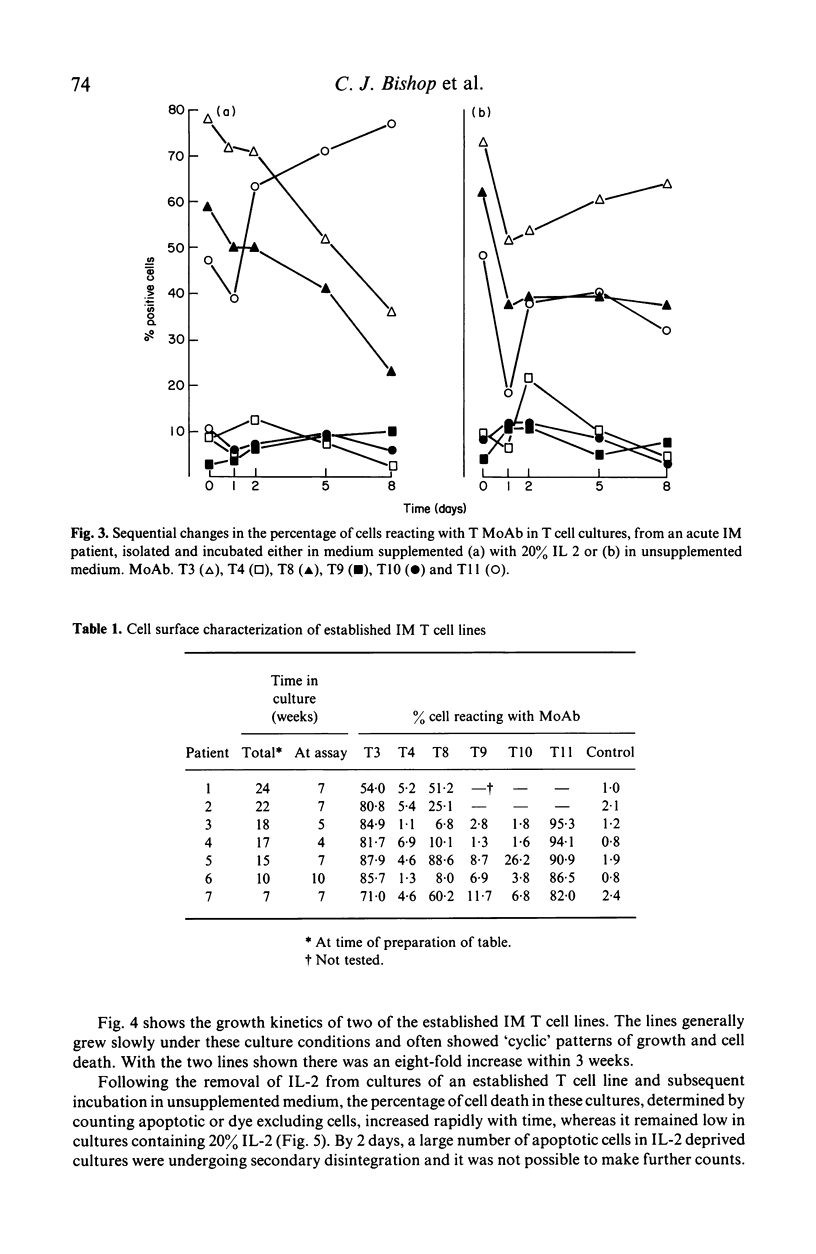
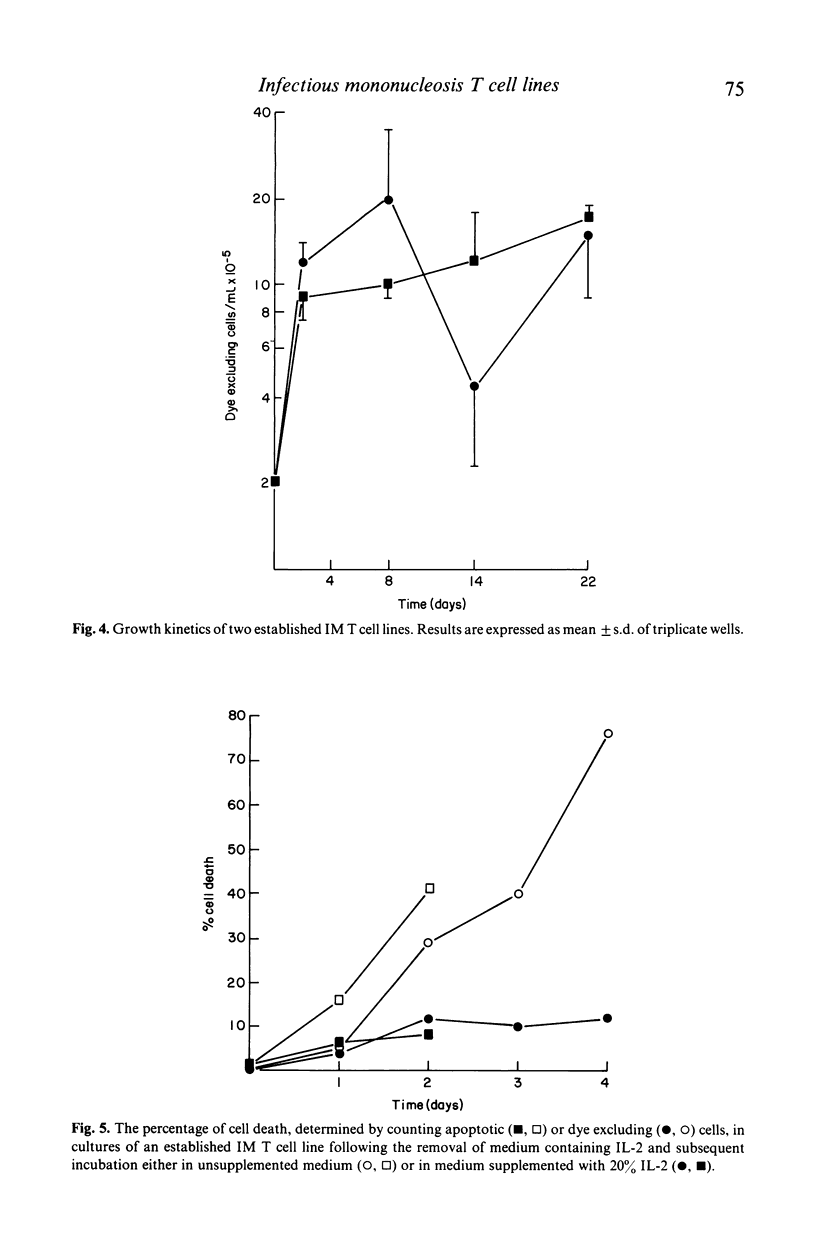
The addition of 20% interleukin-2 (IL-2) significantly reduced the percentage of T lymphocytes dying in vitro after being isolated from the peripheral blood of acute infectious mononucleosis (IM) patients. Moreover, the immediate addition of 20% IL-2 to freshly isolated blood allowed IM T cell lines to be readily established from the peripheral blood of acute IM patients. Characterization of seven of these IM T cell lines showed them to be T3+, T11+, T4-, T9- and generally T10-. Over half of the lines characterized were T8+. It will now be possible to re-evaluate IM T cell effector functions as previous assays of IM T cell functions may have been influenced by the presence of rapid and extensive T cell death in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- De Waele M., Thielemans C., Van Camp B. K. Characterization of immunoregulatory T cells in EBV-induced infectious mononucleosis by monoclonal antibodies. N Engl J Med. 1981 Feb 19;304(8):460–462. doi: 10.1056/NEJM198102193040804. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Schooley R. T., Payling-Wright C. R., Grouse J. E., Dolin R., Fauci A. S. Emergence of suppressor cells of immunoglobulin synthesis during acute Epstein-Barr virus-induced infectious mononucleosis. J Immunol. 1979 Nov;123(5):2095–2101. [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Klein G., Svedmyr E., Jondal M., Persson P. O. EBV-determined nuclear antigen (EBNA)-positive cells in the peripheral blood of infectious mononucleosis patients. Int J Cancer. 1976 Jan 15;17(1):21–26. doi: 10.1002/ijc.2910170105. [DOI] [PubMed] [Google Scholar]
- Lipinski M., Fridman W. H., Tursz T., Vincent C., Pious D., Fellous M. Absence of allogeneic restriction in human T-cell-mediated cytotoxicity to Epstein-Barr virus-infected target cells. Demonstration of an HLA-linked control at the effector level. J Exp Med. 1979 Dec 1;150(6):1310–1322. doi: 10.1084/jem.150.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko I. S., Pope J. H., Hütter R., Soszynski T. D., Kane R. G. HLA-DR-antigen-associated restriction of EBV-specific cytotoxic T-cell colonies. Int J Cancer. 1984 Feb 15;33(2):239–243. doi: 10.1002/ijc.2910330212. [DOI] [PubMed] [Google Scholar]
- Moss D. J., Bishop C. J., Burrows S. R., Ryan J. M. T lymphocytes in infectious mononucleosis. I. T cell death in vitro. Clin Exp Immunol. 1985 Apr;60(1):61–69. [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Pope J. H. Assay of the infectivity of Epstein-Barr virus by transformation of human leucocytes in vitro. J Gen Virol. 1972 Nov;17(2):233–236. doi: 10.1099/0022-1317-17-2-233. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Klein G., Henle W., Henle G. The establishment of lymphoblastoid lines from adult and fetal human lymphoid tissue and its dependence on EBV. Int J Cancer. 1971 Nov 15;8(3):443–450. doi: 10.1002/ijc.2910080312. [DOI] [PubMed] [Google Scholar]
- Papamichail M., Sheldon P. J., Holborow E. J. T- and B-cell subpopulations in infectious mononucleosis. Clin Exp Immunol. 1974 Sep;18(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- Pope J. H. Establishment of cell lines from peripheral leucocytes in infectious mononucleosis. Nature. 1967 Nov 25;216(5117):810–811. doi: 10.1038/216810a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., O'Brien C., Rosenthal P., Schlossman S. F. The cellular basis for viral-induced immunodeficiency: analysis by monoclonal antibodies. J Immunol. 1980 Sep;125(3):1269–1274. [PubMed] [Google Scholar]
- Searle J., Kerr J. F., Bishop C. J. Necrosis and apoptosis: distinct modes of cell death with fundamentally different significance. Pathol Annu. 1982;17(Pt 2):229–259. [PubMed] [Google Scholar]
- Sheridan J. W., Bishop C. J., Simmons R. J. Biophysical and morphological correlates of kinetic change and death in a starved human melanoma cell line. J Cell Sci. 1981 Jun;49:119–137. doi: 10.1242/jcs.49.1.119. [DOI] [PubMed] [Google Scholar]
- Svedmyr E., Jondal M. Cytotoxic effector cells specific for B Cell lines transformed by Epstein-Barr virus are present in patients with infectious mononucleosis. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1622–1626. doi: 10.1073/pnas.72.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhorst C., van Agthoven A., LeClair K., Snow P., Reinherz E., Schlossman S. Biochemical studies of the human thymocyte cell-surface antigens T6, T9 and T10. Cell. 1981 Mar;23(3):771–780. doi: 10.1016/0092-8674(81)90441-4. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]



