Abstract
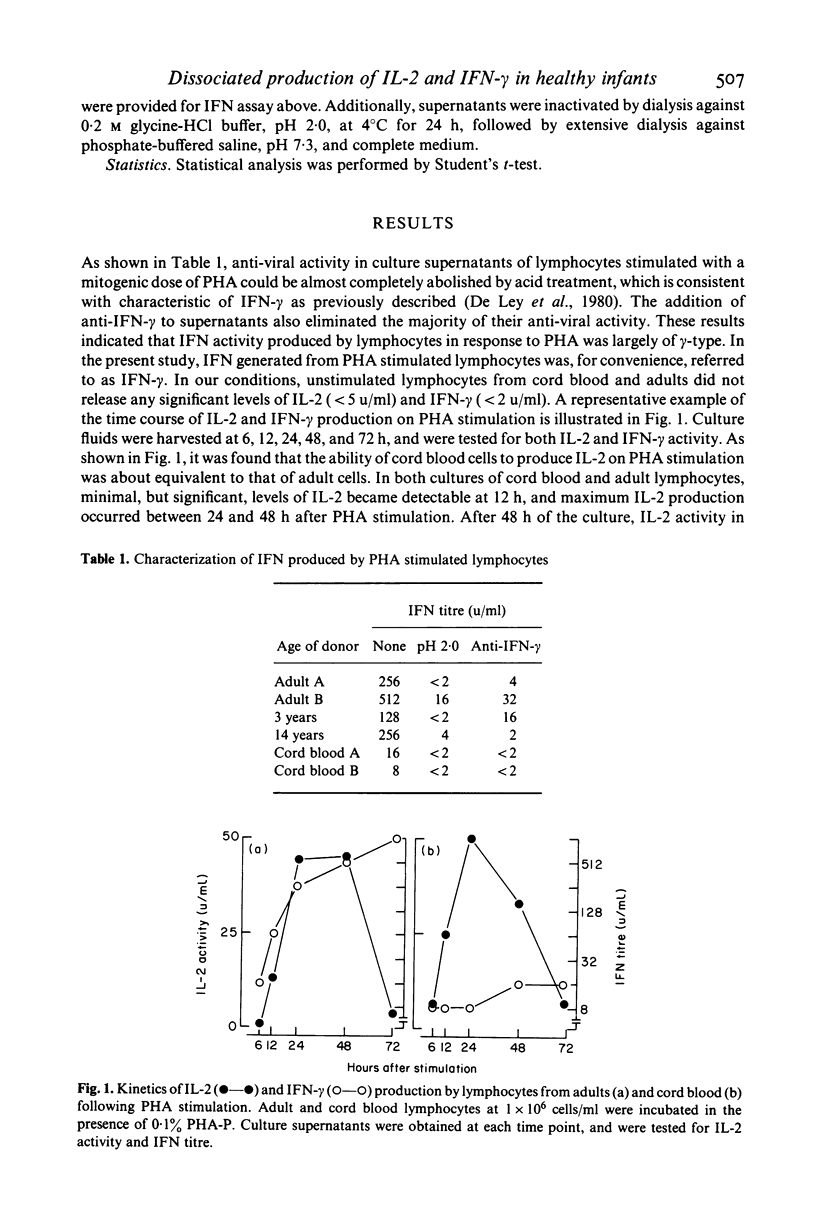
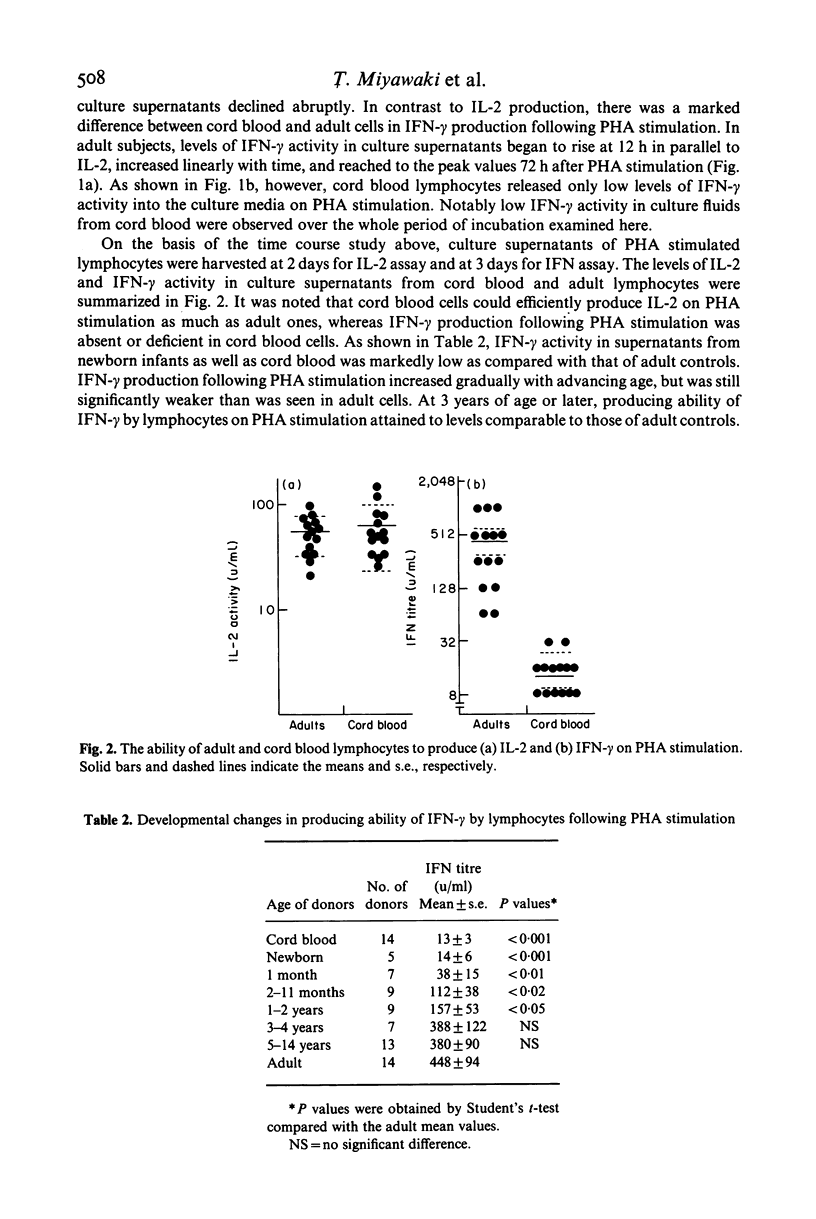
Cord blood lymphocytes were stimulated with phytohaemagglutinin (PHA) to produce interleukin-2 (IL-2) and immune interferon (IFN-gamma). On PHA stimulation, cord blood lymphocytes produced efficiently IL-2 as much as adult ones. Antiviral activity generated on PHA stimulation was shown to consist mainly of IFN-gamma as assessed by the sensitivity to pH 2.0 treatment and neutralization with anti-human IFN-gamma antibody. In contrast to IL-2 production, cord blood lymphocytes released extremely low levels of IFN-gamma following PHA stimulation. The producing ability of IFN-gamma by lymphocytes on PHA stimulation gradually increased with child growth, but was significantly low at 1-2 years of age as compared with adult controls. Around 3 years of age or later, the producing ability of IFN-gamma by lymphocytes on PHA stimulation attained levels comparable to those of adult cells. These results suggested that IL-2 producing ability of lymphocytes appeared to be at a mature stage at birth, whereas lymphocytes in the early human life might be relatively deficient in their ability to produce IFN-gamma.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Cooper M. D., Balch C. M. Postnatal expansion of the natural killer and keller cell population in humans identified by the monoclonal HNK-1 antibody. J Exp Med. 1982 Jan 1;155(1):321–326. doi: 10.1084/jem.155.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J., Rodríguez J., López-Botet M., Silva A., De Landazuri M. O. Removal of PHA from supernatants containing T-cell growth factor. J Immunol Methods. 1981;40(3):289–296. doi: 10.1016/0022-1759(81)90360-4. [DOI] [PubMed] [Google Scholar]
- Bryson Y. J., Winter H. S., Gard S. E., Fischer T. J., Stiehm E. R. Deficiency of immune interferon production by leukocytes of normal newborns. Cell Immunol. 1980 Sep 15;55(1):191–200. doi: 10.1016/0008-8749(80)90150-1. [DOI] [PubMed] [Google Scholar]
- Campbell A. C., Waller C., Wood J., Aynsley-Green A., Yu V. Lymphocyte subpopulations in the blood of newborn infants. Clin Exp Immunol. 1974 Dec;18(4):469–482. [PMC free article] [PubMed] [Google Scholar]
- Carr M. C., Stites D. P., Fudenberg H. H. Cellular immune aspects of the human fetal-maternal relationship. I. In vitro response of cord blood lymphocytes to phytohemagglutinin. Cell Immunol. 1972 Sep;5(1):21–29. doi: 10.1016/0008-8749(72)90080-9. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Testa D., Kung P. C., Perry L., Dreskin H. J., Goldstein G. Cellular origin and interactions involved in gamma-interferon production induced by OKt3 monoclonal antibody. J Immunol. 1982 Feb;128(2):585–589. [PubMed] [Google Scholar]
- Farrar J. J., Benjamin W. R., Hilfiker M. L., Howard M., Farrar W. L., Fuller-Farrar J. The biochemistry, biology, and role of interleukin 2 in the induction of cytotoxic T cell and antibody-forming B cell responses. Immunol Rev. 1982;63:129–166. doi: 10.1111/j.1600-065x.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Johnson H. M., Farrar J. J. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin 2. J Immunol. 1981 Mar;126(3):1120–1125. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hayward A. R., Kurnick J. Newborn T cell suppression: early appearance, maintenance in culture, and lack of growth factor suppression. J Immunol. 1981 Jan;126(1):50–53. [PubMed] [Google Scholar]
- Johnson H. M., Farrar W. L. The role of a gamma interferon-like lymphokine in the activation of T cells for expression of interleukin 2 receptors. Cell Immunol. 1983 Jan;75(1):154–159. doi: 10.1016/0008-8749(83)90314-3. [DOI] [PubMed] [Google Scholar]
- Kasahara T., Djeu J. Y., Dougherty S. F., Oppenheim J. J. Capacity of human large granular lymphocytes (LGL) to produce multiple lymphokines: interleukin 2, interferon, and colony stimulating factor. J Immunol. 1983 Nov;131(5):2379–2385. [PubMed] [Google Scholar]
- Kasahara T., Hooks J. J., Dougherty S. F., Oppenheim J. J. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983 Apr;130(4):1784–1789. [PubMed] [Google Scholar]
- Langford M. P., Weigent D. A., Georgiades J. A., Johnson H. M., Stanton G. J. Antibody to staphylococcal enterotoxin A-induced human immune interferon (IFN gamma). J Immunol. 1981 Apr;126(4):1620–1623. [PubMed] [Google Scholar]
- Linker-Israeli M., Bakke A. C., Kitridou R. C., Gendler S., Gillis S., Horwitz D. A. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE). J Immunol. 1983 Jun;130(6):2651–2655. [PubMed] [Google Scholar]
- López-Botet M., Fontán G., Garcia Rodriguez M. C., de Landázuri M. O. Relationship between IL 2 synthesis and the proliferative response to PHA in different primary immunodeficiencies. J Immunol. 1982 Feb;128(2):679–683. [PubMed] [Google Scholar]
- Miyawaki T., Seki H., Kubo M., Taniguchi N. Suppressor activity of T lymphocytes from infants assessed by co-culture with unfractionated adult lymphocytes in the pokeweed mitogen system. J Immunol. 1979 Sep;123(3):1092–1096. [PubMed] [Google Scholar]
- Miyawaki T., Yachie A., Ohzeki S., Nagaoki T., Taniguchi N. Cyclosporin A does not prevent expression of Tac antigen, a probable TCGF receptor molecule, on mitogen-stimulated human T cells. J Immunol. 1983 Jun;130(6):2737–2742. [PubMed] [Google Scholar]
- Nogueira N., Kaplan G., Levy E., Sarno E. N., Kushner P., Granelli-Piperno A., Vieira L., Colomer Gould V., Levis W., Steinman R. Defective gamma interferon production in leprosy. Reversal with antigen and interleukin 2. J Exp Med. 1983 Dec 1;158(6):2165–2170. doi: 10.1084/jem.158.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley J. A., Nussbaum-Blumenson A., Sheedy D., Grossmayer B. J., Ozer H. Identification of the T cell subset that produces human gamma interferon. J Immunol. 1982 Jun;128(6):2522–2526. [PubMed] [Google Scholar]
- Ratliff T. L., MacDermott R. P., Poepping N. J., Oakley D. M., Shapiro A., Catalona W. J. Production of gamma interferon by human T and null cells and its regulation by macrophages. Cell Immunol. 1982 Nov 15;74(1):111–119. doi: 10.1016/0008-8749(82)90011-9. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Gillis S., Baker P. E., McKenzie D., Ruscetti F. W. T-cell growth factor-mediated T-cell proliferation. Ann N Y Acad Sci. 1979;332:423–432. doi: 10.1111/j.1749-6632.1979.tb47136.x. [DOI] [PubMed] [Google Scholar]
- Stiehm E. R., Winter H. S., Bryson Y. J. Cellular (T cell) immunity in the human newborn. Pediatrics. 1979 Nov;64(5 Pt 2 Suppl):814–821. [PubMed] [Google Scholar]
- Torres B. A., Farrar W. L., Johnson H. M. Interleukin 2 regulates immune interferon (IFN gamma) production by normal and suppressor cell cultures. J Immunol. 1982 May;128(5):2217–2219. [PubMed] [Google Scholar]
- Virelizier J. L., Lenoir G., Griscelli C. Persistent Epstein-Barr virus infection in a child with hypergammaglobulinaemia and immunoblastic proliferation associated with a selective defect in immune interferon secretion. Lancet. 1978 Jul 29;2(8083):231–234. doi: 10.1016/s0140-6736(78)91744-0. [DOI] [PubMed] [Google Scholar]
- de Ley M., van Damme J., Claeys H., Weening H., Heine J. W., Billiau A., Vermylen C., de Somer P. Interferon induced in human leukocytes by mitogens: production, partial purification and characterization. Eur J Immunol. 1980 Nov;10(11):877–883. doi: 10.1002/eji.1830101113. [DOI] [PubMed] [Google Scholar]


