Abstract
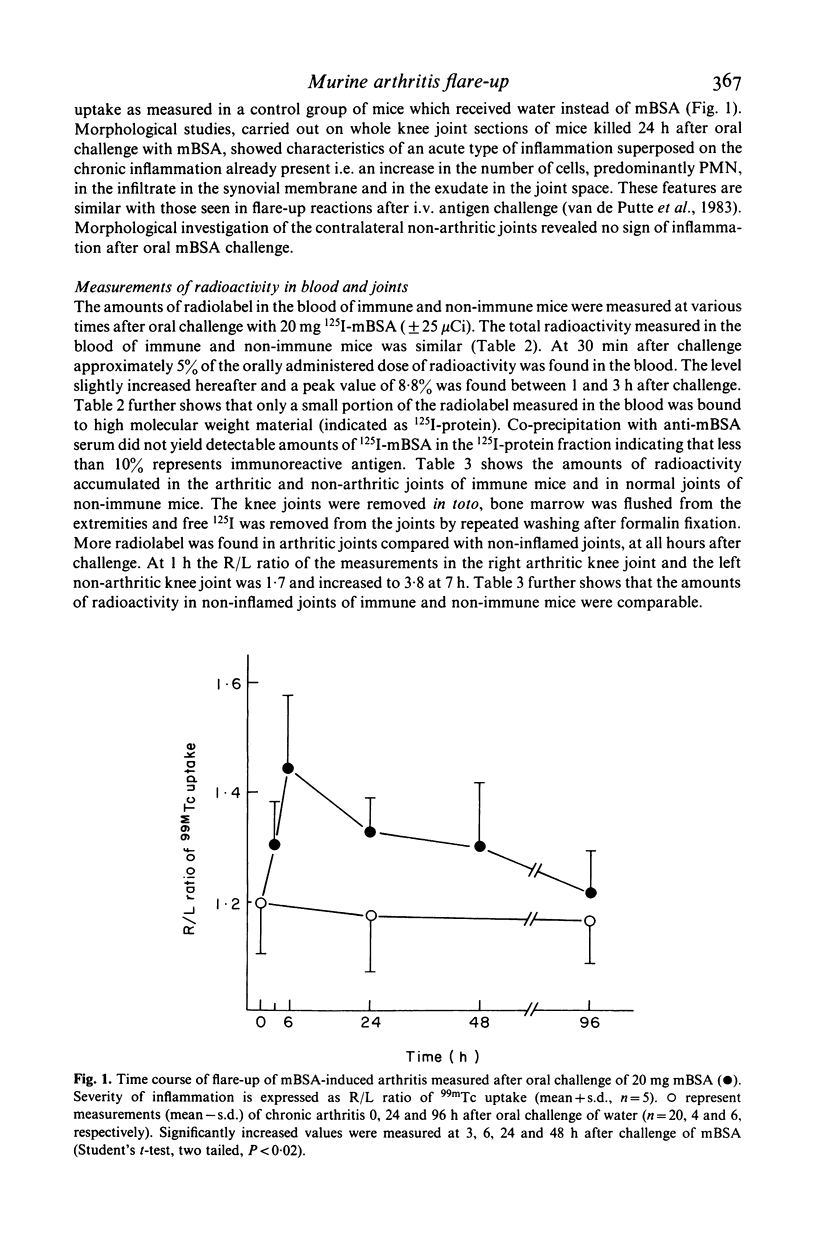
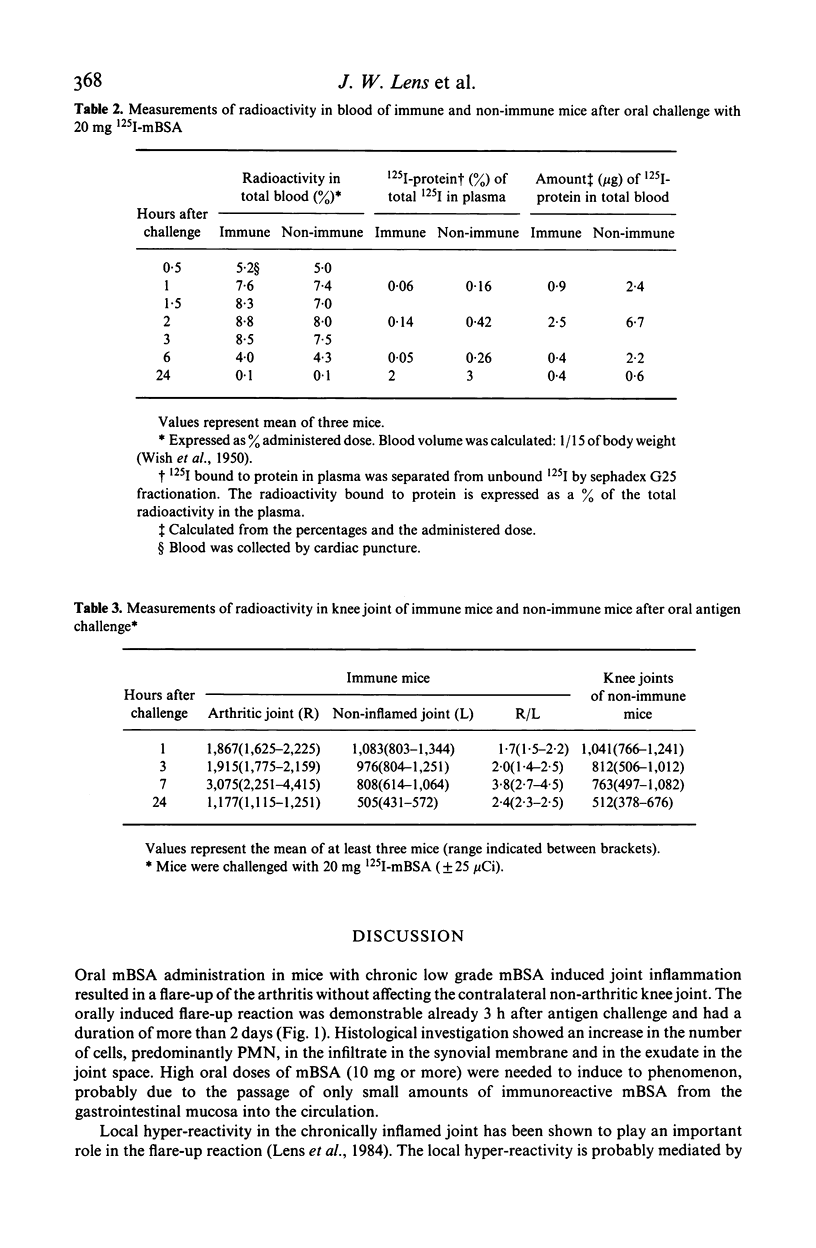
Mice with unilateral chronic mBSA-induced arthritis were orally challenged with mBSA. Three hours after antigen challenge clear flare-up of the chronic arthritis was demonstrable as detected by an increase in the 99mTc uptake of the knee joint and the reaction continued for at least 2 days. The contralateral non-arthritic knee joint was not affected. The dose of mBSA needed to induce a flare-up in nearly all mice within a group was in the order of 20 mg. After oral challenge with 10 or 5 mg of mBSA the incidence was lower and flare-up reactions were only rarely observed after challenge with 2.5 or 1.25 mg mBSA. Histology of knee joints taken at 24 h after oral challenge of 20 mg mBSA revealed an increase in the number of cells in the infiltrate in the synovial tissue and exudate in the joint space, the most conspicuous sign being the increase of PMN. Passage of macromolecules through the gastrointestinal mucosa may be an important principle in the perpetuation of human chronic arthritis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloch K. J., Bloch D. B., Stearns M., Walker W. A. Intestinal uptake of macromolecules. VI. Uptake of protein antigen in vivo in normal rats and in rats infected with Nippostrongylus brasiliensis or subjected to mild systemic anaphylaxis. Gastroenterology. 1979 Nov;77(5):1039–1044. [PubMed] [Google Scholar]
- Brackertz D., Mitchell G. F., Mackay I. R. Antigen-induced arthritis in mice. I. Induction of arthritis in various strains of mice. Arthritis Rheum. 1977 Apr;20(3):841–850. doi: 10.1002/art.1780200314. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C., Brandeis W. E., Pudifin D. J., Day N. K., Good R. A. Autoimmunity in selective IgA deficiency: relationship to anti-bovine protein antibodies, circulating immune complexes and clinical disease. Clin Exp Immunol. 1981 Aug;45(2):299–304. [PMC free article] [PubMed] [Google Scholar]
- Dannaeus A., Inganäs M., Johansson S. G., Foucard T. Intestinal uptake of ovalbumin in malabsorption and food allergy in relation to serum IgG antibody and orally administered sodium cromoglycate. Clin Allergy. 1979 May;9(3):263–270. doi: 10.1111/j.1365-2222.1979.tb01552.x. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Kilshaw P. J., Slade H. Passage of ingested protein into the blood during gastrointestinal hypersensitivity reactions: experiments in the preruminant calf. Clin Exp Immunol. 1980 Sep;41(3):575–582. [PMC free article] [PubMed] [Google Scholar]
- Kruijsen M. W., van den Berg W. B., van de Putte L. B., van den Broek W. J. Detection and quantification of experimental joint inflammation in mice by measurement of 99mTc-pertechnetate uptake. Agents Actions. 1981 Dec;11(6-7):640–642. doi: 10.1007/BF01978775. [DOI] [PubMed] [Google Scholar]
- Lens J. W., van den Berg W. B., van de Putte L. B., Berden J. H., Lems S. P. Flare-up of antigen-induced arthritis in mice after challenge with intravenous antigen: effects of pre-treatment with cobra venom factor and anti-lymphocyte serum. Clin Exp Immunol. 1984 Sep;57(3):520–528. [PMC free article] [PubMed] [Google Scholar]
- Lens J. W., van den Berg W. B., van de Putte L. B. Flare-up of antigen-induced arthritis in mice after challenge with intravenous antigen: studies on the characteristics of and mechanisms involved in the reaction. Clin Exp Immunol. 1984 Feb;55(2):287–294. [PMC free article] [PubMed] [Google Scholar]
- Lens J. W., van den Berg W. B., van de Putte L. B. Quantitation of arthritis by 99mTc-uptake measurements in the mouse knee-joint: correlation with histological joint inflammation scores. Agents Actions. 1984 Jun;14(5-6):723–728. doi: 10.1007/BF01978915. [DOI] [PubMed] [Google Scholar]
- Little C. H., Stewart A. G., Fennessy M. R. Platelet serotonin release in rheumatoid arthritis: a study in food-intolerant patients. Lancet. 1983 Aug 6;2(8345):297–299. doi: 10.1016/s0140-6736(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Moment G. B. Aging, arthritis and food allergies: a research opportunity revisited. Growth. 1980 Sep;44(3):155–159. [PubMed] [Google Scholar]
- Pang K. Y., Walker W. A., Bloch K. J. Intestinal uptake of macromolecules. Differences in distribution and degradation of protein antigen in control and immunised rats. Gut. 1981 Dec;22(12):1018–1024. doi: 10.1136/gut.22.12.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke A. L., Hughes G. R. Rheumatoid arthritis and food: a case study. Br Med J (Clin Res Ed) 1981 Jun 20;282(6281):2027–2029. doi: 10.1136/bmj.282.6281.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S. A., Reinhardt M. C., Paganelli R., Levinsky R. J. Specific antigen exclusion and non-specific facilitation of antigen entry across the gut in rats allergic to food proteins. Clin Exp Immunol. 1981 Jul;45(1):131–136. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Stein H. B., Schlappner O. L., Boyko W., Gourlay R. H., Reeve C. E. The intestinal bypass: arthritis-dermatitis syndrome. Arthritis Rheum. 1981 May;24(5):684–690. doi: 10.1002/art.1780240509. [DOI] [PubMed] [Google Scholar]
- Stokes C. R., Swarbrick E. T., Soothill J. F. Genetic differences in immune exclusion and partial tolerance to ingested antigens. Clin Exp Immunol. 1983 Jun;52(3):678–684. [PMC free article] [PubMed] [Google Scholar]
- Swarbrick E. T., Stokes C. R., Soothill J. F. Absorption of antigens after oral immunisation and the simultaneous induction of specific systemic tolerance. Gut. 1979 Feb;20(2):121–125. doi: 10.1136/gut.20.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udall J. N., Bloch K. J., Fritze L., Walker W. A. Binding of exogenous protein fragments to native proteins: possible explanation for the overestimation of uptake of extrinsically labelled macromolecules from the gut. Immunology. 1981 Feb;42(2):251–257. [PMC free article] [PubMed] [Google Scholar]
- WISH L., FURTH J., STOREY R. H. Direct determinations of plasma, cell, and organ-blood volumes in normal and hypervolemic mice. Proc Soc Exp Biol Med. 1950 Jul;74(3):644–648. doi: 10.3181/00379727-74-18003. [DOI] [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Williams R. Rheumatoid arthritis and food: a case study. Br Med J (Clin Res Ed) 1981 Aug 22;283(6290):563–563. doi: 10.1136/bmj.283.6290.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff M. Diet in the treatment of rheumatoid arthritis. Arthritis Rheum. 1983 Apr;26(4):457–461. doi: 10.1002/art.1780260402. [DOI] [PubMed] [Google Scholar]
- van de Putte L. B., Lens J. W., van den Berg W. B., Kruijsen M. W. Exacerbation of antigen-induced arthritis after challenge with intravenous antigen. Immunology. 1983 May;49(1):161–167. [PMC free article] [PubMed] [Google Scholar]


