Abstract
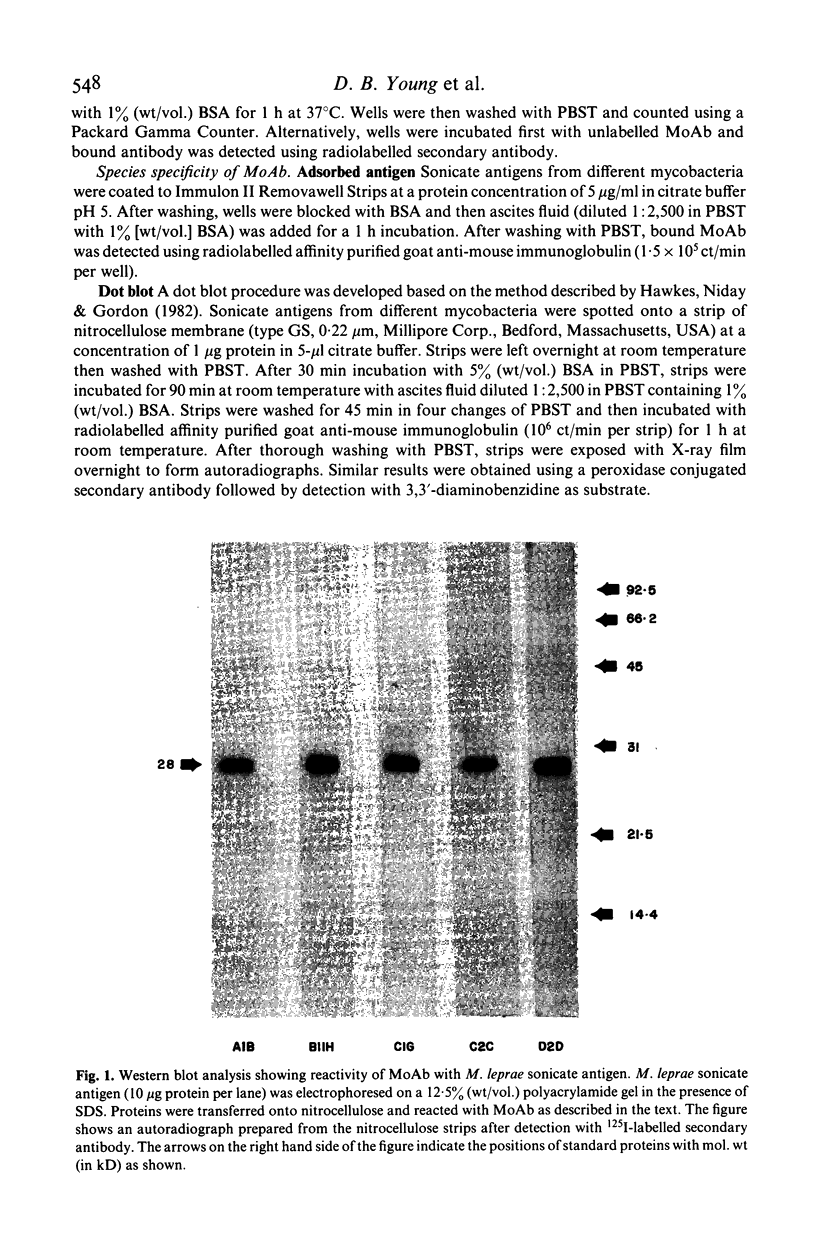
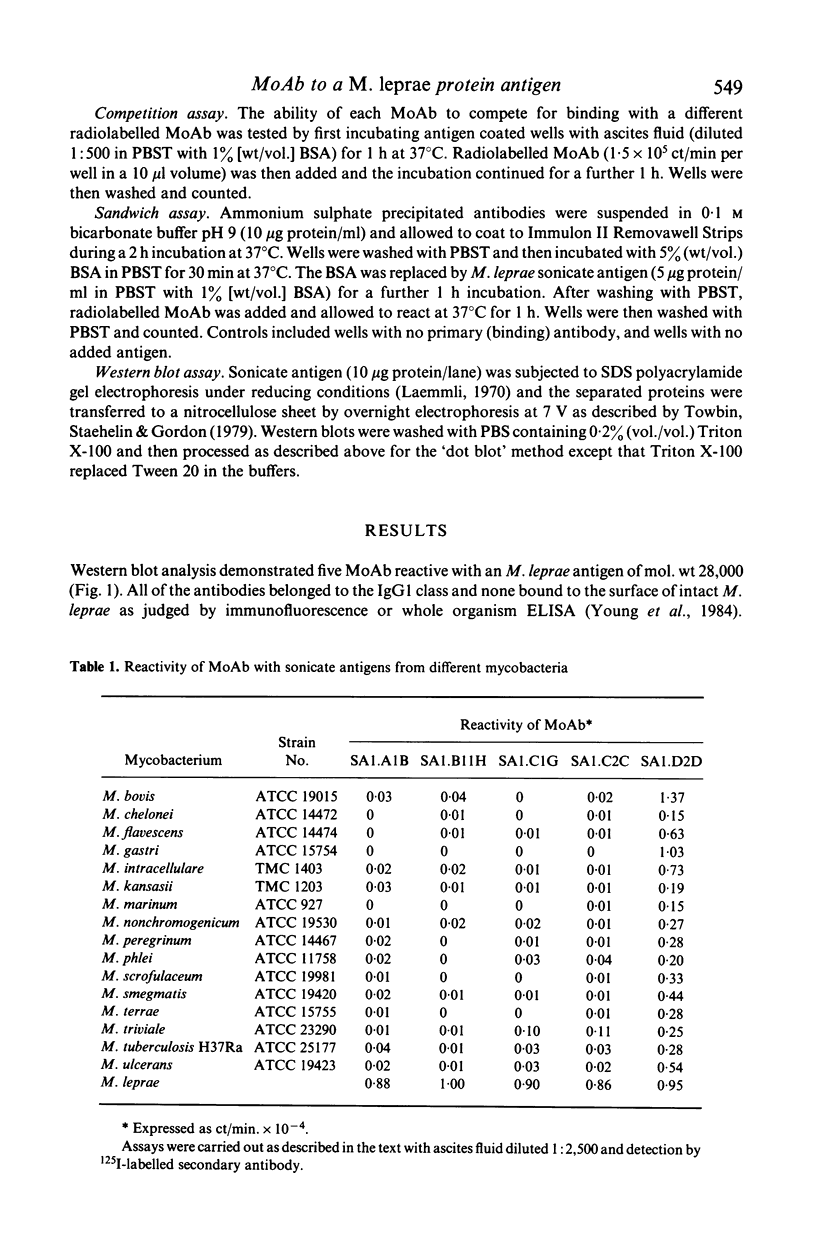
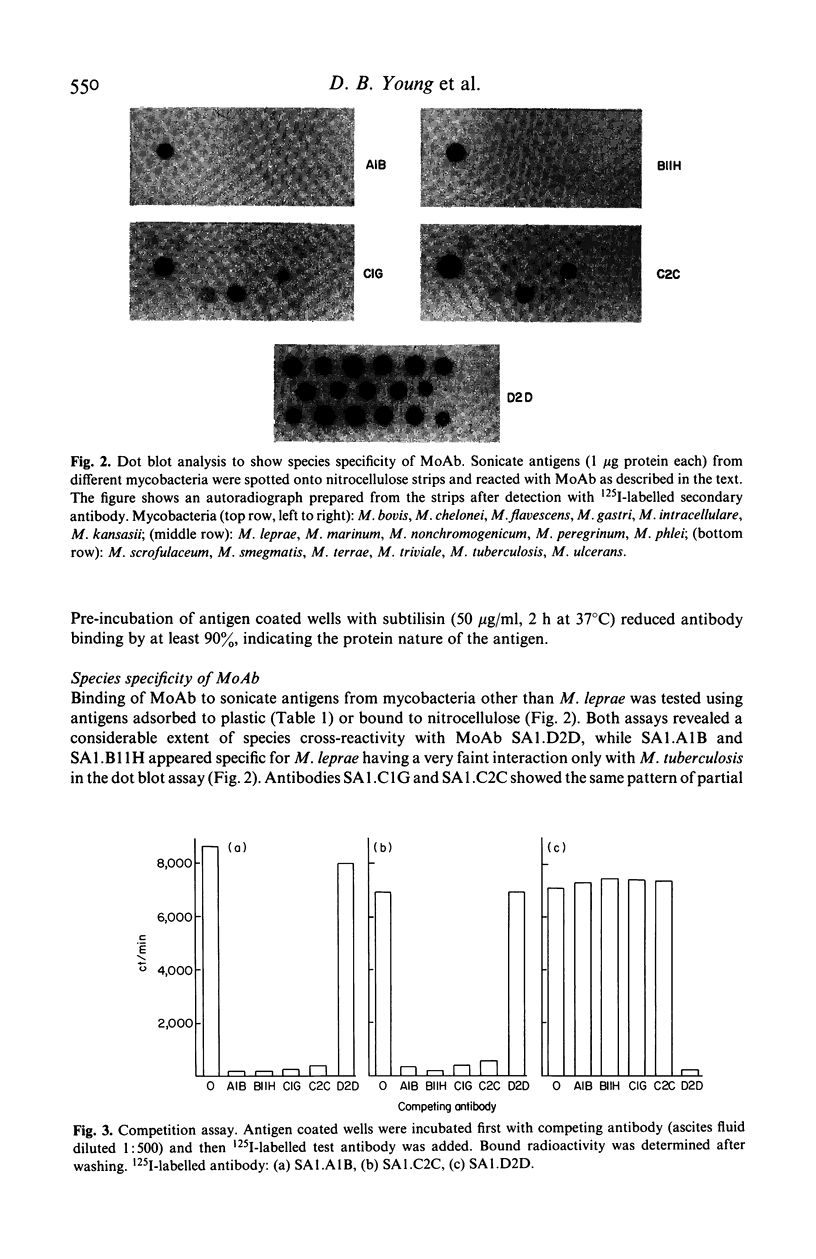
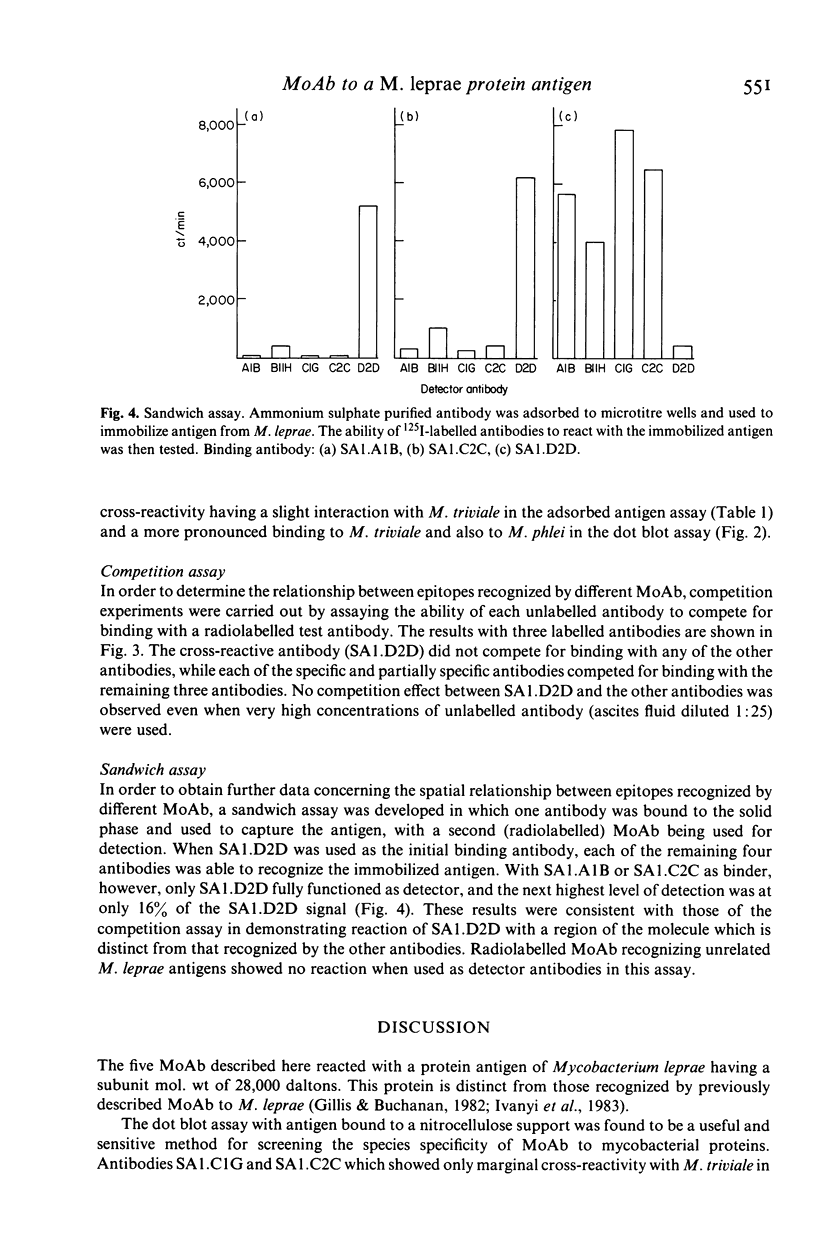
Monoclonal antibodies (MoAb) have been used to analyse a protein antigen from Mycobacterium leprae with a subunit mol. wt of 28,000 daltons. Three different patterns of species specificity were observed with two antibodies being specific for M. leprae, two partially specific, and one broadly cross-reactive amongst all mycobacteria. Competitive binding and sandwich assays demonstrated that the specific and partially specific antibodies recognized closely related regions of the molecule while the cross-reactive antibody recognized a spatially separate epitope on the same polypeptide chain. Identification of specific and cross-reactive epitopes on a single antigenic molecule may be of considerable importance for understanding the functioning of the cell-mediated immune system during leprosy infection and the use of MoAb for such analyses is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R., Godal T. Selective primary health care: strategies for control of disease in the developing world. V. Leprosy. Rev Infect Dis. 1983 Jul-Aug;5(4):765–780. doi: 10.1093/clinids/5.4.765. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis T. P., Buchanan T. M. Production and partial characterization of monoclonal antibodies to Mycobacterium leprae. Infect Immun. 1982 Jul;37(1):172–178. doi: 10.1128/iai.37.1.172-178.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Sinha S., Aston R., Cussell D., Keen M., Sengupta U. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin Exp Immunol. 1983 Jun;52(3):528–536. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Sinha S., Sengupta U., Ramu G., Ivanyi J. A serological test for leprosy based on competitive inhibition of monoclonal antibody binding to the MY2a determinant of Mycobacterium leprae. Trans R Soc Trop Med Hyg. 1983;77(6):869–871. doi: 10.1016/0035-9203(83)90309-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Khanolkar S. R., Barg L. L., Buchanan T. M. Generation and characterization of monoclonal antibodies to the phenolic glycolipid of Mycobacterium leprae. Infect Immun. 1984 Jan;43(1):183–188. doi: 10.1128/iai.43.1.183-188.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]




