Abstract
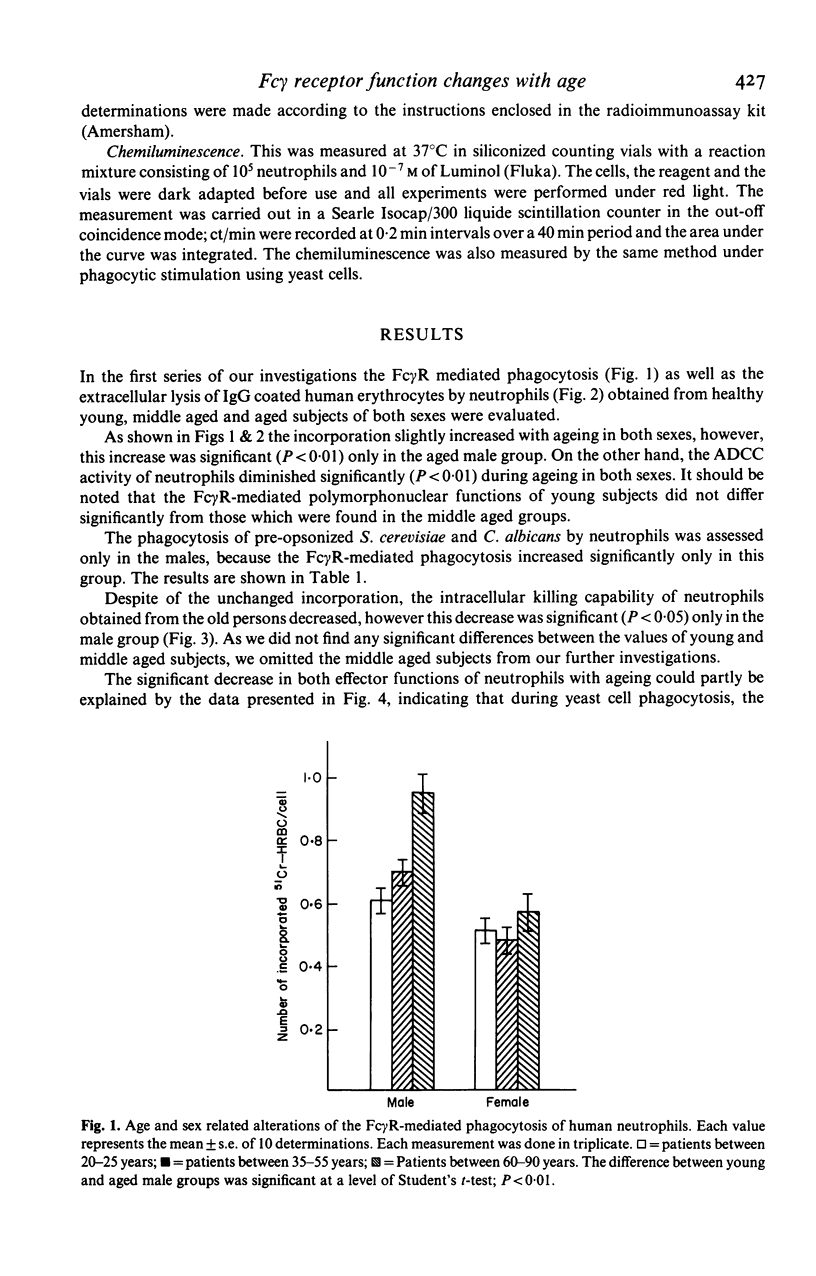
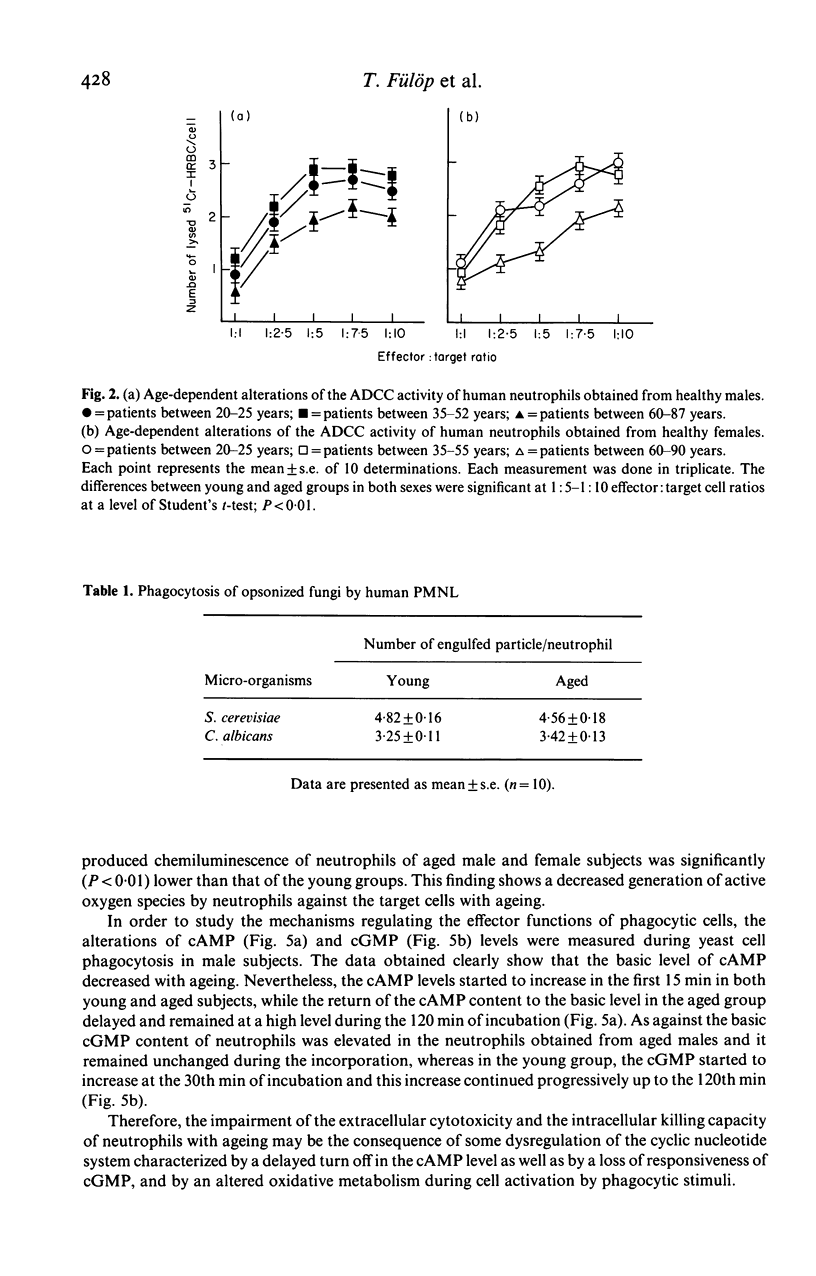
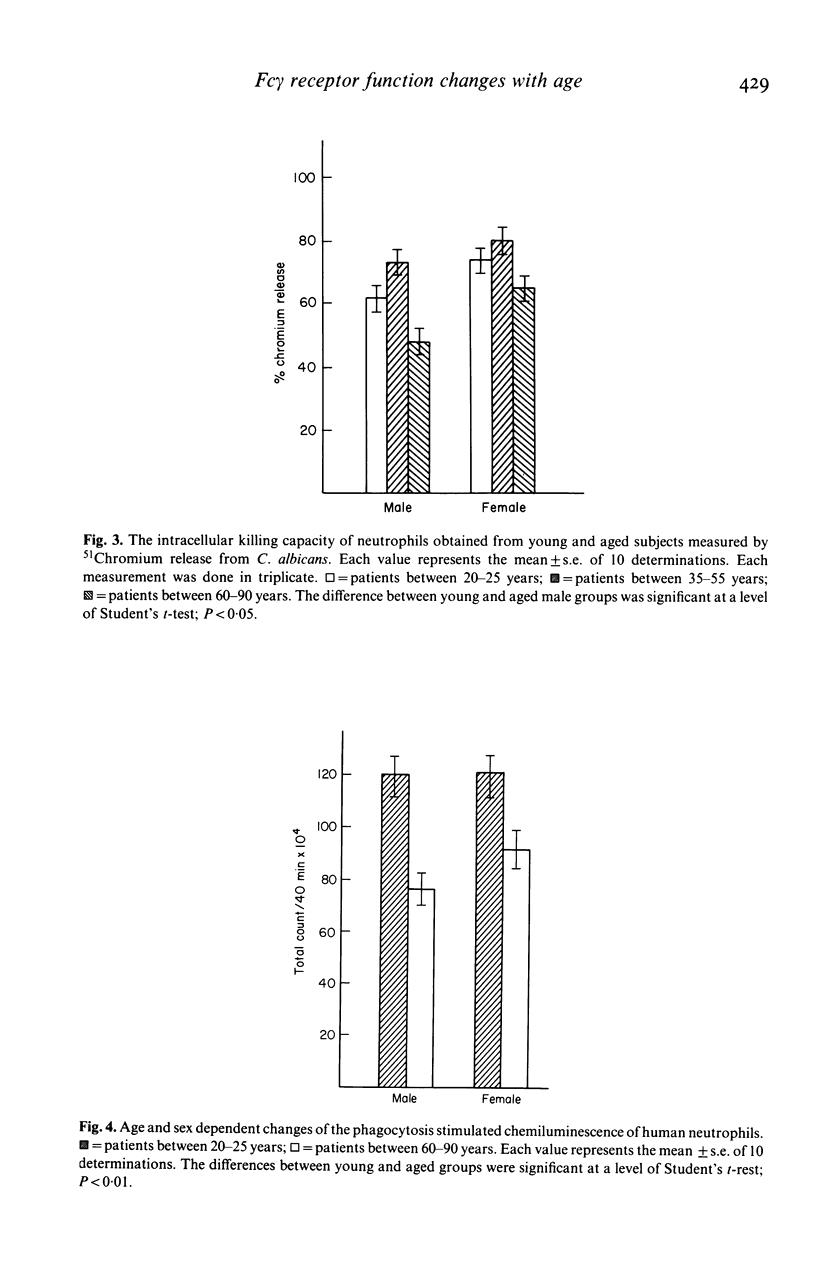
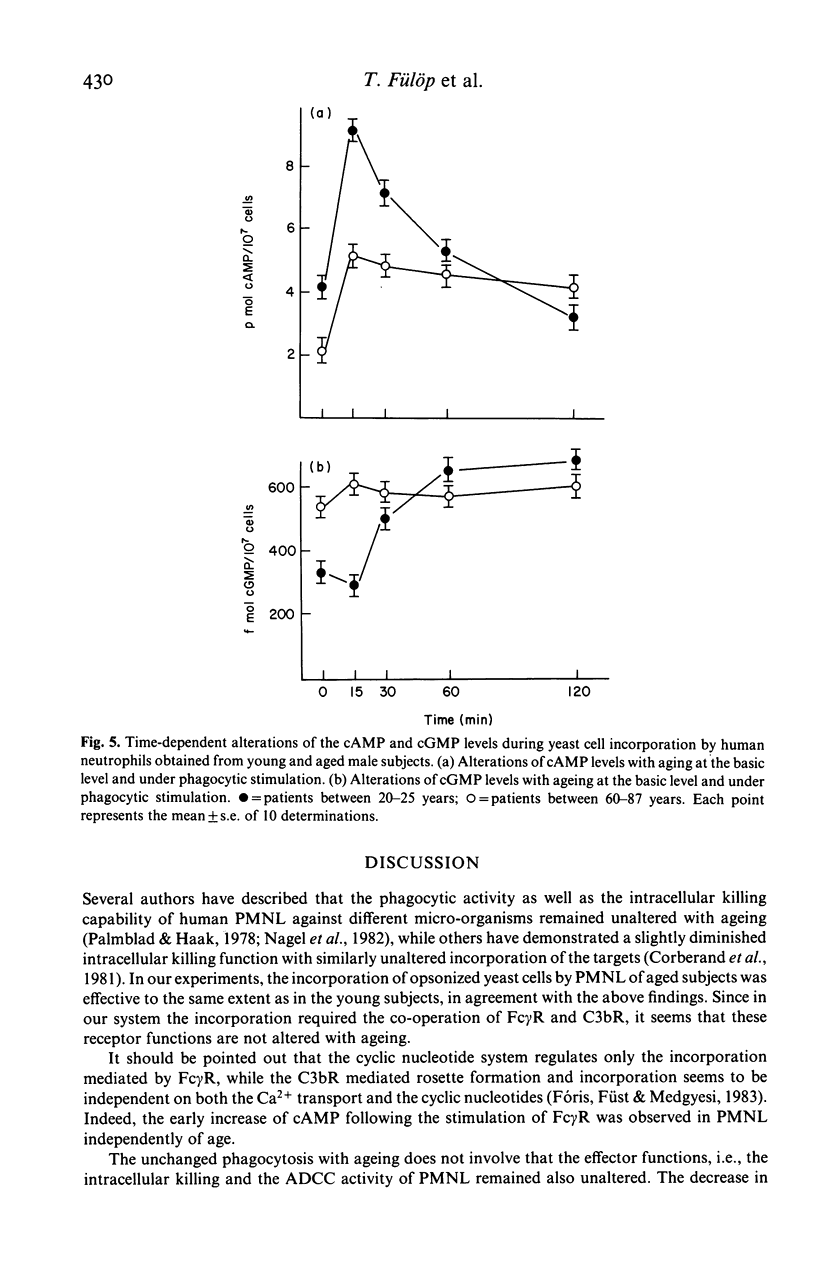
Changes in the effector functions in polymorphonuclear leucocytes (PMNL), harvested from blood of young and aged healthy subjects of both sexes, were studied. FC gamma-receptor (Fc gamma R)-mediated incorporation of IgG coated 51Cr-HRBC significantly increased in the aged male group, while the phagocytosis of pre-opsonized fungi (Saccharomyces cerevisiae and Candida albicans) was independent of both the age and sex. However, the intracellular killing capacity of neutrophils obtained from aged male subjects significantly decreased toward 51Cr-labelled c. albicans. The antibody-dependent cellular cytotoxicity (ADCC) was also impaired with ageing in both sexes. The age-dependent decrease in the effector functions of PMNL may be explained, among others, by the fact that during yeast cell incorporation the increased cAMP level does not return to the basic level in the old group. On the other hand, the cGMP level which increased in PMNL of aged subjects does not show any progressive increase as in the young subjects, but remains unchanged. The oxidative metabolism producing free radicals being necessary for the effective intracellular killing and ADCC diminished in PMNL of aged subjects of both sexes. The above findings indicate that the adaptation of cyclic nucleotide system and the oxidative burst to the cell activation becomes impaired with ageing.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchinsky S. G. Neurotransmitter receptors in the central nervous system and aging: pharmacological aspect (review). Exp Gerontol. 1984;19(4):227–239. doi: 10.1016/0531-5565(84)90018-4. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975 Jun 1;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Studies on the mechanism of antibody-dependent polymorphonuclear leukocyte-mediated cytotoxicity. J Immunol. 1977 Oct;119(4):1413–1418. [PubMed] [Google Scholar]
- Corberand J., Ngyen F., Laharrague P., Fontanilles A. M., Gleyzes B., Gyrard E., Senegas C. Polymorphonuclear functions and aging in humans. J Am Geriatr Soc. 1981 Sep;29(9):391–397. doi: 10.1111/j.1532-5415.1981.tb02376.x. [DOI] [PubMed] [Google Scholar]
- Gale R. P., Zighelboim J. Modulation of polymorphonuclear leukocyte-mediated antibody-dependent cellular cytotoxicity. J Immunol. 1974 Dec;113(6):1793–1800. [PubMed] [Google Scholar]
- Gardner I. D. The effect of aging on susceptibility to infection. Rev Infect Dis. 1980 Sep-Oct;2(5):801–810. doi: 10.1093/clinids/2.5.801. [DOI] [PubMed] [Google Scholar]
- Hafeman D. G., Lucas Z. J. Polymorphonuclear leukocyte-mediated, antibody-dependent, cellular cytotoxicity against tumor cells: dependence on oxygen and the respiratory burst. J Immunol. 1979 Jul;123(1):55–62. [PubMed] [Google Scholar]
- Katz P., Simone C. B., Henkart P. A., Fauci A. S. Mechanisms of antibody-dependent cellular cytotoxicity: the use of effector cells from chronic granulomatous disease patients as investigative probes. J Clin Invest. 1980 Jan;65(1):55–63. doi: 10.1172/JCI109660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall J. F., Connelly-Fittingoff M., Tuck M. L. Lymphocyte adenylate cyclase and human aging. Proc Soc Exp Biol Med. 1983 Sep;173(4):475–480. doi: 10.3181/00379727-173-41673. [DOI] [PubMed] [Google Scholar]
- Lehmeyer J. E., Johnston R. B., Jr Effect of anti-inflammatory drugs and agents that elevate intracellular cyclic AMP on the release of toxic oxygen metabolites by phagocytes: studies in a model of tissue-bound IgG. Clin Immunol Immunopathol. 1978 Apr;9(4):482–490. doi: 10.1016/0090-1229(78)90144-7. [DOI] [PubMed] [Google Scholar]
- Mascart-Lemone F., Delespesse G., Servais G., Kunstler M. Characterization of immunoregulatory T lymphocytes during ageing by monoclonal antibodies. Clin Exp Immunol. 1982 Apr;48(1):148–154. [PMC free article] [PubMed] [Google Scholar]
- Muschel R. J., Rosen N., Rosen O. M., Bloom B. R. Modulation of Fc-mediated phagocytosis by cyclic AMP and insulin in a macrophage-like cell line. J Immunol. 1977 Nov;119(5):1813–1820. [PubMed] [Google Scholar]
- Nagel J. E., Pyle R. S., Chrest F. J., Adler W. H. Oxidative metabolism and bactericidal capacity of polymorphonuclear leukocytes from normal young and aged adults. J Gerontol. 1982 Sep;37(5):529–534. doi: 10.1093/geronj/37.5.529. [DOI] [PubMed] [Google Scholar]
- Ogmundsdóttir H. M., Weir D. M. Mechanisms of macrophage activation. Clin Exp Immunol. 1980 May;40(2):223–234. [PMC free article] [PubMed] [Google Scholar]
- Palmblad J., Haak A. Ageing does not change blood granulocyte bactericidal capacity and levels of complement factors 3 and 4. Gerontology. 1978;24(5):381–385. doi: 10.1159/000212275. [DOI] [PubMed] [Google Scholar]
- Rossi F., Zatti M. Effect of phagocytosis on the carbohydrate metabolism of polymorphonuclear leucocytes. Biochim Biophys Acta. 1966 May 26;121(1):110–119. doi: 10.1016/0304-4165(66)90353-9. [DOI] [PubMed] [Google Scholar]
- Schneider E. L. Infectious diseases in the elderly. Ann Intern Med. 1983 Mar;98(3):395–400. doi: 10.7326/0003-4819-98-3-395. [DOI] [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. Increased levels of cyclic adenosine-3',5'-monophosphate in human polymorphonuclear leukocytes after surface stimulation. J Clin Invest. 1980 May;65(5):1077–1085. doi: 10.1172/JCI109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabinsky Y., Bar-Shavit Z., Fridkin M., Goldman R. On the mechanism of action of the phagocytosis-stimulating peptide tuftsin. Mol Cell Biochem. 1980 Apr 18;30(2):71–77. doi: 10.1007/BF00227920. [DOI] [PubMed] [Google Scholar]
- Tam C. F., Walford R. L. Alterations in cyclic nucleotides and cyclase-specific activities in T lymphocytes of aging normal humans and patients with Down's syndrome. J Immunol. 1980 Oct;125(4):1665–1670. [PubMed] [Google Scholar]
- Vogel S. N., Weedon L. L., Oppenheim J. J., Rosenstreich D. L. Defective Fc-mediated phagocytosis in C3H/HeJ macrophages. II. Correction by cAMP agonists. J Immunol. 1981 Feb;126(2):441–445. [PubMed] [Google Scholar]
- Weissmann G., Dukor P., Zurier R. B. Effect of cyclic AMP on release of lysosomal enzymes from phagocytes. Nat New Biol. 1971 Jun 2;231(22):131–135. doi: 10.1038/newbio231131a0. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Boler J., Valdimarsson H. A chromium release assay for phagocytic killing of Candida albicans. J Immunol Methods. 1976;13(3-4):227–233. doi: 10.1016/0022-1759(76)90069-7. [DOI] [PubMed] [Google Scholar]


