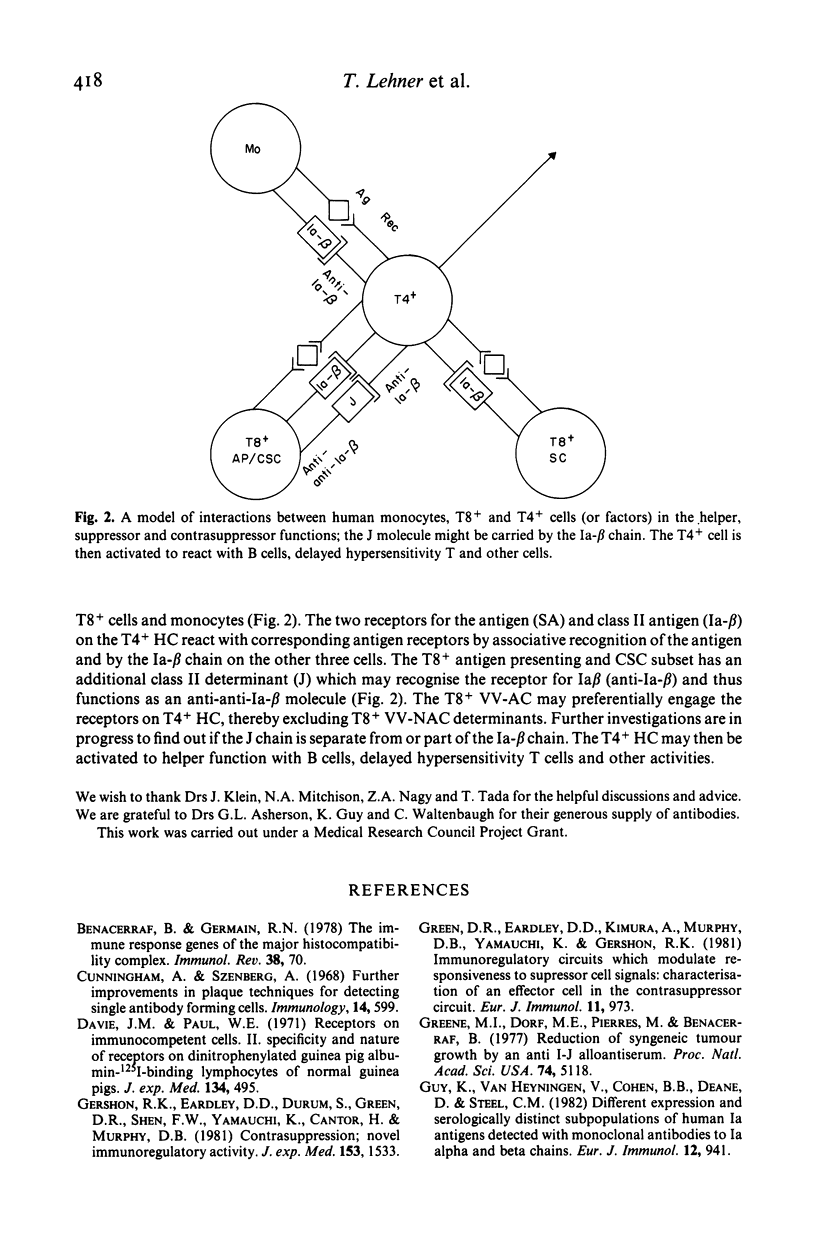
Abstract
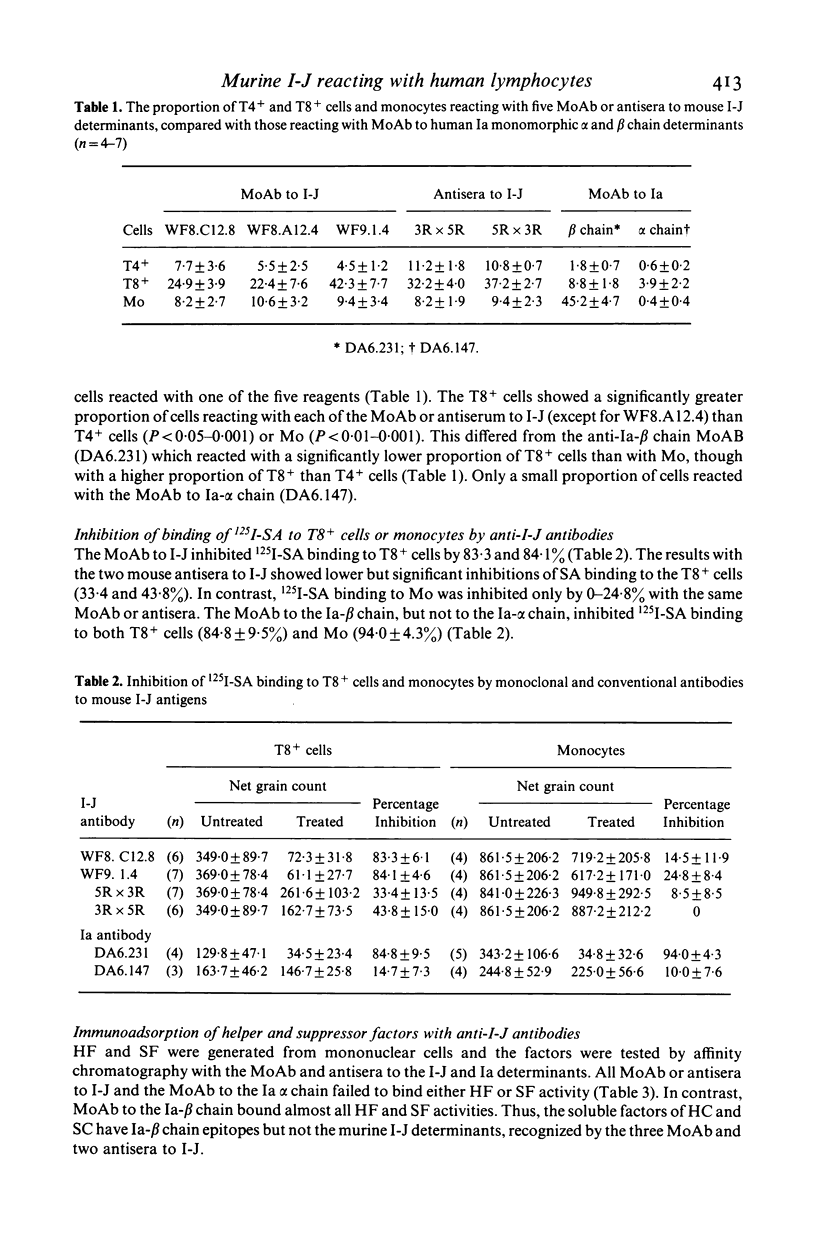
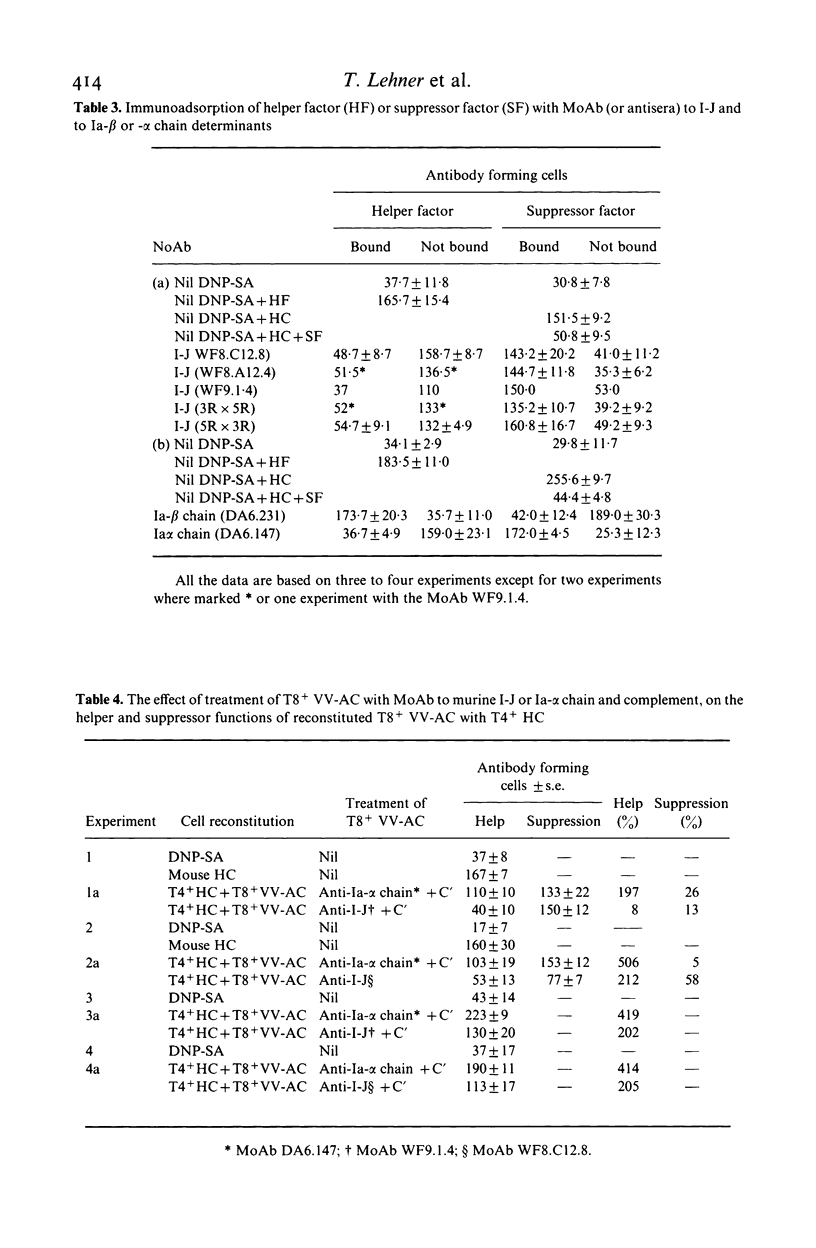
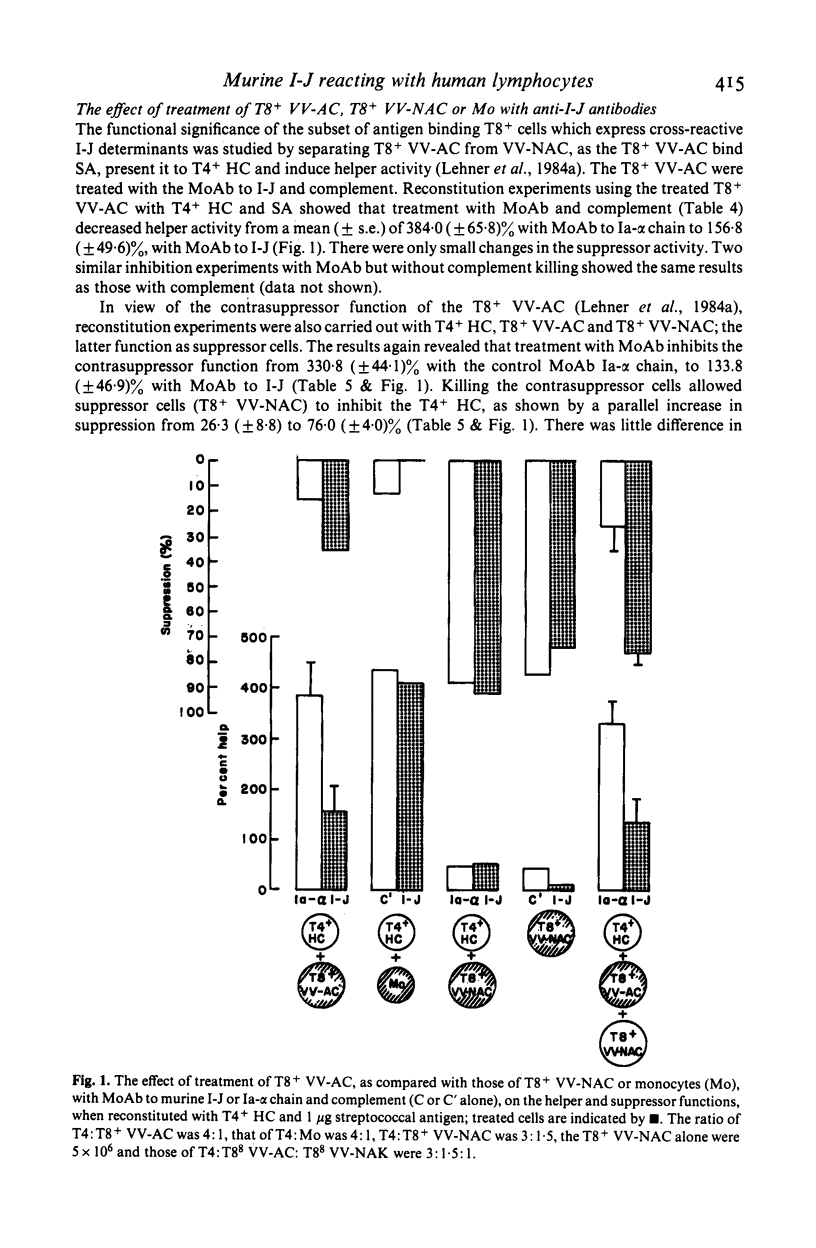
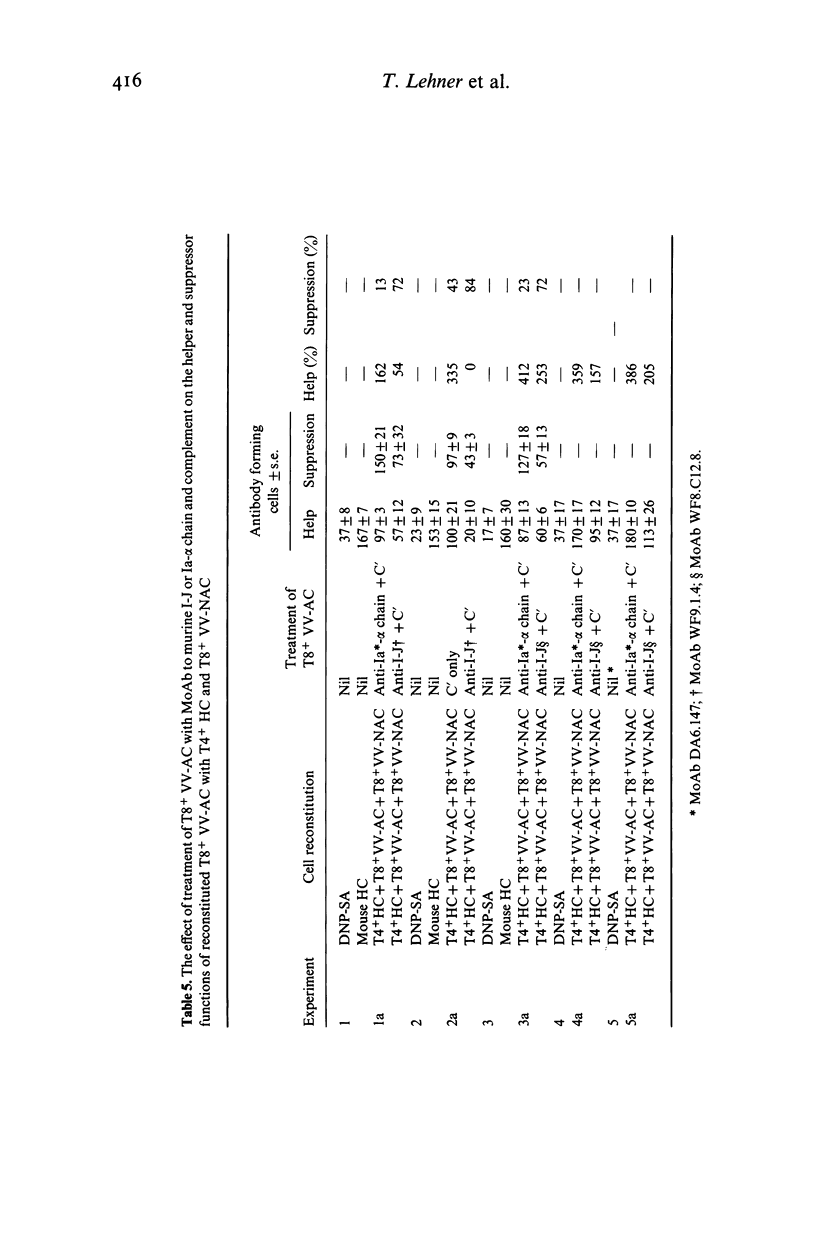
Murine I-J gene products have been found in T suppressor cells (SC) and factors, macrophages and contrasuppressor cells (CSC). However, a human counterpart of the murine I-J has not been reported. As there is strong evidence that some murine anti-Ia antisera cross-react with human Ia antigens, the possibility was tested that mouse anti-I-J antibodies might cross-react with corresponding human class II determinants. Indeed, this revealed that three anti-I-J monoclonal antibodies (MoAb) and two antisera tested react with human mononuclear cells and that a significantly greater proportion of T8+ than T4+ cells or monocytes (Mo) react with the I-J antibodies. This was corroborated by autoradiography with significant inhibition of 125I-SA (streptococcal antigen) binding to T8+ cells but not to Mo by the MoAb or antisera to murine I-J. Functional reconstitution experiments of T4+ helper cells with the SA binding and presenting T8+ Vicia villosa adherent cells (VV-AC) and assessment of specific antibody forming cells to SA suggest that the antigen presenting function of this T8+ subset can be significantly inhibited by killing with the MoAb to I-J and complement. Furthermore, the subset of T8+ VV-AC also functions as CSC, for killing with MoAb to I-J and complement significantly inhibited the contrasuppressor function. This is consistent with the presence of I-J gene products in murine CSC. However, similar treatment of T8+ VV-NAC (non-adherent cells) or monocytes (Mo) failed to affect the suppressor or accessory helper function of these cells, respectively. Phenotypic characterization, inhibition of 125I-SA binding and reconstitution experiments for helper and suppressor functions, suggest that a subset of T8+ antigen binding, presenting and CSC may express determinants cross-reacting with murine I-J molecules.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B., Germain R. N. The immune response genes of the major histocompatibility complex. Immunol Rev. 1978;38:70–119. doi: 10.1111/j.1600-065x.1978.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Receptors on immunocompetent cells. II. Specificity and nature of receptors on dinitrophenylated guinea pig albumin- 125 I-binding lymphocytes of normal guinea pigs. J Exp Med. 1971 Aug 1;134(2):495–516. doi: 10.1084/jem.134.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Eardley D. D., Durum S., Green D. R., Shen F. W., Yamauchi K., Cantor H., Murphy D. B. Contrasuppression. A novel immunoregulatory activity. J Exp Med. 1981 Jun 1;153(6):1533–1546. doi: 10.1084/jem.153.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. R., Eardley D. D., Kimura A., Murphy D. B., Yamauchi K., Gershon R. K. Immunoregulatory circuits which modulate responsiveness to suppressor cell signals: characterization of an effector cell in the contrasuppressor circuit. Eur J Immunol. 1981 Dec;11(12):973–980. doi: 10.1002/eji.1830111205. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Dorf M. E., Pierres M., Benacerraf B. Reduction of syngeneic tumor growth by an anti-I-J-alloantiserum. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5118–5121. doi: 10.1073/pnas.74.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hayes C. E., Klyczek K. K., Krum D. P., Whitcomb R. M., Hullett D. A., Cantor H. Chromosome 4 Jt gene controls murine T cell surface I-J expression. Science. 1984 Feb 10;223(4636):559–563. doi: 10.1126/science.6607530. [DOI] [PubMed] [Google Scholar]
- Ikezawa Z., Baxevanis C. N., Arden B., Tada T., Waltenbaugh C. R., Nagy Z. A., Klein J. Evidence for two suppressor factors secreted by a single cell suggests a solution to the J-locus paradox. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6637–6641. doi: 10.1073/pnas.80.21.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezawa Z., Nonaka M., Abe R., Tada T., Nagy Z. A., Klein J. Induction of T cell responses in nonresponder mice by abolishing suppression with monoclonal antibodies recognizing A region-controlled, T cell-specific determinants. J Immunol. 1983 Oct;131(4):1646–1649. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Lehner T. Antigen-binding human T suppressor cells and their association with the HLA-DR locus. Eur J Immunol. 1983 May;13(5):370–378. doi: 10.1002/eji.1830130505. [DOI] [PubMed] [Google Scholar]
- Lehner T. Characterization of human helper and suppressor factors with special reference to HLA-DR determinants. Immunology. 1983 Apr;48(4):695–702. [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Jones T. The role of MHC class II antigenic determinants in the function of human antigen binding T8+ cells, monocytes and helper and suppressor factors. Clin Exp Immunol. 1984 Jun;56(3):683–693. [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Lamb J. R., Welsh K. L., Batchelor R. J. Association between HLA-DR antigens and helper cell activity in the control of dental caries. Nature. 1981 Aug 20;292(5825):770–772. doi: 10.1038/292770a0. [DOI] [PubMed] [Google Scholar]
- Lehner T. The relationship between human helper and suppressor factors to a streptococcal protein antigen. J Immunol. 1982 Nov;129(5):1936–1940. [PubMed] [Google Scholar]
- Lunney J. K., Mann D. L., Sachs D. H. Sharing Ia antigens between species. III. Ia specificities shared between mice and human beings. Scand J Immunol. 1979;10(5):403–413. doi: 10.1111/j.1365-3083.1979.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Malkovský M., Asherson G. L., Chandler P., Colizzi V., Watkins M. C., Zembala M. Nonspecific inhibitor of DNA synthesis elaborated by T acceptor cells. I. Specific hapten- and I-J-driven liberation of an inhibitor of cell proliferation by Lyt-1-2+ cyclophosphamide-sensitive T acceptor cells armed with a product of Lyt-1+2+-specific suppressor cells. J Immunol. 1983 Feb;130(2):785–790. [PubMed] [Google Scholar]
- Murphy D. B., Herzenberg L. A., Okumura K., Herzenberg L. A., McDevitt H. O. A new I subregion (I-J) marked by a locus (Ia-4) controlling surface determinants on suppressor T lymphocytes. J Exp Med. 1976 Sep 1;144(3):699–712. doi: 10.1084/jem.144.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierres M., Rebouah J. P., Kourilsky F. M., Dosseto M., Mercier P., Mawas C., Malissen B. Cross-reactions between mouse Ia and human HLA-D/DR antigens analyzed with monoclonal alloantibodies. J Immunol. 1981 Jun;126(6):2424–2429. [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Lehner T. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23(1):7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., David C. S. Properties of the antigen-specific suppressive T-cell factor in the regulation of antibody response of the mouse. IV. Special subregion assignment of the gene(s) that codes for the suppressive T-cell factor in the H-2 histocompatibility complex. J Exp Med. 1976 Sep 1;144(3):713–725. doi: 10.1084/jem.144.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltenbaugh C. Regulation of immune responses by I-J gene products. I. Production and characterization of anti-I-J monoclonal antibodies. J Exp Med. 1981 Nov 1;154(5):1570–1583. doi: 10.1084/jem.154.5.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]


