Abstract
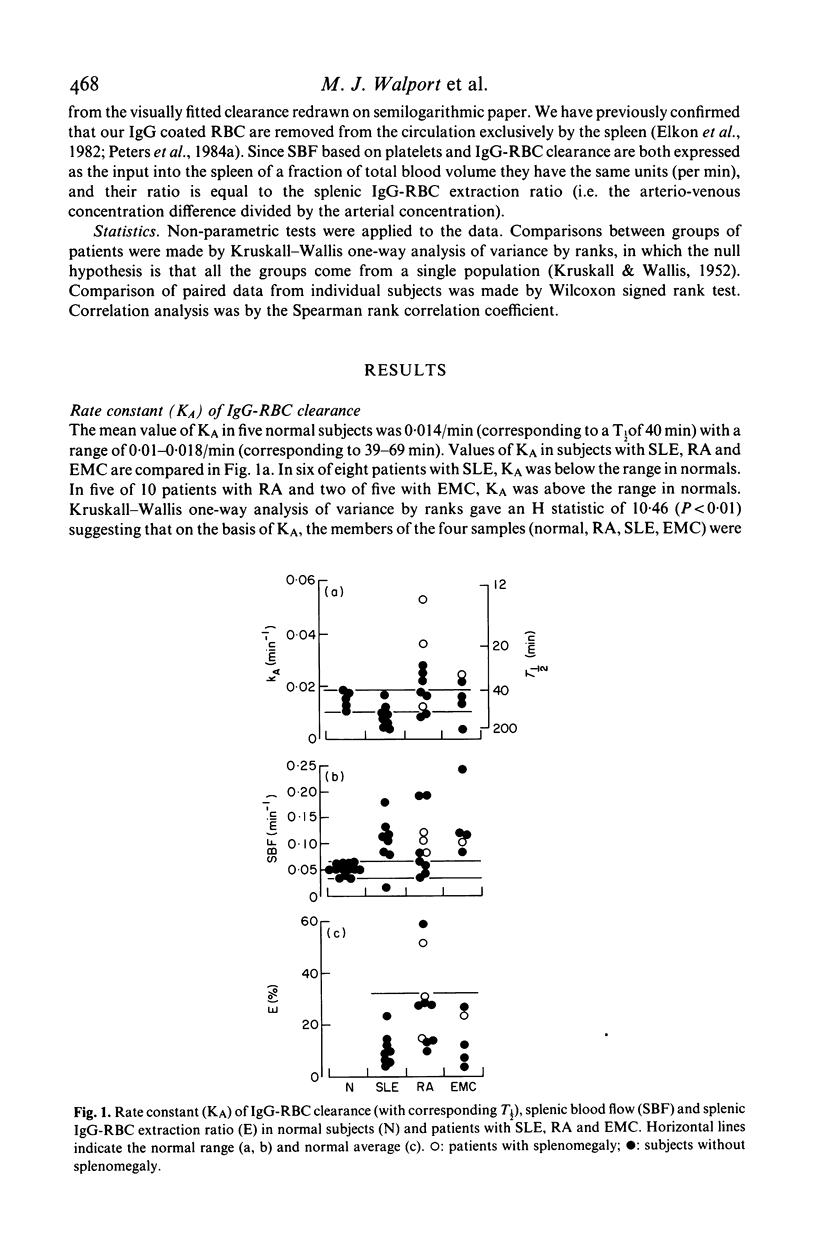
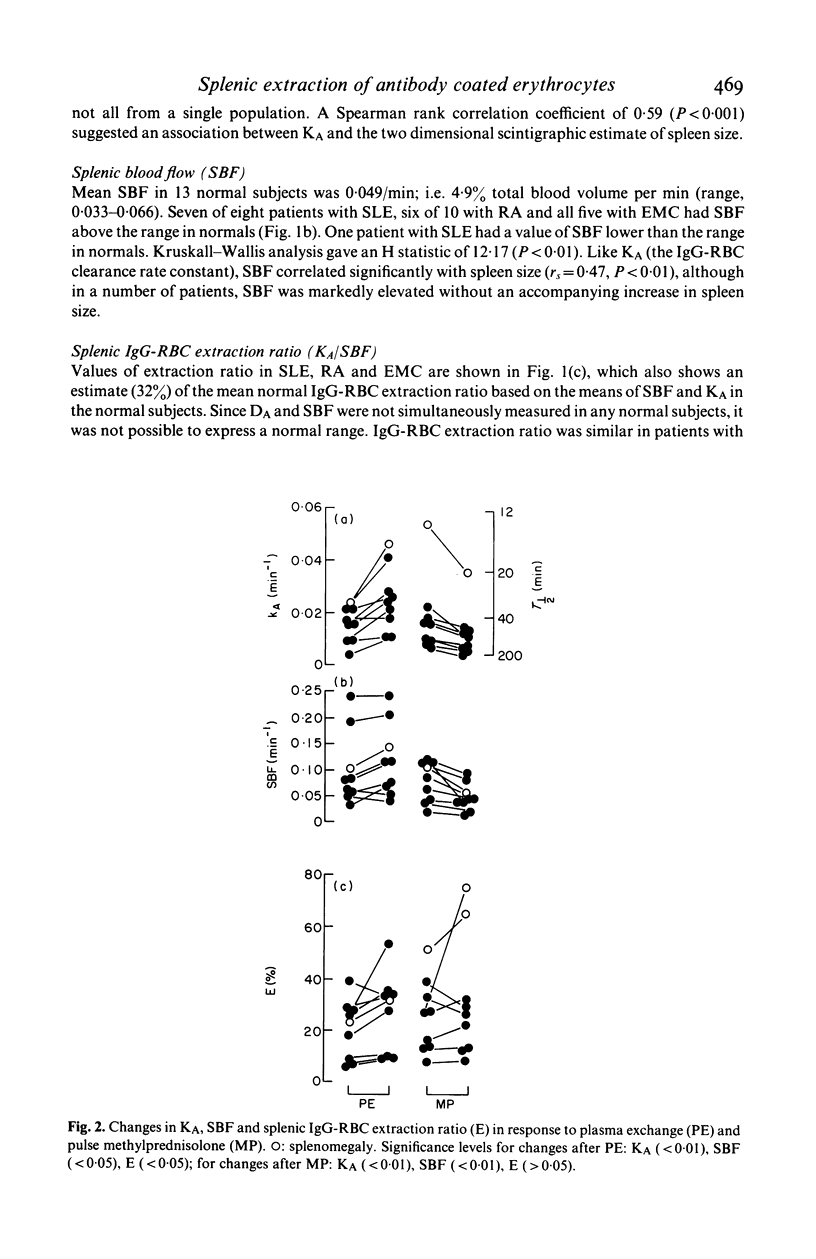
Splenic blood flow was measured in a series of normal subjects and patients with connective tissue diseases by measuring the rate of equilibration and the partition of 111In-labelled autologous platelets between blood and spleen. These data were used to quantify the role of splenic blood flow in determining the splenic clearance of IgG coated erythrocytes (IgG-RBC) from the circulation. Previous studies have interpreted the clearance rates of IgG-RBC only in terms of splenic reticuloendothelial function. Splenic blood flow was increased in seven of eight patients with systemic lupus erythematosus (SLE), six of 10 patients with rheumatoid arthritis (RA) and in all five patients with essential mixed cryoglobulinaemia (EMC) compared with a series of thirteen normal subjects. Expressing the rate constant of clearance of IgG-RBC as a fraction of splenic blood flow gave a value for the 'extraction ratio' of IgG-RBC (a specific measurement of reticuloendothelial function, corrected for splenic blood flow). Normal splenic extraction ratio of IgG-RBC was calculated to be 32%. All the patients with SLE and with EMC had reduced extraction ratios (in seven out of 13 patients less than 10%). In RA the extraction ratio tended to be normal (average 27.3%) but variable (9-59%). Following plasma exchange in nine patients, a significant increase in IgG-RBC extraction ratio (average of 39% with respect to pre-exchange values, P less than 0.05) was found. In contrast there was no significant change in extraction ratio following pulse methylprednisolone therapy in a further nine patients. Although the rate constant of clearance of IgG-RBC decreased by an average of 33% (P less than 0.01) in the latter group, it was matched by an equal decrease of splenic blood flow (average 37%, P less than 0.01) and so extraction ratio showed no change. These data indicate that quantification of splenic reticuloendothelial function requires measurement of both IgG-RBC clearance and of splenic blood flow.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armas R. R., Thakur M. L., Gottschalk A. A simplified method of selective spleen scintigraphy with Tc-99m-labeled erythrocytes: clinical applications. Concise communication. J Nucl Med. 1980 May;21(5):413–416. [PubMed] [Google Scholar]
- Atkinson J. P., Schreiber A. D., Frank M. M. Effects of corticosteroids and splenectomy on the immune clearance and destruction of erythrocytes. J Clin Invest. 1973 Jun;52(6):1509–1517. doi: 10.1172/JCI107325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENACERRAF B., SEBESTYEN M., COOPER N. S. The clearance of antigen antibody complexes from the blood by the reticuloendothelial system. J Immunol. 1959 Feb;82(2):131–137. [PubMed] [Google Scholar]
- Blendis L. M., Banks D. C., Ramboer C., Williams R. Spleen blood flow and splanchnic haemodynamics in blood dyscrasia and other splenomegalies. Clin Sci. 1970 Jan;38(1):73–84. doi: 10.1042/cs0380073. [DOI] [PubMed] [Google Scholar]
- Dambuyant C., Thivolet J., Viala J. J., Ville D., Boyer J. Clearance mediated by splenic macrophage membrane receptors for immune complexes in cutaneous vasculitis. J Invest Dermatol. 1982 Mar;78(3):194–199. doi: 10.1111/1523-1747.ep12506441. [DOI] [PubMed] [Google Scholar]
- Elkon K. B., Ferjencik P. P., Walport M. J., Peters A. M., Lewis S. M., Hughes G. R. Evaluation of heat-damaged and IgG-coated red cells for testing reticuloendothelial function. J Immunol Methods. 1982 Dec 17;55(2):253–263. doi: 10.1016/0022-1759(82)90037-0. [DOI] [PubMed] [Google Scholar]
- Frank M. M., Hamburger M. I., Lawley T. J., Kimberly R. P., Plotz P. H. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med. 1979 Mar 8;300(10):518–523. doi: 10.1056/NEJM197903083001002. [DOI] [PubMed] [Google Scholar]
- Fries L. F., Brickman C. M., Frank M. M. Monocyte receptors for the Fc portion of IgG increase in number in autoimmune hemolytic anemia and other hemolytic states and are decreased by glucocorticoid therapy. J Immunol. 1983 Sep;131(3):1240–1245. [PubMed] [Google Scholar]
- Gordon P. A., Davis P., Russell A. S., Coates J. E., Rothwell R. S., LeClercq S. M. Splenic reticuloendothelial function in patients with active rheumatoid arthritis. J Rheumatol. 1981 May-Jun;8(3):490–493. [PubMed] [Google Scholar]
- Haakenstad A. O., Case J. B., Mannik M. Effect of cortisone on the disappearance kinetics and tissue localization of soluble immune complexes. J Immunol. 1975 Apr;114(4):1153–1160. [PubMed] [Google Scholar]
- Haakenstad A. O., Mannik M. Saturation of the reticuloendothelial system with soluble immune complexes. J Immunol. 1974 May;112(5):1939–1948. [PubMed] [Google Scholar]
- Hamburger M. I., Gorevic P. D., Lawley T. J., Franklin E. C., Frank M. M. Mixed cryoglobulinemia: association of glomerulonephritis with defective reticuloendothelial system Fc receptor function. Trans Assoc Am Physicians. 1979;92:104–112. [PubMed] [Google Scholar]
- Hamburger M. I., Lawley T. J., Kimberly R. P., Plotz P. H., Frank M. M. A serial study of splenic reticuloendothelial system Fc receptor functional activity in systemic lupus erythematosus. Arthritis Rheum. 1982 Jan;25(1):48–54. doi: 10.1002/art.1780250108. [DOI] [PubMed] [Google Scholar]
- Henderson J. M., Bell D. A., Harth M., Chamberlain M. J. Reticuloendothelial function in rheumatoid arthritis: correlation with disease activity and circulating immune complexes. J Rheumatol. 1981 May-Jun;8(3):486–489. [PubMed] [Google Scholar]
- Jaffe C. J., Vierling J. M., Jones E. A., Lawley T. J., Frank M. M. Receptor specific clearance by the reticuloendothelial system in chronic liver diseases. Demonstration of defective C3b-specific clearance in primary biliary cirrhosis. J Clin Invest. 1978 Nov;62(5):1069–1077. doi: 10.1172/JCI109212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J., Mollison P. L. A simple and efficient method of labelling red cells with 99mTc for determination of red cell volume. Br J Haematol. 1978 Jan;38(1):141–148. doi: 10.1111/j.1365-2141.1978.tb07116.x. [DOI] [PubMed] [Google Scholar]
- Lockwood C. M., Rees A. J., Pearson T. A., Evans D. J., Peters D. K., Wilson C. B. Immunosuppression and plasma-exchange in the treatment of Goodpasture's syndrome. Lancet. 1976 Apr 3;1(7962):711–715. doi: 10.1016/s0140-6736(76)93089-0. [DOI] [PubMed] [Google Scholar]
- Lockwood C. M., Worlledge S., Nicholas A., Cotton C., Peters D. K. Reversal of impaired splenic function in patients with nephritis or vasculitis (or both) by plasma exchange. N Engl J Med. 1979 Mar 8;300(10):524–530. doi: 10.1056/NEJM197903083001003. [DOI] [PubMed] [Google Scholar]
- NIH conference. Pathophysiology of immune hemolytic anemia. Ann Intern Med. 1977 Aug;87(2):210–222. doi: 10.7326/0003-4819-87-2-210. [DOI] [PubMed] [Google Scholar]
- Parris T. M., Kimberly R. P., Inman R. D., McDougal J. S., Gibofsky A., Christian C. L. Defective Fc receptor-mediated function of the mononuclear phagocyte system in lupus nephritis. Ann Intern Med. 1982 Oct;97(4):526–532. doi: 10.7326/0003-4819-97-4-526. [DOI] [PubMed] [Google Scholar]
- Peters A. M., Klonizakis I., Lavender J. P., Lewis S. M. Use of 111Indium-labeled platelets to measure spleen function. Br J Haematol. 1980 Dec;46(4):587–593. doi: 10.1111/j.1365-2141.1980.tb06016.x. [DOI] [PubMed] [Google Scholar]
- Peters A. M., Lavender J. P. Factors controlling the intrasplenic transit of platelets. Eur J Clin Invest. 1982 Jun;12(3):191–195. doi: 10.1111/j.1365-2362.1982.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Peters A. M., Lavender J. P. Platelet kinetics with indium-111 platelets: comparison with chromium-51 platelets. Semin Thromb Hemost. 1983 Apr;9(2):100–114. doi: 10.1055/s-2007-1005016. [DOI] [PubMed] [Google Scholar]
- Peters A. M., Mathie R. T., Walport M. J., Reavy H. J., Bell R. N., Lavender J. P. Measurement of splenic blood flow in the anaesthetised dog using electromagnetic flowmetry and indium labelled platelets. Cardiovasc Res. 1983 Nov;17(11):710–718. doi: 10.1093/cvr/17.11.710. [DOI] [PubMed] [Google Scholar]
- Peters A. M., Walport M. J., Bell R. N., Lavender J. P. Methods of measuring splenic blood flow and platelet transit time with In-111-labeled platelets. J Nucl Med. 1984 Jan;25(1):86–90. [PubMed] [Google Scholar]
- Peters A. M., Walport M. J., Elkon K. B., Reavy H. J., Ferjencik P. P., Lavender J. P., Hughes G. R. The comparative blood clearance kinetics of modified radiolabelled erythrocytes. Clin Sci (Lond) 1984 Jan;66(1):55–62. doi: 10.1042/cs0660055. [DOI] [PubMed] [Google Scholar]
- Rinehart J. J., Balcerzak S. P., Sagone A. L., LoBuglio A. F. Effects of corticosteroids on human monocyte function. J Clin Invest. 1974 Dec;54(6):1337–1343. doi: 10.1172/JCI107880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn H., Silvester D. J. Simplified cell labelling with indium-111 acetylacetone. Br J Radiol. 1979 Sep;52(621):758–759. doi: 10.1259/0007-1285-52-621-758. [DOI] [PubMed] [Google Scholar]
- Williams B. D., Lockwood C. M., Pussell B. A. Inhibition of reticuloendothelial function by gold and its relation to postinjection reactions. Br Med J. 1979 Jul 28;2(6184):235–238. doi: 10.1136/bmj.2.6184.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R., Condon R. E., Williams H. S., Blendis L. M., Kreel L. Splenic blood flow in cirrhosis and portal hypertension. Clin Sci. 1968 Jun;34(3):441–452. [PubMed] [Google Scholar]


