Abstract
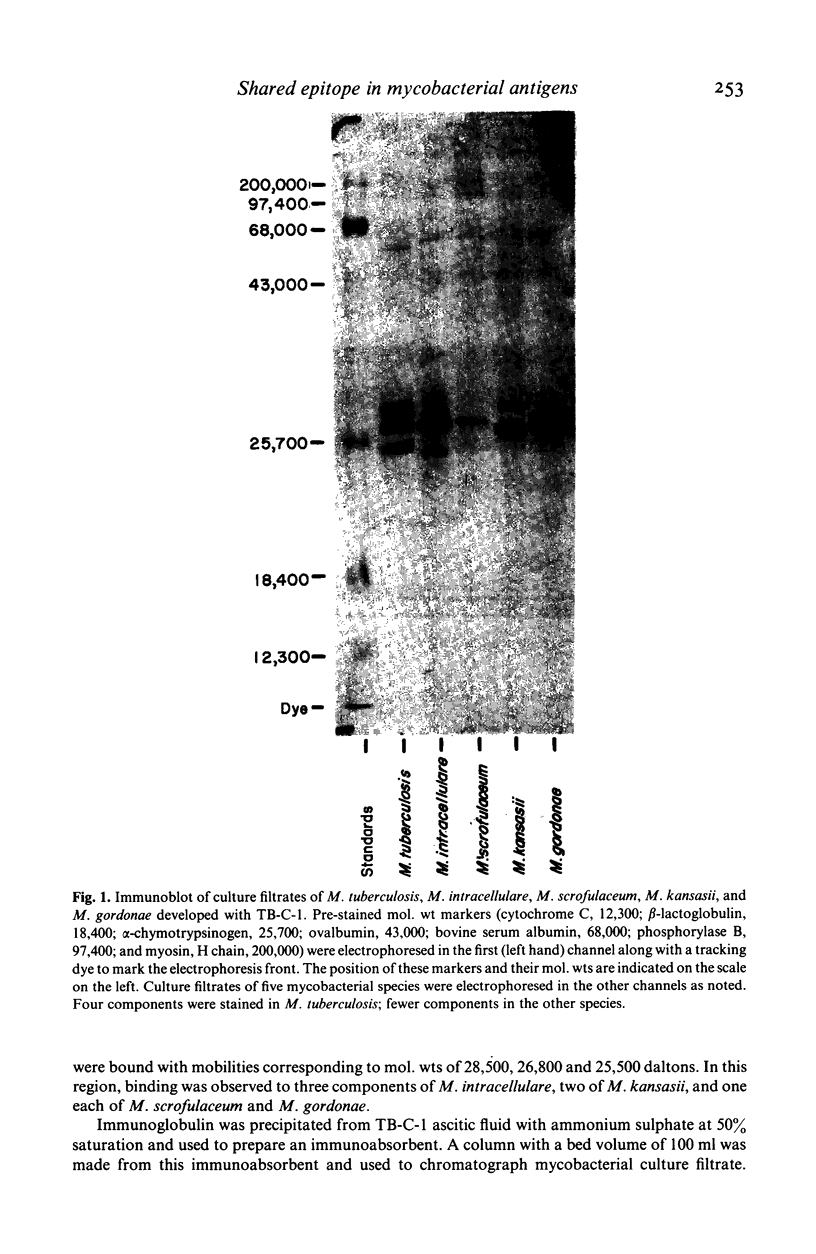
An IgM monoclonal antibody designated TB-C-1 which is broadly reactive with mycobacteria has been studied to characterize the antigens with which it reacts. Enzyme linked immunosorbent assay (ELISA) demonstrated reactivity not only with culture filtrates of several mycobacterial species but with several purified antigens of Mycobacterium tuberculosis, including protein antigens 5 and 6 and polysaccharides arabinogalactan and arabinomannan. Immunoblotting demonstrated reactivity with four distinct components of M. tuberculosis. Reactions with components of similar mol. wt were demonstrated for several other mycobacterial species, although fewer components bound with TB-C-1 in these other mycobacteria than in M. tuberculosis. Immunoabsorbents were prepared from TB-C-1 and used to isolate antigens with which the antibody reacted. Multiple antigens were identified in the eluates from M. tuberculosis, including protein antigens 6 and 7, arabinomannan, and arabinogalactan. Fewer components were recovered from other species of mycobacteria. Affinity of binding of immunoabsorbents was similar for all antigens bound. These results indicate that a common epitope is widely shared among antigens of M. tuberculosis and other mycobacteria and they suggest that species specificity of mycobacterial antigens may rest with individual epitopes rather than intact antigenic molecules.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. A., Hoffenbach A. A monoclonal antibody against Mycobacterium lepraemurium which recognizes a cross-reacting mycobacterial antigen. Ann Immunol (Paris) 1983 May-Jun;134C(3):301–309. doi: 10.1016/s0769-2625(83)80124-x. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Benjamin R. G., Daniel T. M. Serodiagnosis of tuberculosis using the enzyme-linked immunoabsorbent assay (ELISA) of antibody to Mycobacterium tuberculosis antigen 5. Am Rev Respir Dis. 1982 Dec;126(6):1013–1016. doi: 10.1164/arrd.1982.126.6.1013. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Anderson P. A. The isolation by immunoabsorbent affinity chromatography and physicochemical characterization of Mycobacterium tuberculosis antigen 5. Am Rev Respir Dis. 1978 Mar;117(3):533–539. doi: 10.1164/arrd.1978.117.3.533. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Anderson P. A. The use of immunoabsorbents for the purification of mycobacterial antigens. J Lab Clin Med. 1977 Aug;90(2):354–360. [PubMed] [Google Scholar]
- Daniel T. M., Balestrino E. A., Balestrino O. C., Davidson P. T., Debanne S. M., Kataria S., Kataria Y. P., Scocozza J. B. The tuberculin specificity in humans of Mycobacterium tuberculosis antigen 5. Am Rev Respir Dis. 1982 Oct;126(4):600–606. doi: 10.1164/arrd.1982.126.4.600. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., DeMuth R. W. Immunochemical analyses of a major antigen of Mycobacterium szulgai. J Infect Dis. 1977 May;135(5):778–786. doi: 10.1093/infdis/135.5.778. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Ellner J. J., Todd L. S., McCoy D. W., Payne V. D., Anderson P. A., Bhe F. T. Immunobiology and species distribution of Mycobacterium tuberculosis antigen 5. Infect Immun. 1979 Apr;24(1):77–82. doi: 10.1128/iai.24.1.77-82.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. M., Ferguson L. E. Purification and Characterization of Two Proteins from Culture Filtrates of Mycobacterium tuberculosis H(37)Ra Strain. Infect Immun. 1970 Feb;1(2):164–168. doi: 10.1128/iai.1.2.164-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. M., Gonchoroff N. J., Katzmann J. A., Olds G. R. Specificity of Mycobacterium tuberculosis antigen 5 determined with mouse monoclonal antibodies. Infect Immun. 1984 Jul;45(1):52–55. doi: 10.1128/iai.45.1.52-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. M., Good R. C., Janicki B. W. Immunoelectrophoresis of Mycobacterium tuberculosis antigens. Comparative analysis of cell extract and culture filtrate antigens. Am Rev Respir Dis. 1975 Nov;112(5):639–644. doi: 10.1164/arrd.1975.112.5.639. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Janicki B. W. Mycobacterial antigens: a review of their isolation, chemistry, and immunological properties. Microbiol Rev. 1978 Mar;42(1):84–113. doi: 10.1128/mr.42.1.84-113.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. M., Misaki A. Carbohydrate analysis of concanavalin A-reactive and concanavalin A-nonreactive mycobacterial polysaccharides. Am Rev Respir Dis. 1976 May;113(5):705–706. doi: 10.1164/arrd.1976.113.5.705. [DOI] [PubMed] [Google Scholar]
- Daniel T. M. The purification of mycobacterial polysaccharides with concanavalin A. Am Rev Respir Dis. 1974 Nov;110(5):634–640. doi: 10.1164/arrd.1974.110.5.634. [DOI] [PubMed] [Google Scholar]
- Daniels T. M., Affronti L. F. Immunoelectrophoretic analysis of antigenic constituents of Seibert fractions of mycobacterial culture filtrates. Identification with the proposed United States-Japan reference nomenclature. Am Rev Respir Dis. 1973 Nov;108(5):1244–1248. doi: 10.1164/arrd.1973.108.5.1244. [DOI] [PubMed] [Google Scholar]
- Gillis T. P., Buchanan T. M. Production and partial characterization of monoclonal antibodies to Mycobacterium leprae. Infect Immun. 1982 Jul;37(1):172–178. doi: 10.1128/iai.37.1.172-178.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J., Coates A. R., Mitchison D. A., Ivanyi J. The use of murine monoclonal antibodies without purification of antigen in the serodiagnosis of tuberculosis. J Immunol Methods. 1982 Dec 17;55(2):205–211. doi: 10.1016/0022-1759(82)90032-1. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Sinha S., Aston R., Cussell D., Keen M., Sengupta U. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin Exp Immunol. 1983 Jun;52(3):528–536. [PMC free article] [PubMed] [Google Scholar]
- Janicki B. W., Chaparas S. D., Daniel T. M., Kubica G. P., Wright G. L., Yee G. S. A reference system for antigens of Mycobacterium tuberculosis. Am Rev Respir Dis. 1971 Oct;104(4):602–604. doi: 10.1164/arrd.1971.104.4.602. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ma Y., Daniel T. M. Immunochemical analysis of tuberculin purified protein derivative with special reference to United States-Japan antigen 7. J Infect Dis. 1983 Sep;148(3):500–509. doi: 10.1093/infdis/148.3.500. [DOI] [PubMed] [Google Scholar]
- OSSERMAN E. F. A modified technique of immunoelectrophoresis facilitating the identification of specific precipitin arcs. J Immunol. 1960 Jan;84:93–97. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Yoneda M., Fukui Y. Isolation, purification, and characterization of extracellular antigens of Mycobacterium tuberculosis. Am Rev Respir Dis. 1965 Dec;92(6):9–18. doi: 10.1164/arrd.1965.92.6P2.9. [DOI] [PubMed] [Google Scholar]
- Young D. B., Khanolkar S. R., Barg L. L., Buchanan T. M. Generation and characterization of monoclonal antibodies to the phenolic glycolipid of Mycobacterium leprae. Infect Immun. 1984 Jan;43(1):183–188. doi: 10.1128/iai.43.1.183-188.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



