Abstract
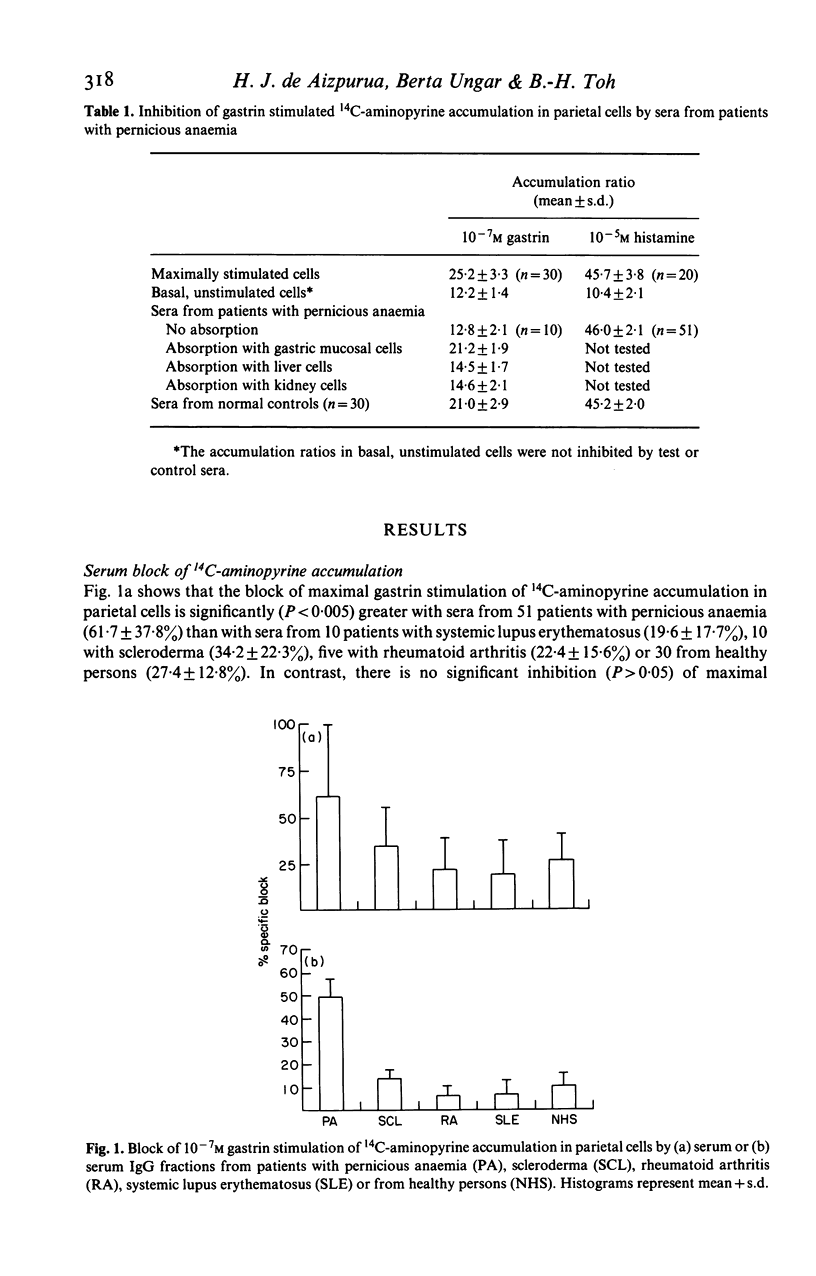
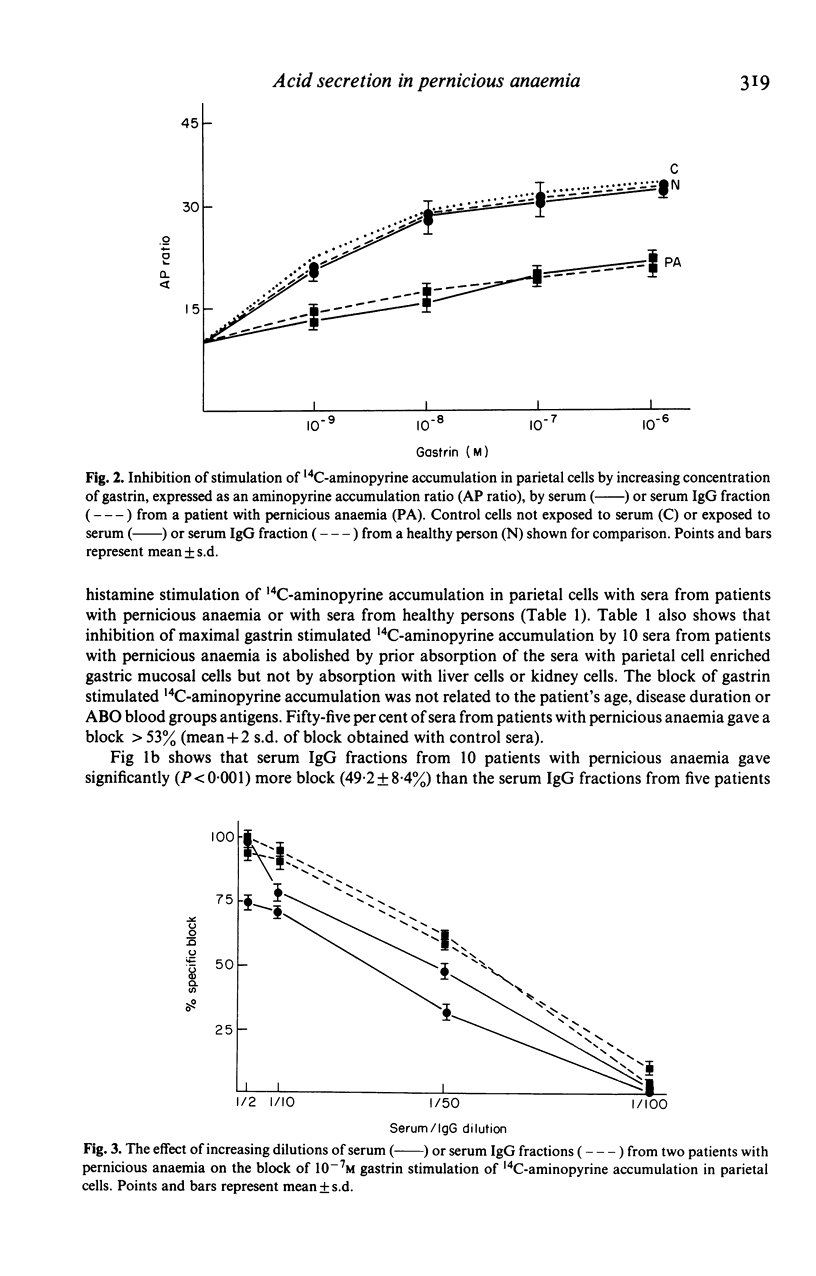
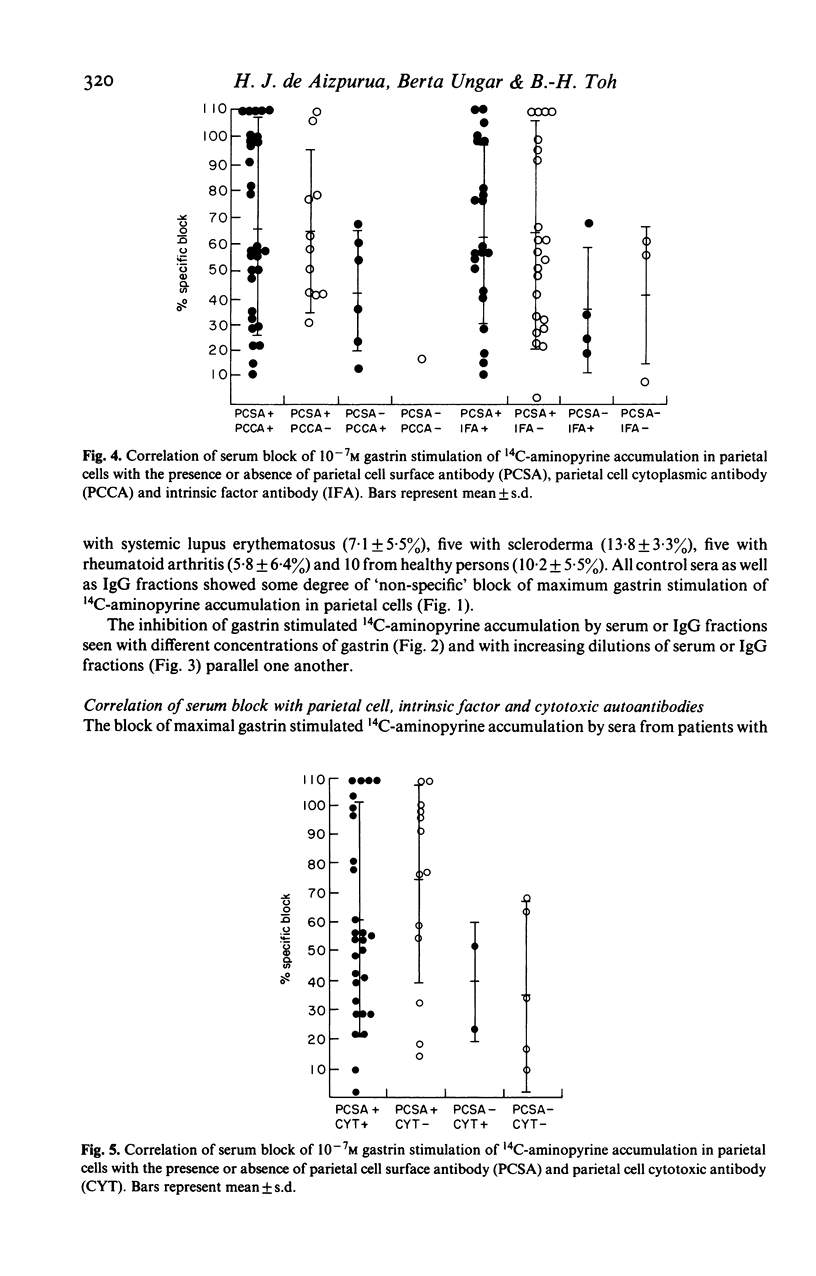
We examined 51 sera from patients with pernicious anaemia for their capacity to block maximal gastrin stimulation of acid secretion by isolated rodent gastric parietal cells. 14C-aminopyrine accumulation was used as the index of acid secretion in vitro. Sera from patients with pernicious anaemia gave significantly (P less than 0.005) more block of maximal gastrin stimulation of acid secretion (61.7 +/- 37.8%) than sera from 10 patients with systemic lupus erythematosus (19.6 +/- 17.7%), 10 with scleroderma (34.2 +/- 22.3%), five with rheumatoid arthritis (22.4 +/- 15.6%) or 30 from healthy persons (27.4 +/- 12.8%). Maximal histamine stimulation of acid secretion was not inhibited. The blocking factor was present in serum IgG fractions, and serum and IgG fractions gave parallel dose-response and dilution curves. The serum block was abolished by absorption with gastric mucosal cells and correlated with the presence of parietal cell surface autoantibody. We conclude that serum immunoglobulin in pernicious anaemia can block gastrin stimulation of acid secretion and suggest that this block may be mediated by competition with gastrin for surface receptors on parietal cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. D., Kennedy T. H. Evidence to suggest that LATS protector stimulates the human thyroid gland. J Clin Endocrinol Metab. 1971 Jul;33(1):47–51. doi: 10.1210/jcem-33-1-47. [DOI] [PubMed] [Google Scholar]
- Beall G. N., Chopra I. J., Solomon D. H., Kruger S. R. Serum protein inhibition of thyrotropin binding to human thyroid tissue. J Clin Endocrinol Metab. 1978 Nov;47(5):967–973. doi: 10.1210/jcem-47-5-967. [DOI] [PubMed] [Google Scholar]
- Berglindh T., Helander H. F., Obrink K. J. Effects of secretagogues on oxygen consumption, aminopyrine accumulation and morphology in isolated gastric glands. Acta Physiol Scand. 1976 Aug;97(4):401–414. doi: 10.1111/j.1748-1716.1976.tb10281.x. [DOI] [PubMed] [Google Scholar]
- Bins M., Burgers P. I., Schuyt-Van Etten T., Selbach S., van Wettum T. B., Poels L. G., Lamers C. B., van Tongeren J. H. Parietal cell antibodies in relation to basal serum gastrin in a normal population. Clin Exp Immunol. 1983 Sep;53(3):619–622. [PMC free article] [PubMed] [Google Scholar]
- Carnegie P. R., Mackay I. R. Vulnerability of cell-surface receptors to autoimmune reactions. Lancet. 1975 Oct 11;2(7937):684–687. doi: 10.1016/s0140-6736(75)90779-5. [DOI] [PubMed] [Google Scholar]
- Chew C. S., Hersey S. J. Gastrin stimulation of isolated gastric glands. Am J Physiol. 1982 May;242(5):G504–G512. doi: 10.1152/ajpgi.1982.242.5.G504. [DOI] [PubMed] [Google Scholar]
- De Aizpurua H. J., Cosgrove L. J., Ungar B., Toh B. H. Autoantibodies cytotoxic to gastric parietal cells in serum of patients with pernicious anemia. N Engl J Med. 1983 Sep 15;309(11):625–629. doi: 10.1056/NEJM198309153091102. [DOI] [PubMed] [Google Scholar]
- De Aizpurua H. J., Ungar B., Toh B. H. Flow microfluorimetric analysis of autoantibody reactions with parietal cell surface membranes in pernicious anaemia. Clin Exp Immunol. 1983 Nov;54(2):405–410. [PMC free article] [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Roth J., Bar R. S. Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science. 1975 Oct 3;190(4209):63–65. doi: 10.1126/science.170678. [DOI] [PubMed] [Google Scholar]
- JEFFRIES G. H. RECOVERY OF GASTRIC MUCOSAL STRUCTURE AND FUNCTION IN PERNICIOUS ANEMIA DURING PREDNISOLONE THERAPY. Gastroenterology. 1965 Mar;48:371–378. [PubMed] [Google Scholar]
- Kaye M. D., Whorwell P. J., Wright R. Gastric mucosal lymphocyte subpopulations in pernicious anemia and in normal stomach. Clin Immunol Immunopathol. 1983 Sep;28(3):431–440. doi: 10.1016/0090-1229(83)90110-1. [DOI] [PubMed] [Google Scholar]
- La Brooy J. T., Rowley D., Shearman D. J. Measurement of intestinal antibody by radioimmunoassay. Clin Exp Immunol. 1980 Aug;41(2):281–289. [PMC free article] [PubMed] [Google Scholar]
- Lennon V. A., Carnegie P. R. Immunopharmacological disease: a break in tolerance to receptor sites. Lancet. 1971 Mar 27;1(7700):630–633. doi: 10.1016/s0140-6736(71)91557-1. [DOI] [PubMed] [Google Scholar]
- Loveridge N., Bitensky L., Chayen J., Hausamen T. U., Fisher J. M., Taylor K. B., Gardner J. D., Bottazzo G. F., Doniach D. Inhibition of parietal cell function by human gammaglobulin containing gastric parietal cell antibodies. Clin Exp Immunol. 1980 Aug;41(2):264–270. [PMC free article] [PubMed] [Google Scholar]
- McGuigan J. E., Trudeau W. L. Serum gastrin concentrations in pernicious anemia. N Engl J Med. 1970 Feb 12;282(7):358–361. doi: 10.1056/NEJM197002122820703. [DOI] [PubMed] [Google Scholar]
- Mittag T., Kornfeld P., Tormay A., Woo C. Detection of anti-acetylcholine receptor factors in serum and thymus from patients with myasthenia gravis. N Engl J Med. 1976 Mar 25;294(13):691–694. doi: 10.1056/NEJM197603252941303. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Amirian D. A., Thomas L. P., Reedy T. J., Elashoff J. D. Gastrin receptors on isolated canine parietal cells. J Clin Invest. 1984 May;73(5):1434–1447. doi: 10.1172/JCI111348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H. Secretagogue stimulation of [14C]aminopyrine accumulation by isolated canine parietal cells. Am J Physiol. 1980 Apr;238(4):G366–G375. doi: 10.1152/ajpgi.1980.238.4.G366. [DOI] [PubMed] [Google Scholar]
- Strickland R. G., Mackay I. R. A reappraisal of the nature and significance of chronic atrophic gastritis. Am J Dig Dis. 1973 May;18(5):426–440. doi: 10.1007/BF01071995. [DOI] [PubMed] [Google Scholar]
- Venter J. C., Fraser C. M., Harrison L. C. Autoantibodies to beta 2-adrenergic receptors: a possible cause of adrenergic hyporesponsiveness in allergic rhinitis and asthma. Science. 1980 Mar 21;207(4437):1361–1363. doi: 10.1126/science.6153472. [DOI] [PubMed] [Google Scholar]
- de Aizpurua H. J., Toh B. H., Ungar B. Parietal cell surface reactive autoantibody in pernicious anaemia demonstrated by indirect membrane immunofluorescence. Clin Exp Immunol. 1983 May;52(2):341–349. [PMC free article] [PubMed] [Google Scholar]


