Abstract
Patients of some rheumatic autoimmune diseases like systemic lupus erythematosus have in their sera autoantibodies to vimentin, the main protein of intermediate sized filaments of the cytoskeleton of connective tissue cells. Immunoprecipitation and Western blotting techniques have been used to detect the interaction between human anti-vimentin autoantibodies and the two peptides that resulted from the cleavage of vimentin by N-chlorosuccinimide. Both methods and also immunoabsorption of sera with the isolated peptides suggest that the interaction of vimentin and immunoglobulins is mainly carried out through a peptide of 30kD mol. wt located close to the amino-terminal part of the vimentin molecule. The same results were obtained when a serum from a rabbit immunized with human vimentin was used.
Full text
PDF

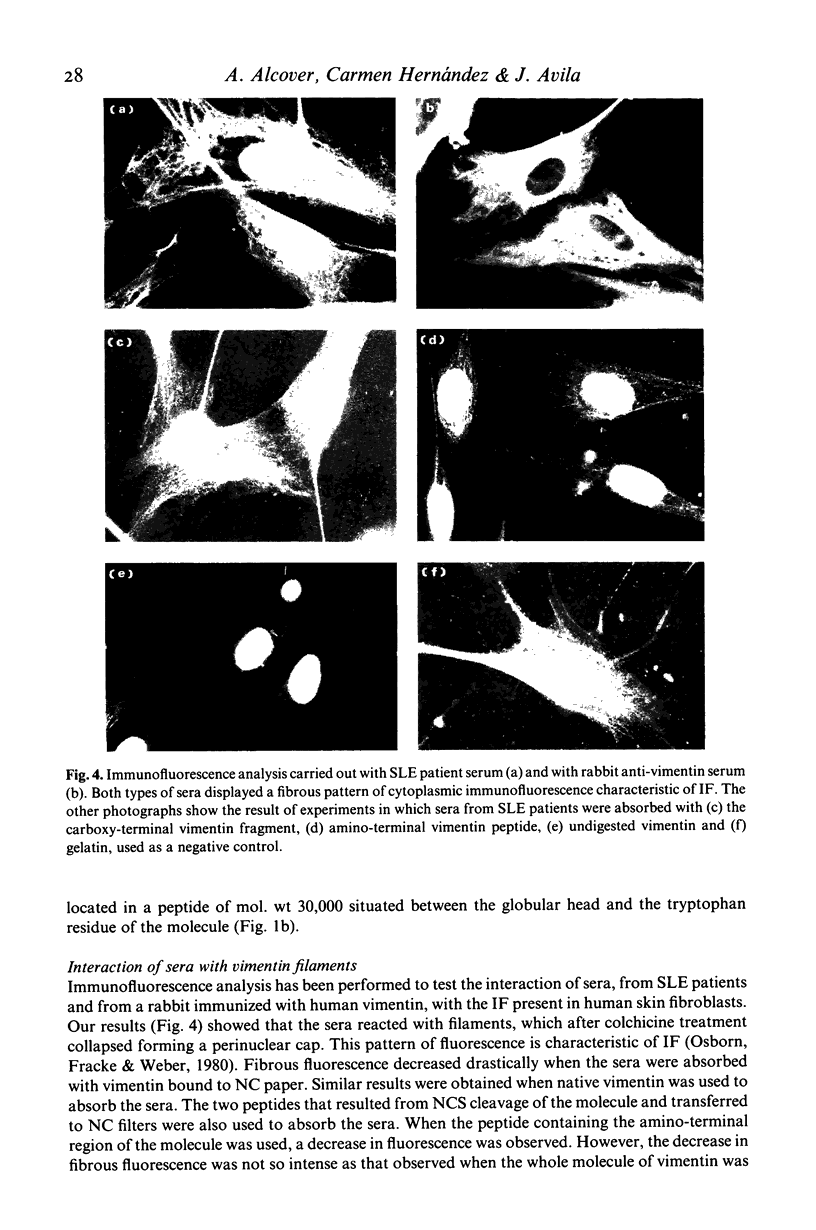


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcover A., Molano J., Renart J., Gil-Aguado A., Nieto A., Avila J. Antibodies to vimentin intermediate filaments in sera from patients with systemic lupus erythematosus. Arthritis Rheum. 1984 Aug;27(8):922–928. doi: 10.1002/art.1780270812. [DOI] [PubMed] [Google Scholar]
- André-Schwartz J., Datta S. K., Shoenfeld Y., Isenberg D. A., Stollar B. D., Schwartz R. S. Binding of cytoskeletal proteins by monoclonal anti-DNA lupus autoantibodies. Clin Immunol Immunopathol. 1984 May;31(2):261–271. doi: 10.1016/0090-1229(84)90246-0. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Weber K. The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filament proteins. EMBO J. 1982;1(12):1649–1656. doi: 10.1002/j.1460-2075.1982.tb01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurki P., Helve T., Virtanen I. Antibodies to cytoplasmic intermediate filaments in rheumatic diseases. J Rheumatol. 1983 Aug;10(4):558–562. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs D. A new method for partial peptide mapping using N-chlorosuccinimide/urea and peptide silver staining in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1982 Dec;127(2):453–457. doi: 10.1016/0003-2697(82)90203-2. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Traub P. Proteolysis of vimentin and desmin by the Ca2+-activated proteinase specific for these intermediate filament proteins. Mol Cell Biol. 1983 Jun;3(6):1146–1156. doi: 10.1128/mcb.3.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Osborn M., Franke W., Weber K. Direct demonstration of the presence of two immunologically distinct intermediate-sized filament systems in the same cell by double immunofluorescence microscopy. Vimentin and cytokeratin fibers in cultured epithelial cells. Exp Cell Res. 1980 Jan;125(1):37–46. doi: 10.1016/0014-4827(80)90186-x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Geisler N., Shaw G., Sharp G., Weber K. Intermediate filaments. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):413–429. doi: 10.1101/sqb.1982.046.01.040. [DOI] [PubMed] [Google Scholar]
- Osung O. A., Chandra M., Holborow E. J. Antibody to intermediate filaments of the cytoskeleton. Ann Rheum Dis. 1982 Feb;41(1):69–73. doi: 10.1136/ard.41.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quax W., Egberts W. V., Hendriks W., Quax-Jeuken Y., Bloemendal H. The structure of the vimentin gene. Cell. 1983 Nov;35(1):215–223. doi: 10.1016/0092-8674(83)90224-6. [DOI] [PubMed] [Google Scholar]
- Senecal J. L., Rothfield N. F., Oliver J. M. Immunoglobulin M autoantibody to vimentin intermediate filaments. J Clin Invest. 1982 Mar;69(3):716–721. doi: 10.1172/JCI110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]