Abstract
Myasthenia gravis (MG) and experimental autoimmune MG (EAMG) are T cell-regulated, antibody-mediated autoimmune diseases. The major autoantigen in MG is the nicotinic acetylcholine receptor (AChR). Two peptides, representing sequences of the human AChR α-subunit, p195–212 and p259–271, were previously shown to be immunodominant T cell epitopes in MG patients as well as, respectively, in SJL and BALB/c mice. A dual analog (termed Lys-262–Ala-207) composed of the tandemly arranged two single amino acid analogs of p195–212 and p259–271 was shown to inhibit, in vitro and in vivo, MG-associated autoimmune responses. Furthermore, the dual analog could down-regulate myasthenogenic manifestations in mice with EAMG that was induced by inoculation of a pathogenic T cell line. In the present study, the ability of the dual analog to treat EAMG induced in susceptible C57BL/6 mice by native Torpedo AChR was evaluated. Mice that were diagnosed to have clinical symptoms of EAMG were treated with the dual analog by oral administration, 500 μg per mouse three times a week for 5–8 weeks. Treatment with the dual analog down-regulated the clinical manifestations of the ongoing disease as assessed by the clinical score, grip strength (measured by a grip strength meter), and electromyography. The effects on the clinical EAMG correlated with a reduced production of anti-AChR antibody as well as a decrease in the secretion of interleukin-2 and, more dramatically, interferon-γ, in response to AChR triggering. Thus, the dual analog is an efficient immunomodulator of EAMG in mice and might be of specific therapeutic potential for MG.
Myasthenia gravis (MG) and its experimental animal model, experimental autoimmune MG (EAMG), are immune disorders characterized by circulating antibodies and lymphocyte autoreactivity to the nicotinic acetylcholine receptor (AChR) leading to a reduced number of AChR molecules at the postsynaptic end plates. Weakness and fatigability of voluntary muscles characterize both MG and EAMG (1). All species of animals tested, including rabbits (2), rats (3), mice (4), and monkeys (5), have been found to be susceptible to EAMG.
Although the symptoms of MG are due to autoantibodies produced by B cells, there is ample evidence to incriminate T cells in the disease process as well (6–8). Furthermore, in both humans and rodents, increased susceptibility to MG is seen with certain major histocompatibility complex haplotypes (9, 10).
Because the α-subunit of the AChR was shown to be predominant for T cell epitopes (11), we have used peptides representing different sequences of the human AChR α-subunit. Two sequences of the latter, namely peptides p195–212 and p257–269, discriminated significantly between MG patients and healthy controls based on proliferation of peripheral blood lymphocytes and binding to sera antibodies (12). Furthermore, peripheral blood lymphocytes of seronegative MG patients responded by either proliferation or interleukin-2 (IL-2) secretion to p195–212 and p259–271 (which elicits immune responses identical to those triggered by p257–269), emphasizing the importance of AChR-specific T cells in MG (13). Peptides p195–212 and p259–271 were further shown to be immunodominant T cell epitopes in SJL and BALB/c mice, respectively (14). T cell lines specific to p195–212 and p259–271 that were inoculated into SJL and BALB/c mice, respectively, were found to be pathogenic and to induce EAMG manifested by the production of antibodies to self (murine) AChR and by decremental compound muscle action potentials consistent with impairment of neuromuscular transmission (15).
Ideally the goal therapy in MG should be the elimination of autoimmune responses to the AChR specifically, without interfering with immune responses to other antigens. To this end, a dual analog composed of the tandemly, reciprocally arranged two single analogs of p195–212 and p259–271 (16–18), namely Lys-262–Ala-207, was prepared and shown to inhibit efficiently the proliferation of T cell lines specific to the myasthenogenic peptides and of lymph node cells that were primed in vivo to either of the myasthenogenic peptides (19). Furthermore, the dual analog inhibited specifically in vitro T cell stimulation to either myasthenogenic peptide in >90% of the responding MG patients (20).
We have previously demonstrated that oral administration of the dual analog to BALB/c mice in which EAMG was induced by the pathogenic p259–271-specific T cell line reversed EAMG manifestations in the treated mice (19). Because C57BL/6 mice are the mouse strain most susceptible to EAMG induced by Torpedo AChR, we addressed the question of whether the dual analog Lys-262–Ala-207 (administered orally) could treat EAMG induced in these mice by immunization with the multideterminant native Torpedo AChR. We show in this study that oral administration of the dual analog to C57BL/6 mice that were diagnosed to have EAMG had beneficial effects on the clinical manifestations characterizing EAMG.
Materials and Methods
Mice.
Female mice of the inbred strain C57BL/6 (The Jackson Laboratory) were used at the age of 8–12 wk.
Torpedo AChR.
AChR was purified from Torpedo marmorata as described (21) and was used for immunizations and in vitro studies.
Synthetic Peptides and Peptide Analogs.
Peptides p195–212 (DTPYLDITYHFVMQRLPL) and p259–271 (VIVELIPSTSSAV), which correspond to sequences of the human AChR α-subunit, were synthesized and characterized as described (19). The dual analog Lys-262–Ala-207 (VIVKLIPSTSSAVDTPYLDITYHFFFVAQRLPL) was designed as described (19) and synthesized (97% purity) by UCB-Bioproducts.
Induction of EAMG.
C57BL/6 mice were immunized and boosted 1 mo later with 20 μg per mouse Torpedo AChR in complete Freund's adjuvant (CFA) enriched with 10 mg/ml Mycobacterium tuberculosis H37 Ra (Difco). A second group was injected with an emulsion of CFA/PBS. The mice were followed for serological and clinical manifestations.
Evaluation of EAMG.
Clinical score.
The mice were tested once a week for signs of clinical muscle weakness (clinical score). The disease symptoms were graded as follows: 0, no definite muscle weakness after exercise; 1, moderate muscle weakness after exercise consisting of 10 consecutive paw grips; 2, grade 1 weakness at rest; and 3, severe muscle weakness, paralysis, dehydration and moribund.
Evaluation of grip strength.
Muscle strength of the experimental mice was also determined by the use of an automated grip strength meter (22). Grip strength was determined following “exercising” the animals by subjecting them to 10 consecutive paw grips. During the instrumental measurement sessions, mice were placed on a platform, allowed to grasp a rectangular ring, and then steadily pulled away until the grip was broken. Grip strength was determined with a computerized electronic pull strain gauge that was fitted directly to the grasping ring. Individual animals were exposed to five successive repeated and recorded measurements.
Electrophysiological evaluations.
Myasthenic decrements were measured and calculated as previously described (19). Recordings were obtained from at least four locations within the muscle and were considered as positive only if a decremental response of 10% or more was obtained in at least two different recording sites within the muscle (23). Electromyographies were performed with the examiner blinded to whether mice belonged to control or experimental groups.
Detection of Antibodies to the Myasthenogenic Peptides (p195–212 and p259–271) and to the Torpedo AChR.
Experimental mice were bled periodically for detection of anti-Torpedo AChR, p195–212, and p259–271 antibodies in their sera by using an ELISA. In brief, Maxisorb plates (Nunc) were coated with the different antigens (15 μg/ml) diluted in 0.1 M NaHCO3. After incubation with different dilutions of sera, goat anti-mouse IgG Fc coupled to horseradish peroxidase (Jackson ImmunoResearch) was added to the plates and was followed by the addition of the peroxidase substrate 2,2′-azinobis(3-ethylbenzthioline)-6-sulfonate (Sigma). Results were determined using a MRX ELISA reader (Dynatech) and a 410-nm filter.
Treatment of Mice with the Dual Analog.
Mice that showed clinical manifestations of EAMG were randomly divided into two groups. One group was given 500 μg per mouse of the dual analog, by oral administration (total volume 300 μl, three times a week), using a ball-type feeding needle. The second group was given the vehicle PBS. The mice were treated for a period of 5–8 wk.
Production and Detection of Cytokines.
Splenocytes (5 × 106 per ml) of the tested mice were stimulated with the Torpedo AChR (10 μg/ml) for a period of 48 hr. The supernatants were collected and analyzed for cytokine content by ELISA, using the relevant standard capture and detecting antibodies (PharMingen).
Statistical Analysis.
ANOVA, χ2, and Student t test were used for statistical analyses of the data.
Results
Antibodies Specific to the Myasthenogenic Peptides in Sera of C57BL/6 Mice Immunized with Torpedo AChR.
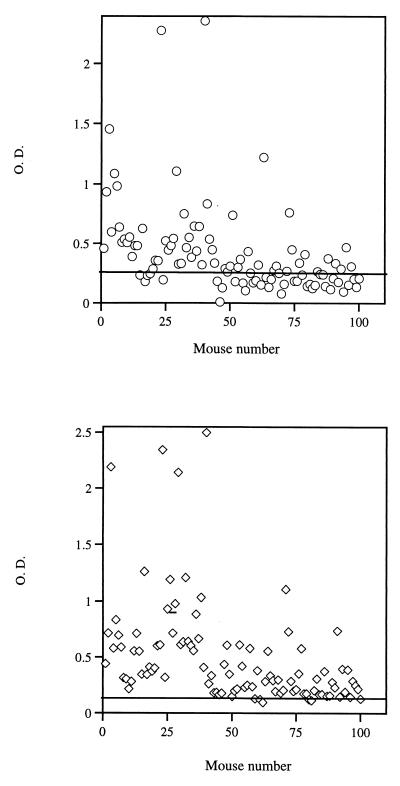
All of the C57BL/6 mice that were immunized with the Torpedo AChR produced high titers of anti-receptor antibodies (data not shown). Because the dual analog used to treat mice with EAMG was synthesized based on the human AChR sequence of p195–212 and p259–271, it was important to find out whether injection of C57BL/6 mice with whole Torpedo AChR leads to production of antibodies directed against the above peptides. Fig. 1 demonstrates the results of 100 sera of Torpedo AChR immunized C57BL/6 mice, tested for the content of antibodies to p195–212 and p259–271. As can be seen, most mice developed antibodies that reacted with the peptides. Thus 66% and 94% of the sera reacted with p195–212 and p259–271, respectively.
Figure 1.
Antibodies specific to p195–212 and p259–271 in sera of C57BL/6 mice immunized with Torpedo AChR. C57BL/6 mice were primed and boosted with Torpedo AChR α-subunit (20 μg per mouse in CFA). Mice were bled 10 days after boost, and their sera were assayed for the presence of antibodies to p195–212 (Upper) or to p259–212 (Lower). OD levels are of sera diluted 1:10. Horizontal lines indicate mean OD in sera of control mice that were injected with PBS/CFA + 2 SD.
Effect of the Dual Analog on EAMG Induced by the Torpedo AChR in C57BL/6.
It was important to find out whether the dual analog is capable of immunomodulating an already existing EAMG induced by the native AChR. To this end, C57BL/6 mice were primed and boosted with the Torpedo AChR in CFA. Approximately 40% of the C57BL/6 mice injected with the Torpedo AChR developed a disease that could be diagnosed by clinical score, electrophysiological evaluation, and grip strength as measured with a grip strength meter. Approximately 30% of the mice that developed EAMG died immediately before starting treatment with the dual analog. The mice diagnosed to have EAMG were divided randomly into two groups and were treated three times per week either with 500 μg per mouse of the analog given orally in PBS (experimental group) or with PBS only (control group). Mice were treated for 5–8 wk. Because a certain percentage of the PBS-treated mice underwent spontaneous remission, most significant differences between groups could be demonstrated after 4–5 wk of treatment. Table 1 summarizes results of three treatment experiments. As can be seen, out of a total of 31 treated mice, 23 (74%) did not show disease manifestations after treatment, 7 of the treated mice (22.6%) remained sick, and 1 mouse (3%) died. These results are significantly different (P = 0.0094) from those obtained with mice treated with PBS only (35% spontaneous remission, 40% sick, and 25% dead; Table 1).
Table 1.
Therapeutic effects of oral administration of the dual analog on an established clinical EAMG in mice
| Treatment | No. of mice
|
|||
|---|---|---|---|---|
| Total | Remission or improvement | Sick | Dead | |
| Dual analog | 31 | 23 (74%) | 7 (23%) | 1 (3%) |
| PBS | 20 | 7 (35%) | 8 (40%) | 5 (25%) |
C57BL/6 mice were immunized and boosted with Torpedo AChR in CFA (see Materials and Methods). After the mice developed clinical EAMG, they were randomly divided into two groups. One group was treated with the dual analog (Lys-262–Ala-207, 500 μg per mouse administered orally in PBS, three times per week), and the second group was treated with PBS. Disease manifestations were determined according to disease score, grip strength measurements, and electromyography (see Materials and Methods). The above results are of three alike experiments. χ2 was used to analyze differences between the dual analog- and PBS-treated groups for all categories (P = 0.0094).
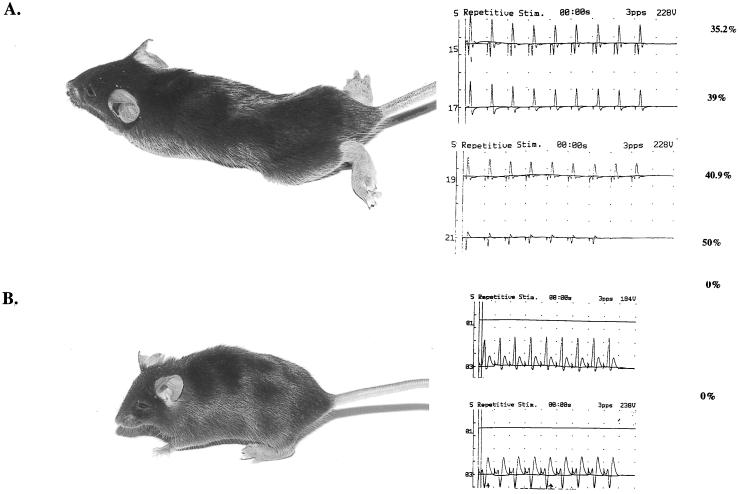
Fig. 2 is a representative picture of a C57BL/6 mouse diagnosed with EAMG before (A) and after (B) treatment with the dual analog. The mouse was given a clinical score of 3 before treatment, whereas after treatment its clinical score was 0. The picture shows also that, in agreement with the clinical symptoms, the significant myasthenic decrement determined for this mouse before treatment could not be detected after treatment with the dual analog.
Figure 2.
Clinical status of a C57BL/6 mouse with EAMG before and after treatment with the dual analog. (A) A representative C57BL/6 mouse after immunization and boost with Torpedo AChR α-subunit. The myasthenic decrements determined at that time by electromyography (see Materials and Methods) are shown. (B) The same mouse after 6 wk of treatment with the dual analog administered orally. No myasthenic decrement could be detected by electromyography.
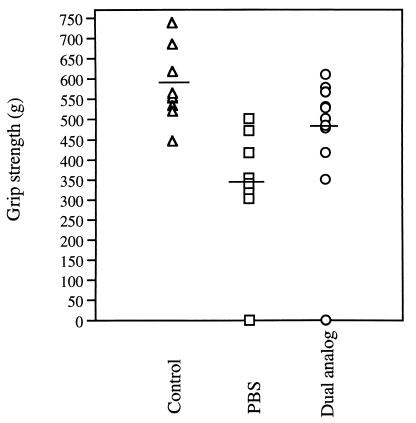
Fig. 3 shows the results of a representative experiment in which the muscle strength was measured with an automated grip strength meter to quantitate muscle weakness. The figure shows the maximal grip strength (out of five measurements) determined for each mouse. It can be seen that two mice, one of the dual analog-treated group and one of the PBS-treated group, were too weak to be evaluated in this test. The comparison of the rest of the mice indicated a significant difference between the mean maximal strength in the dual analog-treated group and that in the PBS-treated group (P < 0.01). The clinical scores matched the results obtained with the grip strength meter. Thus, in the dual analog-treated group only 1 of 12 animals was still sick after 4 wk of treatment, exhibiting a score of 2–3. In the PBS-treated group, on the other hand, 5 of 9 mice had clinical symptoms (P < 0.05). Thus, the above results suggest that treatment with the dual analog has significant beneficial effects on the clinical EAMG.
Figure 3.
Effect of treatment with the dual analog on the grip strength of mice. Individual mice of three groups: (i) Control mice injected with PBS/CFA, (ii) mice with EAMG manifestations that were treated with PBS, and (iii) mice with EAMG that were treated with the dual analog given orally in PBS, were subjected to evaluation using a grip strength meter (see Materials and Methods). Results are of mice tested after 4 wk of treatment. Results are expressed as maximum grip strength of five successive repeated and recorded measurements. Dashes indicate the mean maximal grip strength per group. The group treated with the dual analog differs significantly (P < 0.01) from the PBS-treated group.
Effect of Treatment with the Dual Analog on Immunological Parameters.
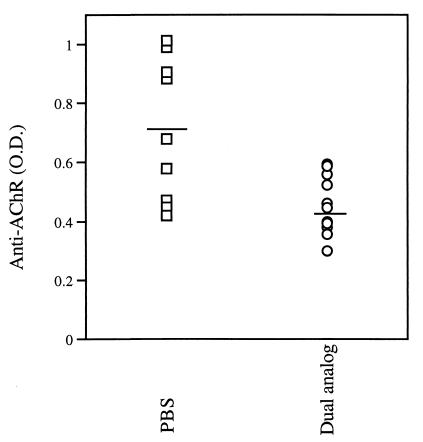
It was of interest to find out whether the clinical improvement after treatment with the dual analog was accompanied by changes in immunological parameters related to EAMG. We first measured the levels of anti-AChR antibodies in the sera of mice. Fig. 4 demonstrates the results of one representative experiment. As can be seen, the treatment led to a significant decrease in the anti-AChR antibody levels (P = 0.005).
Figure 4.
Treatment of EAMG in C57BL/6 mice reduces levels of antibodies specific to AChR. Anti-AChR antibody levels were measured by ELISA. Shown are results obtained with sera of individual mice (dilution 1:107) bled at the end of 4 wk of treatment with the dual analog. Dashes indicate mean anti-AChR antibody titers. Antibody titers of the dual analog-treated group are significantly lower (P = 0.005) than those of mice in the PBS-treated group.
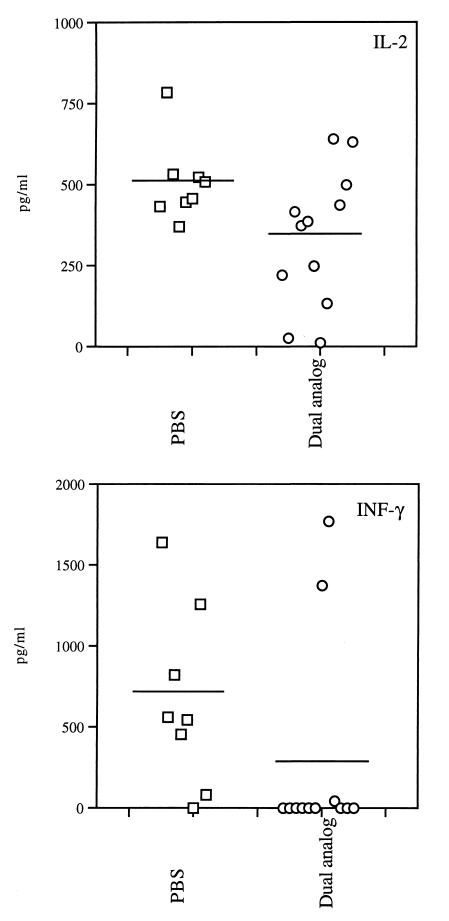
Because cytokines play an important role in MG and EAMG, the possible effect of treatment on the secreted cytokine pattern was tested. Mice were killed at the end of treatment and their spleen cells were stimulated with Torpedo AChR. Supernatants collected 48 hr later were tested for the presence of interferon-γ (IFN-γ), IL-2, IL-4, IL-10, and transforming growth factor β (TGFβ). Fig. 5 demonstrates results of IL-2 and IFN-γ measurements of a representative experiment. A moderate but significant (P = 0.027) reduction in the secretion of IL-2 by splenocytes of the dual analog-treated mice could be observed, as compared with the group of mice that was treated with PBS only. A dramatic difference between the groups could be observed when levels of IFN-γ were measured. Whereas splenocytes of most mice of the PBS-treated group secreted high levels of IFN-γ after their stimulation with Torpedo AChR, only 2 of the 12 tested mice in the dual analog-treated group produced significant levels of IFN-γ (Fig. 5). The latter cytokine could not be detected in supernatants of triggered splenocytes of the 10 additional mice in this group (P = 0.01). No significant differences between dual analog and PBS-treated groups could be detected in the levels of secreted IL-4, IL-10, or TGFβ.
Figure 5.
Effect of treatment with the dual analog on the secretion of Th1-type cytokines. Cytokines were determined by ELISA in supernatants of splenocytes of individual mice after 48 hr of stimulation with Torpedo AChR. Results are of mice killed after 6 wk of treatment with either PBS (control group) or the dual analog (experimental group). (Upper) Levels of IL-2 secretion. (Lower) Results of measurements of IFN-γ secretion. Dashes indicate mean concentration of cytokine per group. Significant lower levels were detected for both IL-2 (P = 0.027) and IFN-γ (P = 0.01) in the dual analog-treated group as compared with the PBS-treated group.
Discussion
The main findings of the present research are that a peptide in which two single amino acid substituted analogs of two myasthenogenic peptides were combined, is capable of immunomodulating an already established EAMG induced by the multideterminant Torpedo AChR. Furthermore, the amelioration of the clinical disease correlates with a reduction in the titers of AchR-specific antibodies and with a decrease in the levels of IL-2 and especially IFN-γ. The latter cytokine is known to play a pathogenic role in MG and EAMG (24, 25).
We have previously shown that the dual analog is capable of inhibiting in vitro and in vivo MG-associated autoimmune responses (19). Moreover, the dual analog could ameliorate manifestations of EAMG induced in BALB/c mice by a pathogenic T cell line specific to the myasthenogenic peptide p259–271 (19). The demonstration in the present report that the dual analog down-regulated disease symptoms induced in C57BL/6 mice by the Torpedo AChR indicates that the effects of the dual analog are not restricted to the strains that are high responders to the myasthenogenic peptides (e.g., BALB/c and SJL mice). Furthermore, the results suggest that the dual analog does not affect responses to the two myasthenogenic T cell epitopes only, but it immunomodulates responses to other determinants within the AChR molecule, probably via epitope spreading. The results of this study also support the idea that the two myasthenogenic peptides are highly immunogenic epitopes of the AChR because most C57BL/6 mice that were immunized with the Torpedo AChR produced antibodies that reacted with either p195–212 or p259–271, or with both (Fig. 1).
The C57BL/6 model of EAMG induced by immunization with Torpedo AChR is a complicated one because of the relatively low incidence of mice with clinical disease (<50%), the acute nature of the disease, and the spontaneous remission that occurs in some of the mice. The fact that under the latter circumstances the dual analog could be shown to have significant beneficial effects on the clinical disease emphasizes its efficacy in down-regulating EAMG. The effect of the dual analog could be shown by the clinical score as well as by objective measurements such as grip strength measured by an automated grip strength meter and electromyography evaluation that measures myasthenic decrements.
The improvement in the clinical symptoms in the dual analog-treated C57BL/6 mice correlated with a significant reduction in the total AchR-specific antibodies. It should be noted that, in spite of the reduction in the anti-AChR antibody response, the titers of the latter were still high. Nevertheless, it has been demonstrated that the severity of EAMG as well as MG does not correlate with the levels of anti-AChR antibodies (11). A significant reduction also could be determined in the secretion of the T helper 1 (Th1)-type cytokines, namely IL-2 and IFN-γ, in splenocytes of the dual analog-treated mice. The observed suppression in IFN-γ production in the dual analog-treated group was very impressive (Fig. 5). Indeed, the pathogenic role of IFN-γ in EAMG has been demonstrated (26, 27), and mice with IFN-γ receptor deficiency were shown to be less susceptible to EAMG (22). In the present study, we did not detect significant differences in levels of IL-4, IL-10, or TGFβ secreted by splenocytes of either the dual analog-treated or the PBS-treated groups. It is noteworthy that in short-term experiments oral administration of the dual analog into mice immunized with either of the myasthenogenic peptides resulted in down-regulation of the Th1-type cytokines, a mild increase in the secretion of Th2-type cytokines, and a significant elevation in the secretion of the immunosuppressive cytokine TGFβ (M.P.-R., A. Faber-Elmann, M.S., and E.M., unpublished results). It is possible that changes in the secretion of the latter cytokines are of a short-term nature, and therefore they could not be detected at the end of treatment experiments that lasted 6–8 wk. Furthermore, the fact that some of the PBS-treated mice underwent spontaneous remission by the time that the mice were killed might have affected the observed differences between the groups. A shift of Th1- to Th2-type cytokines has been reported in some studies in which antigens or peptides were given in tolerogenic doses, whereas in other studies no change in the Th2-type cytokines could be determined (26, 28). Induction of tolerance to Torpedo AChR by either oral or nasal administration into mice or rats was reported to result in elevated production of TGFβ (25, 26).
The current therapy of MG involves mainly the use of immunosuppressive drugs that cause a global suppression of the immune system and various side effects. Therefore, an antigen-specific down-regulation of the autoimmune reactions has been the main goal of multiple studies. Because in the case of MG the autoantigen is known to be the AChR, a number of studies attempted the immunomodulation of EAMG by oral or nasal administration of the entire Torpedo AChR molecule (26, 29, 30). However, because the AChR is highly immunogenic, frequent administrations of the latter might lead to an immune response rather than tolerance. Therefore, short peptides that represent T cell epitopes of the AChR and especially altered T cell epitopes with less immunogenic potential than the native protein, as is the case of the dual analog, might provide the therapy means of choice for the disease. Injection of peptides that are dominant T cell epitopes of the Torpedo AChR either before the immunization with the Torpedo AChR or after priming suppressed disease manifestations (28, 31). However at least one of these studies reported lack of ability of the peptides to treat an ongoing disease (28). In the present study, we demonstrate that the dual analog is capable of immunomodulating efficiently an already established EAMG in C57BL/6 mice.
Mucosal administration of autoantigens has been reported to shut down autoaggressive immune responses (32). Oral and nasal antigen administration have been the main routes used to silence autoreactive T cells (25). Oral immunomodulation of disease manifestations involves multiple mechanisms, including deletion and anergy of antigen-specific T cells after the administration of high antigen dose and induction of regulatory Th2- and Th3-type cells after low dose antigen administration. The exact mechanism by which the dual analog reversed EAMG manifestations has not been completely elucidated yet. Nevertheless, the beneficial effects of the dual analog are associated with a reduction in the production of the pathogenic cytokine in MG, namely, IFN-γ (22, 24–27). Because the dual analog is based on two, single amino acid-substituted peptides of two T cell epitopes of the human AChR, it is likely to down-regulate autoimmune responses to the human AChR at least as efficiently as demonstrated for the murine EAMG. Thus, the orally administered dual analog is a potential candidate for the treatment of human MG.
Acknowledgments
The authors thank Ms. Edna Schechtman of the Computing Center, Weizmann Institute of Science, for statistical evaluations. This research was supported by Teva Pharmaceutical Industries, Netanya, Israel (M.S. and E.M.). Y.P. is supported by a postdoctoral fellowship of the Human Frontier Science Program Organization. J.P.C. thanks the Association Française contre les Myopathies and the College de France for support.
Abbreviations
- AChR
acetylcholine receptor
- MG
myasthenia gravis
- EAMG
experimental autoimmune MG
- CFA
complete Freund's adjuvant
- TGFβ
transforming growth factor β
- IL
interleukin
- Th
T helper
- IFN-γ
interferon-γ
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040554597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040554597
References
- 1.Drachman D B. N Engl J Med. 1994;330:1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 2.Patrick J, Lindstrom J. Science. 1973;180:871–872. doi: 10.1126/science.180.4088.871. [DOI] [PubMed] [Google Scholar]
- 3.Lindstrom J, Einarson B, Lennon V A, Seybold M E. J Exp Med. 1976;144:726–738. doi: 10.1084/jem.144.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christadoss P, Talal N, Lindstrom J, Fernandez G. Cell Immunol. 1984;88:1–8. doi: 10.1016/0008-8749(84)90046-7. [DOI] [PubMed] [Google Scholar]
- 5.Tarrab-Hazdai R, Aharonov A, Silverman I, Fuchs S, Abramsky O. Nature (London) 1975;256:128–130. doi: 10.1038/256128a0. [DOI] [PubMed] [Google Scholar]
- 6.Tournier-Lasserve E, Bach J F. Neuroimmunology. 1993;47:103–114. doi: 10.1016/0165-5728(93)90020-y. [DOI] [PubMed] [Google Scholar]
- 7.Melms A, Shalke B C G, Kirchner T, Mueller-Hermelink H K, Albert E, Wekerle H. J Clin Invest. 1988;81:902–908. doi: 10.1172/JCI113401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manfredi A A, Protti M P, Bellone M, Moiola L, Conti-Tronconi B M. J Lab Clin Med. 1992;120:13–21. [PubMed] [Google Scholar]
- 9.Bell J, Rassenti L, Smoot S, Smith K, Newby C, Hohlfeld R, Toyka K, McDevitt H, Steinman L. Lancet. 1986;i:1058–1060. doi: 10.1016/s0140-6736(86)91330-9. [DOI] [PubMed] [Google Scholar]
- 10.Christadoss P, Lindstrom J M, Melvold R W, Talal N. Immunogenetics. 1985;21:33–38. doi: 10.1007/BF00372239. [DOI] [PubMed] [Google Scholar]
- 11.Lindstrom J, Shelton D, Fuji Y. Adv Immunol. 1988;42:233–284. doi: 10.1016/s0065-2776(08)60847-0. [DOI] [PubMed] [Google Scholar]
- 12.Brocke S, Brautbar L, Steinman L, Abramsky O, Rothbard J, Neumann D, Fuchs S, Mozes E. J Clin Invest. 1988;82:1894–1900. doi: 10.1172/JCI113807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karni A, Zisman E, Katz-Levy Y, Paas-Rozner M, Dayan M, Brautbar C, Abramsky O, Sela M, Mozes E. Neurology. 1997;48:1638–1642. doi: 10.1212/wnl.48.6.1638. [DOI] [PubMed] [Google Scholar]
- 14.Brocke S, Dayan M, Rothbard J, Fuchs S, Mozes E. Immunology. 1990;69:495–500. [PMC free article] [PubMed] [Google Scholar]
- 15.Kirshner S, Katz-Levy Y, Wirguin I, Argov Z, Mozes E. Cell Immunol. 1994;157:11–28. doi: 10.1006/cimm.1994.1201. [DOI] [PubMed] [Google Scholar]
- 16.Katz-Levy Y, Kirshner S L, Sela M, Mozes E. Proc Natl Acad Sci USA. 1993;90:7000–7004. doi: 10.1073/pnas.90.15.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz-Levy Y, Dayan M, Wirguin I, Fridkin M, Sela M, Mozes E. J Neuroimmunol. 1998;85:78–86. doi: 10.1016/s0165-5728(97)00265-8. [DOI] [PubMed] [Google Scholar]
- 18.Kirshner S L, Zisman E, Fridkin M, Sela M, Mozes E. Scand J Immunol. 1996;44:512–521. doi: 10.1046/j.1365-3083.1996.d01-330.x. [DOI] [PubMed] [Google Scholar]
- 19.Katz-Levy Y, Pass-Rozner M, Kirshner S, Dayan M, Zisman E, Fridkin M, Wirguin I, Sela M, Mozes E. Proc Natl Acad Sci USA. 1997;94:3200–3205. doi: 10.1073/pnas.94.7.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zisman E, Katz-Levy Y, Dayan M, Kirshner S L, Paas-Rozner M, Karni A, Abramsky O, Brautbar C, Fridkin M, Sela M, Mozes E. Proc Natl Acad Sci USA. 1996;93:4492–4497. doi: 10.1073/pnas.93.9.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saoudi A, Bernard I, Hoedemaekers A, Cautain B, Martinez K, Druet P, De Baets M, Guery J C. J Immunol. 1999;162:7189–7197. [PubMed] [Google Scholar]
- 22.Zhang G-X, Xiao B-G, Bai X-F, van der Meide P H, Orn A, Link H. J Immunol. 1999;162:3775–3781. [PubMed] [Google Scholar]
- 23.Berman P W, Patrick J. J Exp Med. 1980;151:204–210. doi: 10.1084/jem.151.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mokhtarian F, Shirazian D, Grob D. Ann NY Acad Sci. 1993;681:315–322. doi: 10.1111/j.1749-6632.1993.tb22905.x. [DOI] [PubMed] [Google Scholar]
- 25.Xiao B-G, Link H. Clin Immunol Immunopathol. 1997;85:119–128. doi: 10.1006/clin.1997.4432. [DOI] [PubMed] [Google Scholar]
- 26.Shi F-D, Li H, Wang H, Bai X, van der Meide P H, Link H, Ljunggren H-G. J Immunol. 1999;162:5757–5763. [PubMed] [Google Scholar]
- 27.Balasa B, Deng C, Lee J, Bradley L M, Dalton D K, Christadoss P, Sarvetnick N. J Exp Med. 1997;186:385–391. doi: 10.1084/jem.186.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karachunski P I, Ostlie N S, Okita D K, Garman R, Conti-Fine B M. J Neuroimmunol. 1999;93:108–121. doi: 10.1016/s0165-5728(98)00208-2. [DOI] [PubMed] [Google Scholar]
- 29.Okumura S, McIntosh K, Drachman D B. Ann Neurol. 1994;36:704–713. doi: 10.1002/ana.410360504. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z-Y, Qiao J, Link H. J Neuroimmunol. 1993;44:209–214. doi: 10.1016/0165-5728(93)90045-z. [DOI] [PubMed] [Google Scholar]
- 31.Wu B, Deng C, Goluszko E, Christadoss P. J Immunol. 1997;159:3016–3023. [PubMed] [Google Scholar]
- 32.Weiner H L. Immunol Today. 1997;18:335–343. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]