Abstract
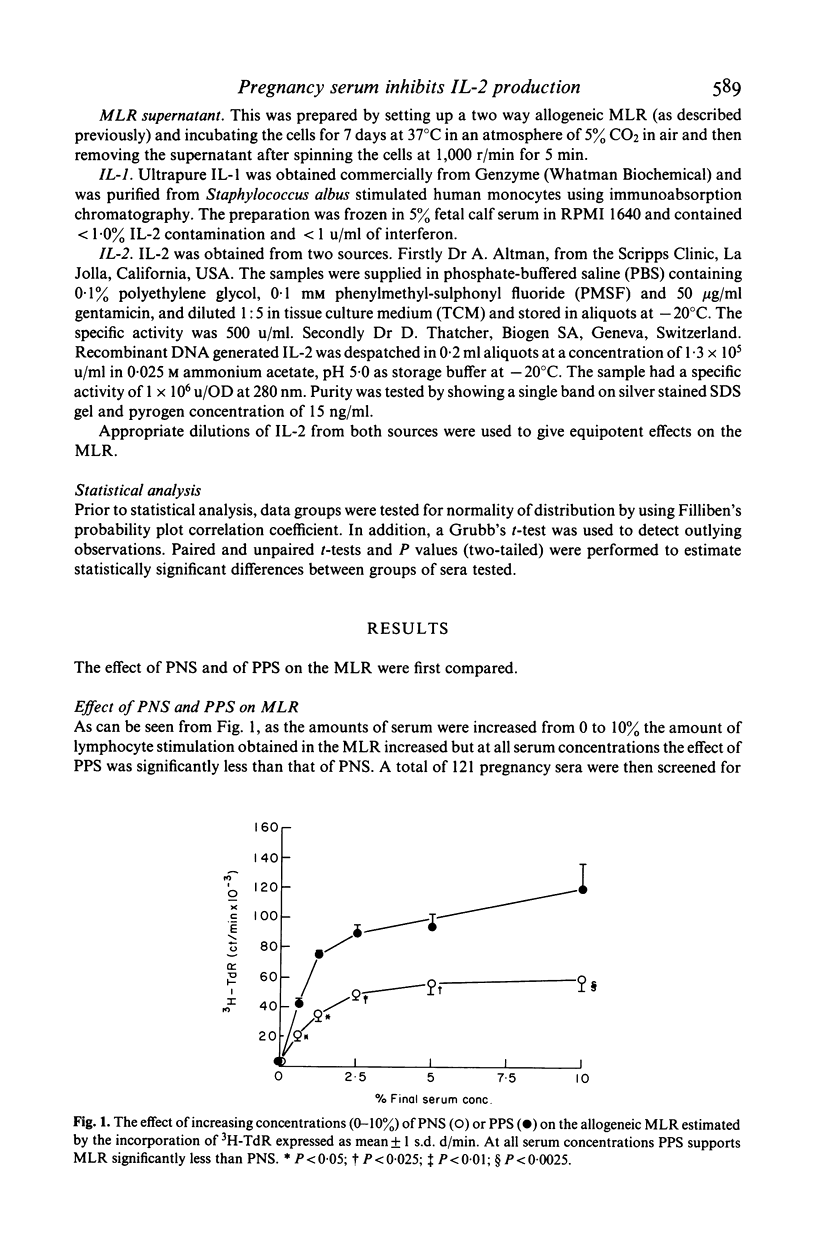
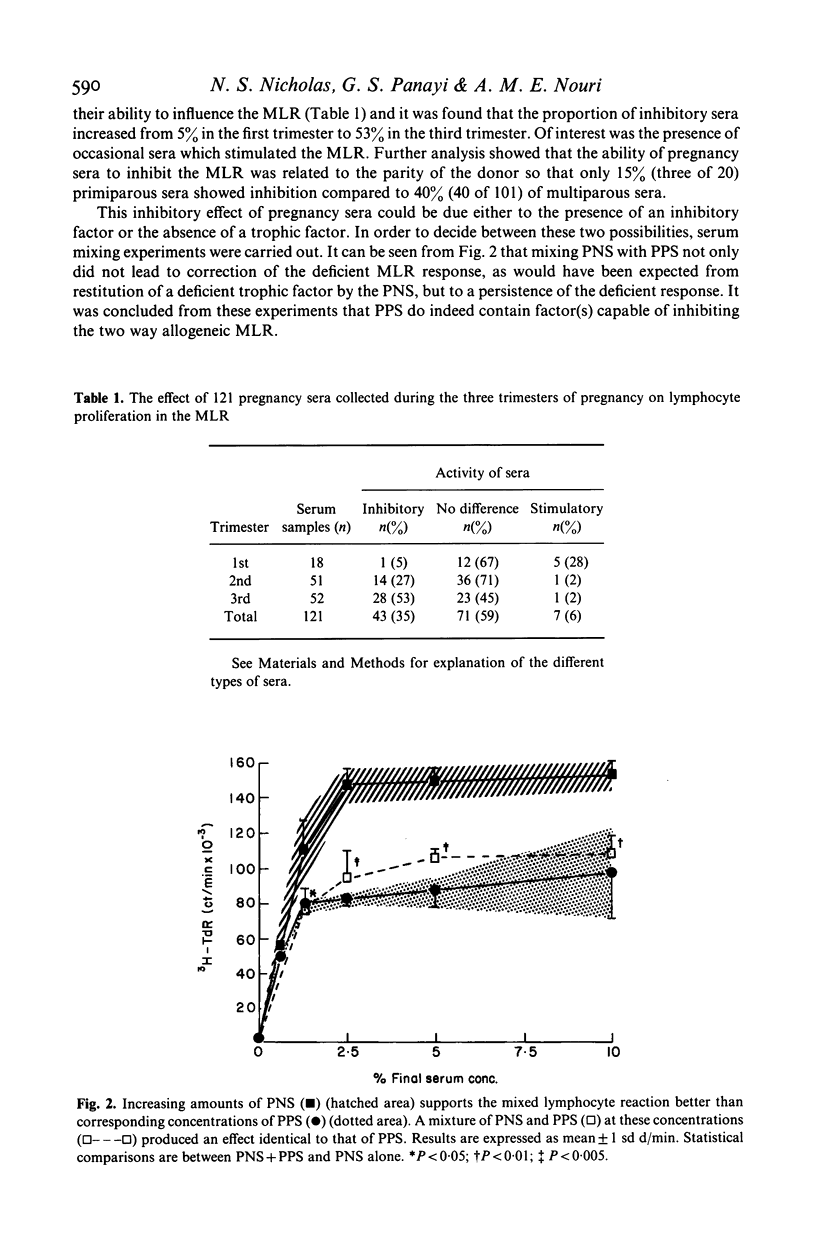
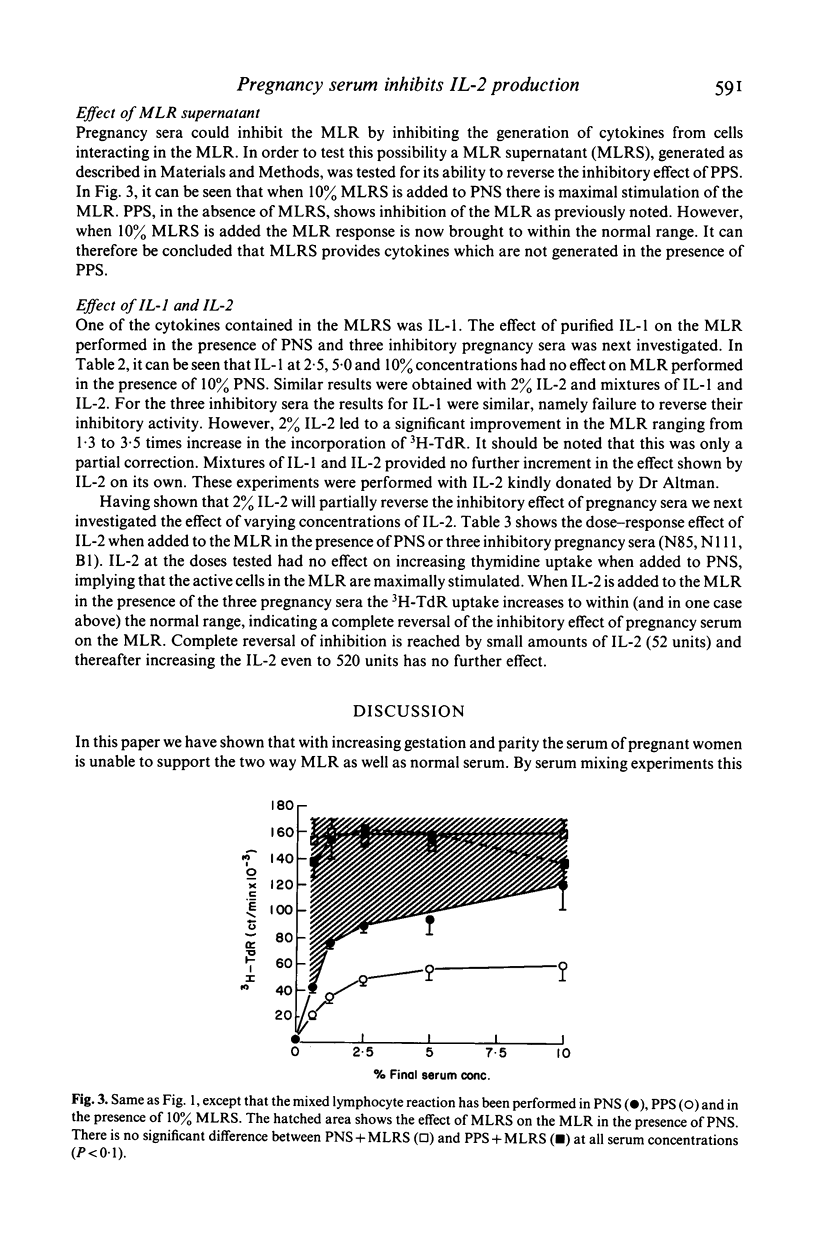
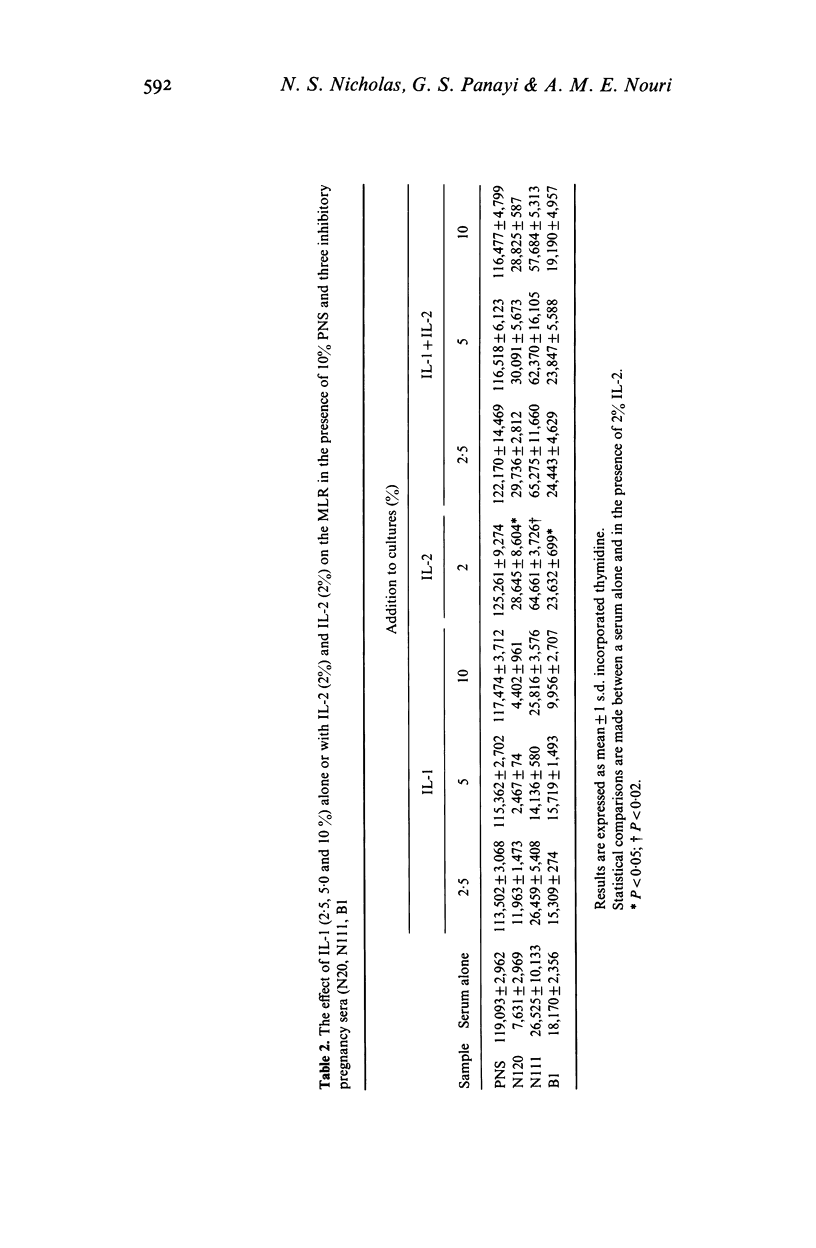
Cell-mediated immunity may be depressed during pregnancy. We used the two way mixed lymphocyte reaction as an in vitro model of cell mediated immunity and studied the effect of pregnancy sera on this system by the amount of tritiated thymidine taken up by activated lymphocytes. We found that: (1) pregnancy sera contain a factor inhibiting the mixed lymphocyte reaction; (2) the inhibition of the mixed lymphocyte reaction induced by sera could be reversed by the addition of the supernatant from allogeneic mixed lymphocyte reaction; (3) pure interleukin-1 could not reverse the inhibitory effect and (4) recombinant interleukin-2 (IL-2) completely reversed the inhibitory effect of pregnancy sera on the mixed lymphocyte reaction. We conclude that a factor (or factors) present in serum from pregnant women is capable of inhibiting the generation of IL-2 during lymphocyte activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRESEN R. H., MONROE C. W. Experimental study of the behavior of adult human skin homografts during pregnancy. A preliminary report. Am J Obstet Gynecol. 1962 Oct 15;84:1096–1103. doi: 10.1016/0002-9378(62)90560-4. [DOI] [PubMed] [Google Scholar]
- Campion P. D., Currey H. L. Cell-mediated immunity in pregnancy. Lancet. 1972 Oct 14;2(7781):830–830. doi: 10.1016/s0140-6736(72)92201-5. [DOI] [PubMed] [Google Scholar]
- Challis J. R., Thorburn G. D. Prenatal endocrine function and the initiation of parturition. Br Med Bull. 1975 Jan;31(1):57–61. doi: 10.1093/oxfordjournals.bmb.a071242. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Sanderson A. R., Temple A. Distribution of MHC antigens in human placental chorionic villi. Transplant Proc. 1977 Jun;9(2):1379–1384. [PubMed] [Google Scholar]
- Faulk W. P., Temple A. Distribution of beta2 microglobulin and HLA in chorionic villi of human placentae. Nature. 1976 Aug 26;262(5571):799–802. doi: 10.1038/262799a0. [DOI] [PubMed] [Google Scholar]
- Finn R., St Hill C. A., Govan A. J., Ralfs I. G., Gurney F. J., Denye V. Immunological responses in pregnancy and survival of fetal homograft. Br Med J. 1972 Jul 15;3(5819):150–152. doi: 10.1136/bmj.3.5819.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garewal G., Sehgal S., Aikat B. K., Gupta A. N. Cell-mediated immunity in pregnant patients with and without a previous history of spontaneous abortions. Br J Obstet Gynaecol. 1978 Mar;85(3):221–224. doi: 10.1111/j.1471-0528.1978.tb10486.x. [DOI] [PubMed] [Google Scholar]
- Hill C. A., Finn R., Denye V. Depression of cellular immunity in pregnancy due to a serum factor. Br Med J. 1973 Sep 8;3(5879):513–514. doi: 10.1136/bmj.3.5879.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. II. discriminating stimulation of lymphocyte subpopulations by phytomitogens and heterologous antilymphocyte sera. Clin Exp Immunol. 1972 Mar;10(3):525–536. [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. M., Hancock K. W. Maternal unresponsiveness to paternal histocompatibility antigens in human pregnancy. Transplantation. 1972 Jun;13(6):618–619. [PubMed] [Google Scholar]
- Jones E., Curzen P., Gaugas J. M. Suppressive activity of pregnancy plasma on the mixed lymphocyte reaction. J Obstet Gynaecol Br Commonw. 1973 Jul;80(7):603–607. doi: 10.1111/j.1471-0528.1973.tb16033.x. [DOI] [PubMed] [Google Scholar]
- Kasakura S. A factor in maternal plasma during pregnancy that suppresses the reactivity of mixed leukocyte cultures. J Immunol. 1971 Nov;107(5):1296–1301. [PubMed] [Google Scholar]
- Kasakura S. Is cortisol responsible for inhibition of MLC reactions by pregnancy plasma? Nature. 1973 Dec 21;246(5434):496–497. doi: 10.1038/246496a0. [DOI] [PubMed] [Google Scholar]
- Liggins G. C., Fairclough R. J., Grieves S. A., Kendall J. Z., Knox B. S. The mechanism of initiation of parturition in the ewe. Recent Prog Horm Res. 1973;29:111–159. doi: 10.1016/b978-0-12-571129-6.50007-5. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Josey W. E., Naib Z. M., Freeman M. G., Fernandez R. J., Wheeler J. H. Perinatal risk associated with maternal genital herpes simplex virus infection. Am J Obstet Gynecol. 1971 Jul 15;110(6):825–837. doi: 10.1016/0002-9378(71)90580-1. [DOI] [PubMed] [Google Scholar]
- Palacios R., Möller G. HLA-DR antigens render resting T cells sensitive to interleukin-2 and induce production of the growth factor in the autologous mixed lymphocyte reaction. Cell Immunol. 1981 Sep 1;63(1):143–153. doi: 10.1016/0008-8749(81)90035-6. [DOI] [PubMed] [Google Scholar]
- Palacios R. Role of individual chains of HLA-DR antigens in activation of T cells induced by alloantigens. Immunogenetics. 1981;14(3-4):309–322. doi: 10.1007/BF00342200. [DOI] [PubMed] [Google Scholar]
- Persson U., Hammarström L., Möller E., Möller G., Smith C. I. The role of adherent cells in B and T lymphocyte activation. Immunol Rev. 1978;40:78–101. doi: 10.1111/j.1600-065x.1978.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Petrucco O. M., Seamark R. F., Holmes K., Forbes I. J., Symons R. G. Changes in lymphocyte function during pregnancy. Br J Obstet Gynaecol. 1976 Mar;83(3):245–250. doi: 10.1111/j.1471-0528.1976.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Poskitt P. K., Kurt E. A., Paul B. B., Selvaraj R. J., Sbarra A. J., Mitchell G. W., Jr Response to mitogen during pregnancy and the postpartum period. Obstet Gynecol. 1977 Sep;50(3):319–323. [PubMed] [Google Scholar]
- Rocklin R. E., Kitzmiller J. L., Carpenter C. B., Garovoy M. R., David J. R. Maternal-fetal relation. Absence of an immunologic blocking factor from the serum of women with chronic abortions. N Engl J Med. 1976 Nov 25;295(22):1209–1213. doi: 10.1056/NEJM197611252952201. [DOI] [PubMed] [Google Scholar]
- SIEGEL M., GREENBERG M. Incidence of poliomyelitis in pregnancy; its relation to maternal age, parity and gestational period. N Engl J Med. 1955 Nov 17;253(20):841–847. doi: 10.1056/NEJM195511172532001. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimson W. H., Blackstock J. C. Identification of an immunosuppressive factor in pregnancy serum. Obstet Gynecol. 1976 Sep;48(3):305–311. [PubMed] [Google Scholar]
- Tomoda Y., Fuma M., Miwa T., Saiki N., Ishizuka N. Cell-mediated immunity in pregnant women. Gynecol Invest. 1976;7(5):280–292. doi: 10.1159/000301389. [DOI] [PubMed] [Google Scholar]


