Abstract
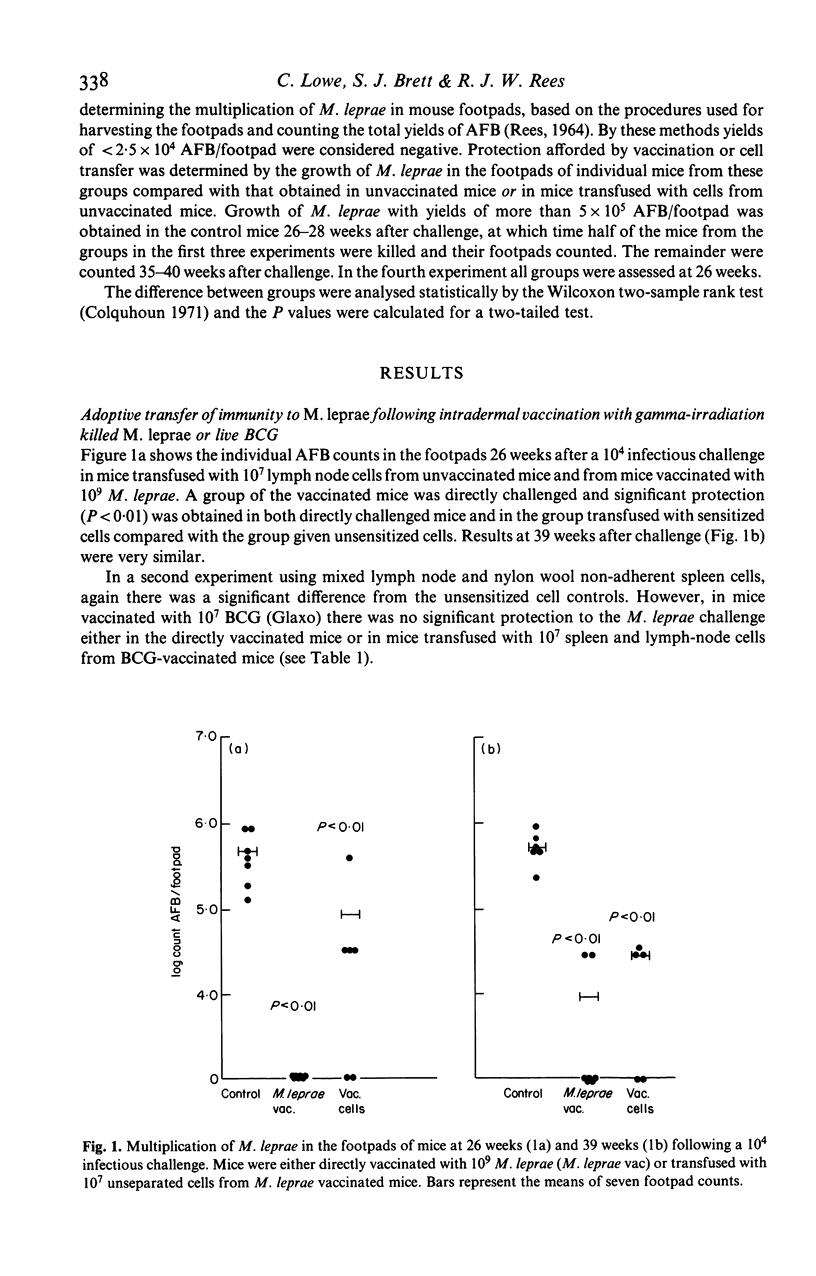
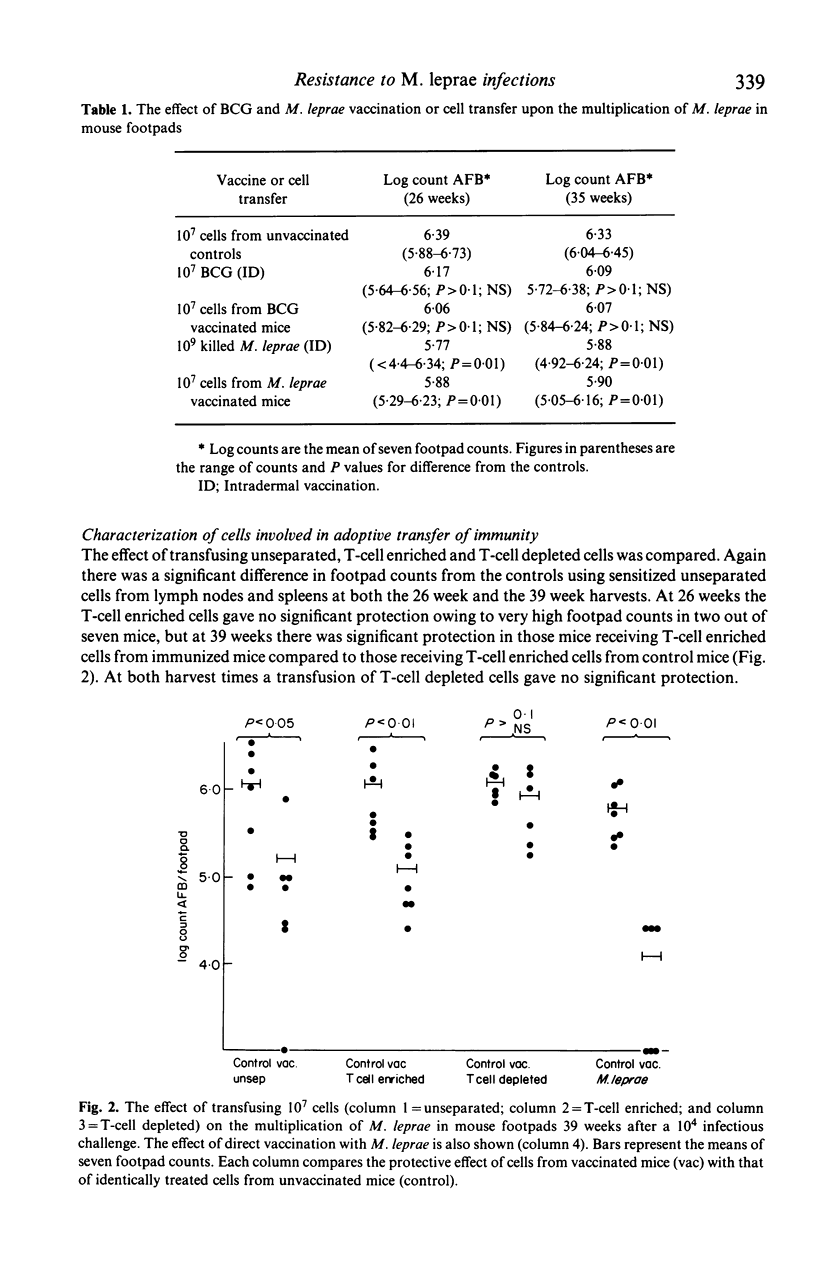
Cells were transferred from mice intradermally vaccinated with killed Mycobacterium leprae to sublethally irradiated recipients. Unseparated cells from lymph nodes or spleens of M. leprae vaccinated mice were found to cause significant inhibition of the growth of a subsequent M. leprae challenge in mouse footpads for up to 26 weeks after vaccination. Vaccination with live BCG and cells transferred from BCG-vaccinated mice caused no significant inhibition of M. leprae growth in mouse footpads. Cell separation into fractions containing predominantly B and T lymphocytes showed that the inhibition of growth was due to M. leprae-sensitized T lymphocytes. M. leprae vaccinated mice were also skin tested with soluble M. leprae antigen and showed maximum delayed hypersensitivity responses 4 weeks after vaccination.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Graham L., Jr, Navalkar R. G. Evaluation of Mycobacterium leprae immunogenicity via adoptive transfer studies. Infect Immun. 1984 Jan;43(1):79–83. doi: 10.1128/iai.43.1.79-83.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mehra V., Bloom B. R. Induction of cell-mediated immunity to Mycobacterium leprae in guinea pigs. Infect Immun. 1979 Mar;23(3):787–794. doi: 10.1128/iai.23.3.787-794.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984 Mar;84(1):113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Patel P. J., Lefford M. J. Specific and nonspecific resistance in mice immunized with irradiated Myobacterium leprae. Infect Immun. 1978 Jun;20(3):692–697. doi: 10.1128/iai.20.3.692-697.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES R. J. LIMITED MULTIPLICATION OF ACID-FAST BACILLI IN THE FOOT-PADS OF MICE INOCULATED WITH MYCOBACTERIUM LEPRAE. Br J Exp Pathol. 1964 Apr;45:207–218. [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C., Walker L. L., Van Landingham R. M., Ye S. Z. Sensitization or tolerance to Mycobacterium leprae antigen by route of injection. Infect Immun. 1982 Nov;38(2):673–680. doi: 10.1128/iai.38.2.673-680.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C., van Landingham R., Walker L. L. Searches among mycobacterial cultures for antileprosy vaccines. Infect Immun. 1980 Sep;29(3):1034–1039. doi: 10.1128/iai.29.3.1034-1039.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelt A. H., Rees R. J., Liew F. Y. Induction of delayed-type hypersensitivity to Mycobacterium leprae in healthy individuals. Clin Exp Immunol. 1981 Jun;44(3):501–506. [PMC free article] [PubMed] [Google Scholar]


