Abstract
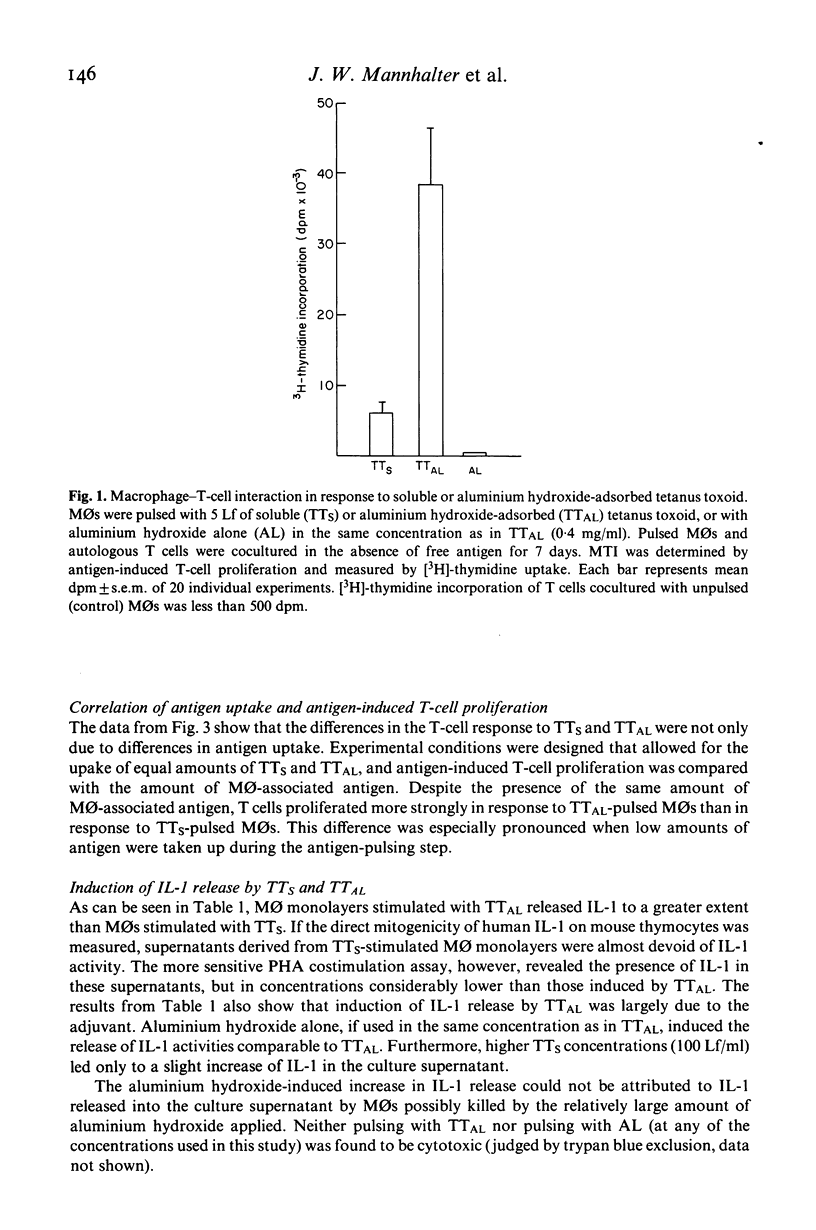
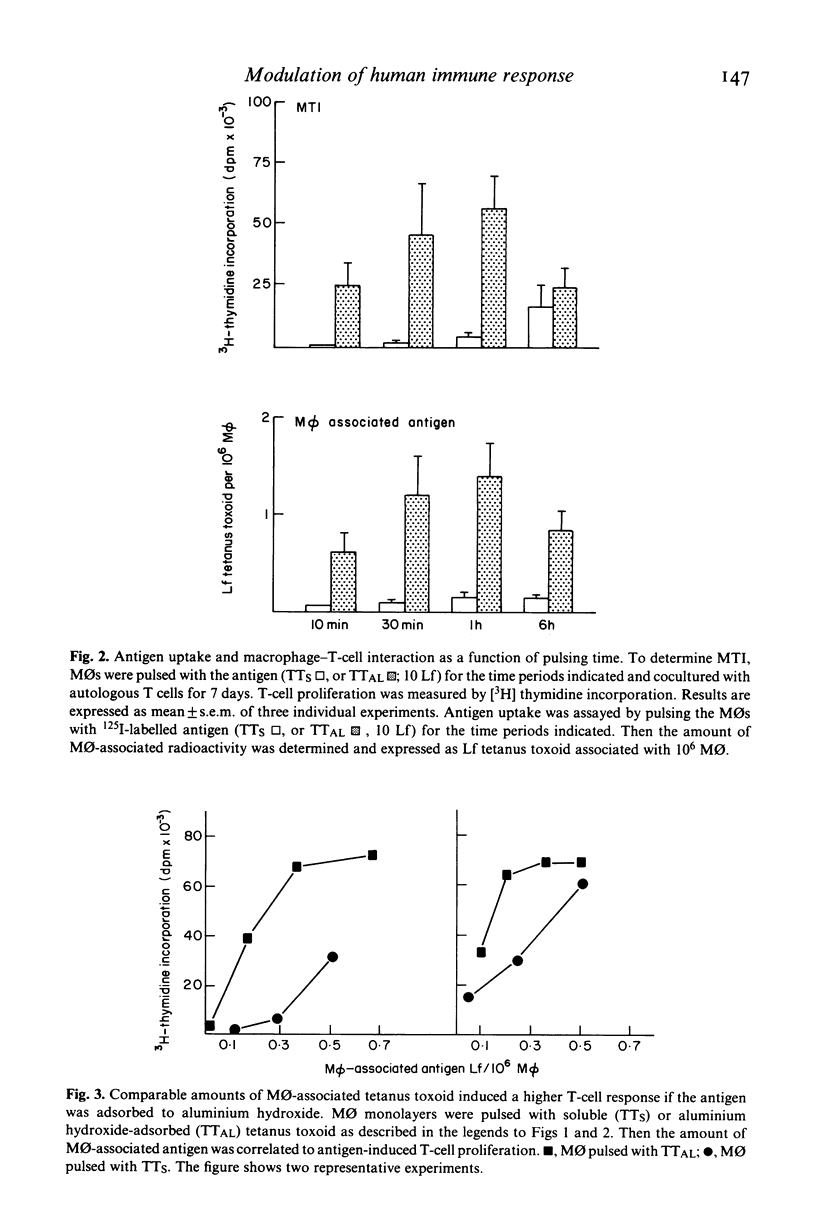
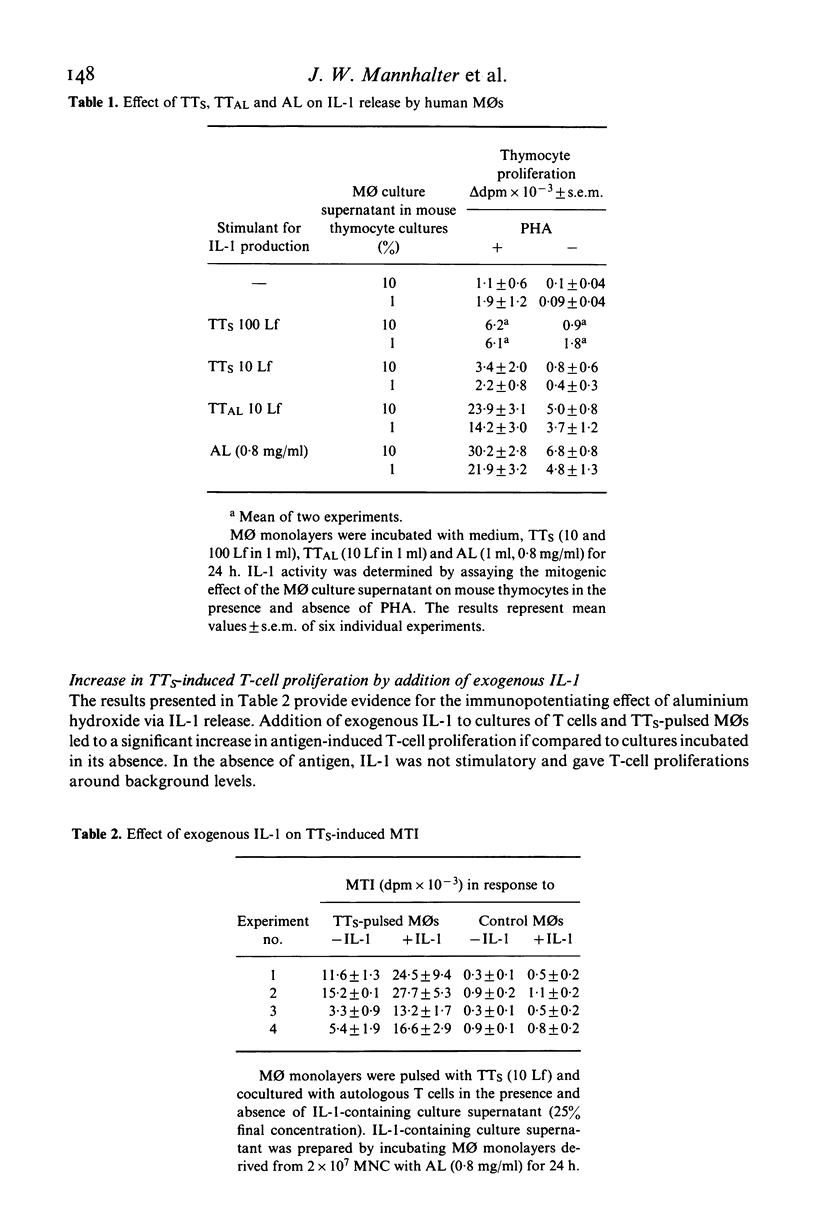
The regulatory effects of an adjuvant (aluminium hydroxide) on the early phase of the immune response have been investigated. Adsorbing a soluble antigen (tetanus toxoid) to aluminium hydroxide led to a significant increase (P less than 0.001) in antigen-induced T-cell proliferation (macrophage-T-cell interaction, MTI) making aluminium hydroxide-adsorbed antigens especially suitable to study immunoregulatory changes in the early phase of the immune response. First studies revealed that this increase was due to an enhancement of antigen uptake by the antigen-presenting cell. However, under conditions allowing for the uptake of comparable amounts of soluble (TTs) or aluminium hydroxide-absorbed (TTAL) antigen, T-cell proliferation in response to TTAL was still higher than in response to TTS. This difference was especially pronounced if suboptimal antigen concentrations were used and could be explained by differences in the TTS-versus TTAL-induced release of interleukin-1 (IL-1). Pulsing with TTAL led to a substantial increase in IL-1 release by monocytes (MO) which then subsequently augmented antigen-induced T-cell proliferation. This was further supported by addition of exogenous IL-1 to cultures of T cells and TTS-pulsed MOs, which also significantly increased the T cells' proliferative response. These findings demonstrate that in the early phase of the immune response, aluminium hydroxide exerts its regulatory effect at the level of the antigen-presenting and mediator-releasing accessory cell.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bomford R. The comparative selectivity of adjuvants for humoral and cell-mediated immunity. I. Effect on the antibody response to bovine serum albumin and sheep red blood cells of Freund's incomplete and complete adjuvants, alhydrogel, Corynebacterium parvum, Bordetella pertussis, muramyl dipeptide and saponin. Clin Exp Immunol. 1980 Feb;39(2):426–434. [PMC free article] [PubMed] [Google Scholar]
- Bomford R. The comparative selectivity of adjuvants for humoral and cell-mediated immunity. II. Effect on delayed-type hypersensitivity in the mouse and guinea pig, and cell-mediated immunity to tumour antigens in the mouse of Freund's incomplete and complete adjuvants, alhydrogel, Corynebacterium parvum, Bordetella pertussis, muramyl dipeptide and saponin. Clin Exp Immunol. 1980 Feb;39(2):435–441. [PMC free article] [PubMed] [Google Scholar]
- Eibl M., Mannhalter J. W., Ahmad R. Macrophage-lymphocyte interaction in response to a bacterial antigen (E. coli). Clin Exp Immunol. 1982 Feb;47(2):260–268. [PMC free article] [PubMed] [Google Scholar]
- Elsbach P. Degradation of microorganisms by phagocytic cells. Rev Infect Dis. 1980 Jan-Feb;2(1):106–128. doi: 10.1093/clinids/2.1.106. [DOI] [PubMed] [Google Scholar]
- Garnham P. C., Humphrey J. H. Problems in leishmaniasis related to immunology. Curr Top Microbiol Immunol. 1969;48:29–42. doi: 10.1007/978-3-642-46163-7_2. [DOI] [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B. Cell-mediated immunity to tumor cells. Adv Cancer Res. 1974;19(0):207–263. doi: 10.1016/s0065-230x(08)60055-x. [DOI] [PubMed] [Google Scholar]
- Lachman L. B. Human interleukin 1: purification and properties. Fed Proc. 1983 Jun;42(9):2639–2645. [PubMed] [Google Scholar]
- Larsh J. E., Jr, Weatherly N. F. Cell-mediated immunity in certain parasitic infections. Curr Top Microbiol Immunol. 1974;67:113–137. doi: 10.1007/978-3-642-65912-6_4. [DOI] [PubMed] [Google Scholar]
- Lawrence H. S., Borkowsky W. A new basis for the immunoregulatory activities of transfer factor--an arcane dialect in the language of cells. Cell Immunol. 1983 Nov;82(1):102–116. doi: 10.1016/0008-8749(83)90145-4. [DOI] [PubMed] [Google Scholar]
- Leclerc C., Audibert F., Chedid L. Influence of a synthetic adjuvant (MDP) on qualitative and quantitative changes of serum globulins. Immunology. 1978 Dec;35(6):963–970. [PMC free article] [PubMed] [Google Scholar]
- Malek T. R., Shevach E. M. Nature of the antigenic complex recognized by T lymphocytes. IX. Direct immunochemical demonstration of nominal antigen on the macrophage cell surface. Eur J Immunol. 1982 Oct;12(10):825–831. doi: 10.1002/eji.1830121006. [DOI] [PubMed] [Google Scholar]
- Mancino D., Ovary Z. Adjuvant effects of amorphous silica and of aluminium hydroxide on IgE and IgG1 antibody production in different inbred mouse strains. Int Arch Allergy Appl Immunol. 1980;61(3):253–258. doi: 10.1159/000232443. [DOI] [PubMed] [Google Scholar]
- Mannhalter J. W., Zlabinger G. J., Ahmad R., Eibl M. M. Human T cell proliferation in response to E. coli presented by autologous macrophages is antigen specific. Clin Exp Immunol. 1983 Oct;54(1):95–102. [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Oppenheim J. J., Togawa A., Chedid L., Mizel S. Components of mycobacteria and muramyl dipeptide with adjuvant activity induce lymphocyte activating factor. Cell Immunol. 1980 Mar 1;50(1):71–81. doi: 10.1016/0008-8749(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Palacios R. Mechanism of T cell activation: role and functional relationship of HLA-DR antigens and interleukins. Immunol Rev. 1982;63:73–110. doi: 10.1111/j.1600-065x.1982.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S. Regulation of the immune response--role of the macrophage. N Engl J Med. 1980 Nov 13;303(20):1153–1156. doi: 10.1056/NEJM198011133032005. [DOI] [PubMed] [Google Scholar]
- Staruch M. J., Wood D. D. The adjuvanticity of interleukin 1 in vivo. J Immunol. 1983 May;130(5):2191–2194. [PubMed] [Google Scholar]
- Sugimoto M., Germain R. N., Chedid L., Benacerraf B. Enhancement of carrier-specific helper T cell function by the synthetic adjuvant, N-acetyl muramyl-L-alanyl-D-isoglutamine (MDP). J Immunol. 1978 Mar;120(3):980–982. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Reinherz E. L., Schlossman S. F. Human macrophage-lymphocyte interaction in proliferation to soluble antigen. I. Specific delection of lymphocyte proliferative activity on macrophage monolayers. Cell Immunol. 1980 Sep 15;55(1):114–123. doi: 10.1016/0008-8749(80)90142-2. [DOI] [PubMed] [Google Scholar]
- Togawa A., Oppenheim J. J., Mizel S. B. Characterization of lymphocyte-activating factor (LAF) produced by human mononuclear cells: biochemical relationship of high and low molecular weight forms of LAF. J Immunol. 1979 May;122(5):2112–2118. [PubMed] [Google Scholar]
- Unanue E. R., Askonas B. A. Persistence of immunogenicity of antigen after uptake by macrophages. J Exp Med. 1968 May 1;127(5):915–926. doi: 10.1084/jem.127.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Kondo S., Kameyama S., Murata R. Studies on adjuvants for human prophylactics. I. Comparison of efficiencies of different adjuvants at various stages of immunization with tetanus and diphtheria toxoids. Jpn J Med Sci Biol. 1978 Jun;31(3):263–276. doi: 10.7883/yoken1952.31.263. [DOI] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]
- Zlabinger G. J., Mannhalter J. W., Ahmad R., Eibl M. M. Reduced antigen-induced proliferation and surface Ia expression of peripheral blood T cells following tetanus booster immunization. Clin Immunol Immunopathol. 1985 Feb;34(2):254–262. doi: 10.1016/0090-1229(85)90029-7. [DOI] [PubMed] [Google Scholar]



