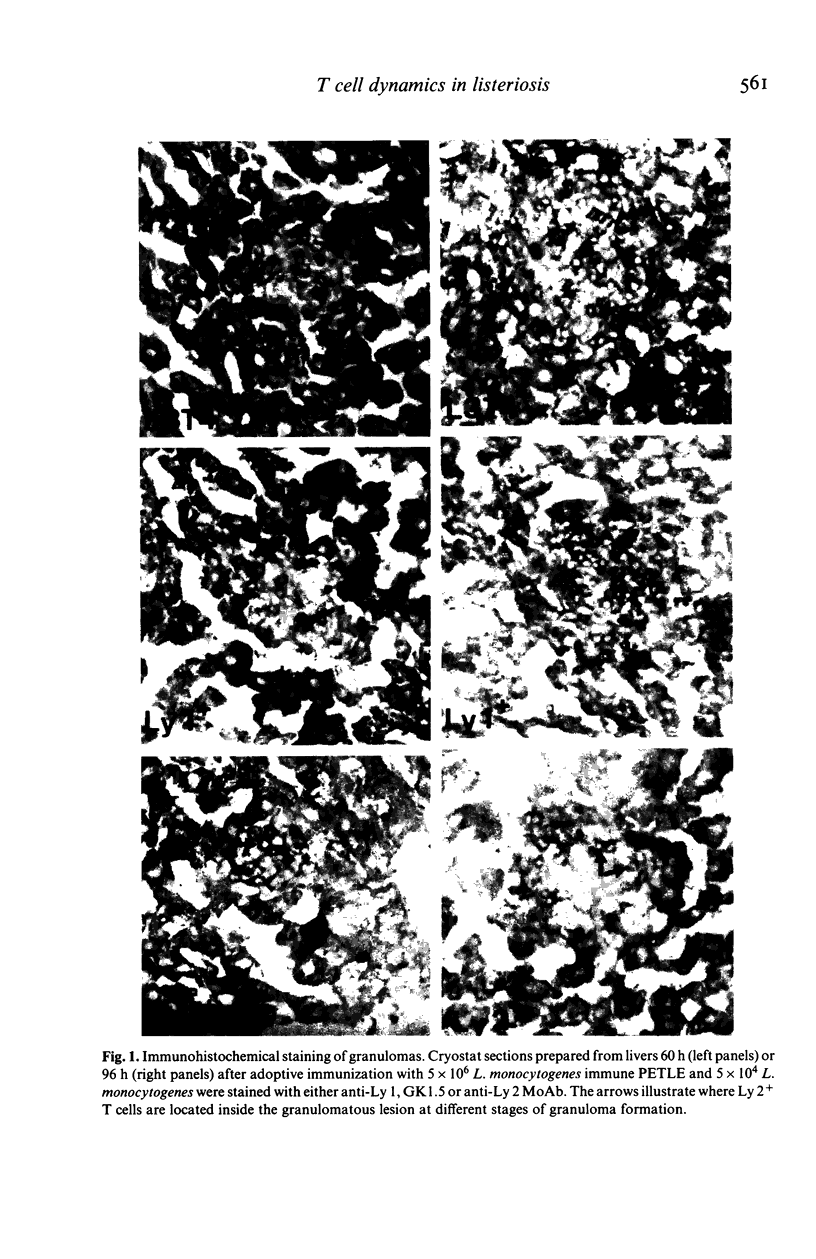
Abstract
Monoclonal antibodies anti-Ly 1, anti-Ly 2 and GK1.5 were applied to determine phenotypes of T cells within granulomas formed as a result of infection of mice with the facultative intracellular bacterium Listeria monocytogenes. Early in granuloma formation, equal numbers of Ly 1+, Ly 2+ and L3T4+ cells were found, T cells of different phenotypes being evenly distributed over the lesions. In mature granulomas, numbers of Ly 1+ and L3T4+ cells about doubled as compared to incipient granulomas, Ly 2+ cells, however, remained constant. Whereas Ly 1+ and L3T4+ cells within mature granulomas still were evenly distributed, Ly 2+ cells were predominantly localized in the periphery of the lesions. The data indicate that both, specific Ly 2+ and L3T4+ T cells, display characteristic dynamics within granulomas: Ly 2+ T cells which most likely mature from Ly 1+2+ T cells over time locate to the periphery. Concomitantly, L3T4+ T cells are enriched maintaining their distribution all over the lesions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dannenberg A. M., Jr, Meyer O. T., Esterly J. R., Kambara T. The local nature of immunity in tuberculosis, illustrated histochemically in dermal BCG lesions. J Immunol. 1968 May;100(5):931–941. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Farr A. G., Wechter W. J., Kiely J. M., Unanue E. R. Induction of cytocidal macrophages after in vitro interactions between Listeria-immune T cells and macrophages--role of H-2. J Immunol. 1979 Jun;122(6):2405–2412. [PubMed] [Google Scholar]
- Godal T., Myklestad B., Samuel D. R., Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971 Dec;9(6):821–831. [PMC free article] [PubMed] [Google Scholar]
- Haregewoin A., Godal T., Mustafa A. S., Belehu A., Yemaneberhan T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature. 1983 May 26;303(5915):342–344. doi: 10.1038/303342a0. [DOI] [PubMed] [Google Scholar]
- Heymer B., Hof H., Emmerling P., Finger H. Morphology and time course of experimental listeriosis in nude mice. Infect Immun. 1976 Sep;14(3):832–835. doi: 10.1128/iai.14.3.832-835.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Specific Lyt 123 cells are involved in protection against Listeria monocytogenes and in delayed-type hypersensitivity to listerial antigens. J Exp Med. 1979 Oct 1;150(4):1033–1038. doi: 10.1084/jem.150.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Gebhard J. F., Taylor C. R., Rea T. H. In situ characterization of T lymphocyte subsets in the reactional states of leprosy. Clin Exp Immunol. 1983 Jul;53(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- North R. J., Spitalny G. Inflammatory lymphocyte in cell-mediated antibacterial immunity: factors governing the accumulation of mediator T cells in peritoneal exudates. Infect Immun. 1974 Sep;10(3):489–498. doi: 10.1128/iai.10.3.489-498.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näher H., Sperling U., Hahn H. Developmental interrelationship of specific Lyt 123 and Lyt 1 cell sets in expression of antibacterial immunity to Listeria monocytogenes. Infect Immun. 1984 May;44(2):252–256. doi: 10.1128/iai.44.2.252-256.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näher H., Sperling U., Hahn H. H-2K-restricted granuloma formation by Ly-2+ T cells in antibacterial protection to facultative intracellular bacteria. J Immunol. 1985 Jan;134(1):569–572. [PubMed] [Google Scholar]
- Simon M. M., Edwards A. J., Hämmerling U., McKenzie I. F., Eichmann K., Simpson E. Generation of effector cells from T cell subsets. III. Synergy between Lyt-1 and Lyt-123/23 lymphocytes in the generation of H-2-restricted and alloreactive cytotoxic T cell. Eur J Immunol. 1981 Mar;11(3):246–250. doi: 10.1002/eji.1830110315. [DOI] [PubMed] [Google Scholar]
- Sperling U., Kaufmann S. H., Hahn H. Production of macrophage-activating and migration-inhibition factors in vitro by serologically selected and cloned Listeria monocytogenes-specific T cells of the Lyt 1+2- phenotype. Infect Immun. 1984 Oct;46(1):111–115. doi: 10.1128/iai.46.1.111-115.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Durant D. A., Potter J. W. Migration of human helper/inducer T cells in response to supernatants from Con A-stimulated suppressor/cytotoxic T cells. J Immunol. 1983 Aug;131(2):697–700. [PubMed] [Google Scholar]
- Van Voorhis W. C., Kaplan G., Sarno E. N., Horwitz M. A., Steinman R. M., Levis W. R., Nogueira N., Hair L. S., Gattass C. R., Arrick B. A. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T-cell phenotypes. N Engl J Med. 1982 Dec 23;307(26):1593–1597. doi: 10.1056/NEJM198212233072601. [DOI] [PubMed] [Google Scholar]