Abstract
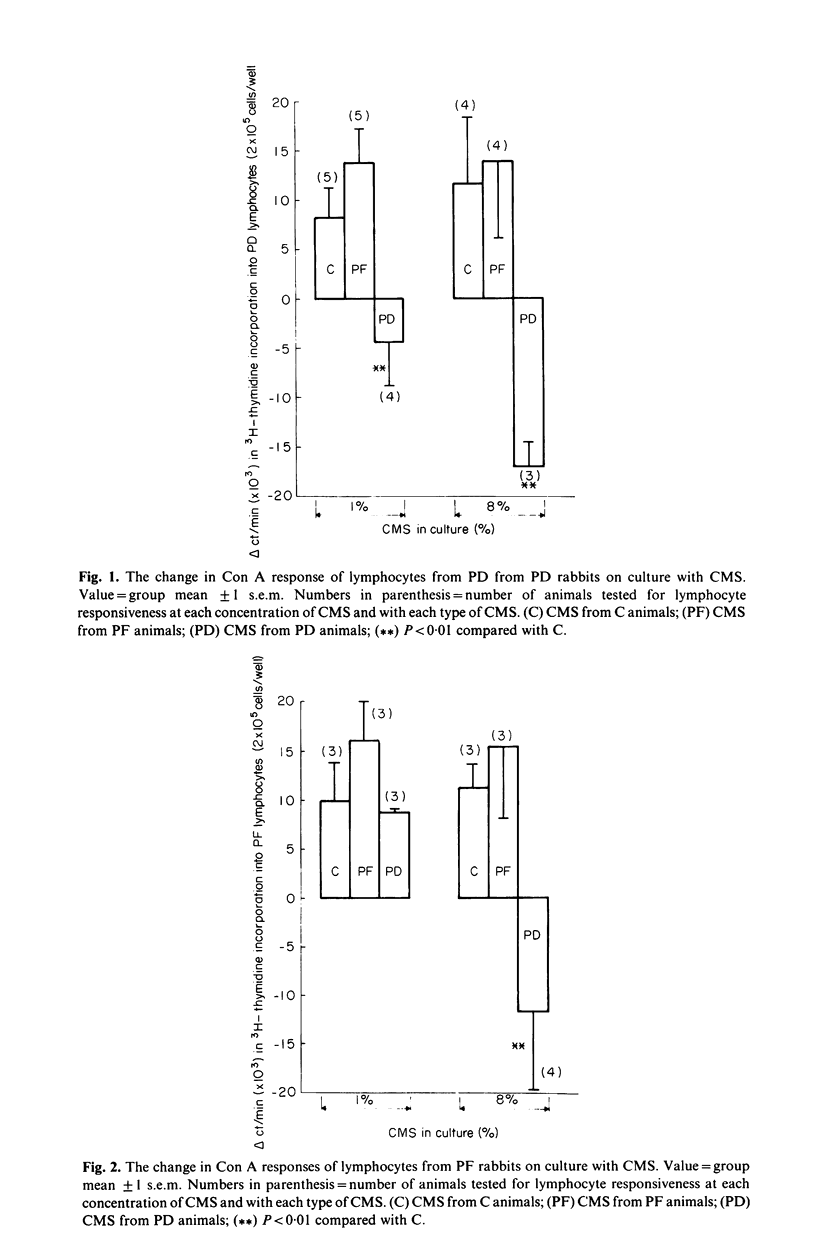
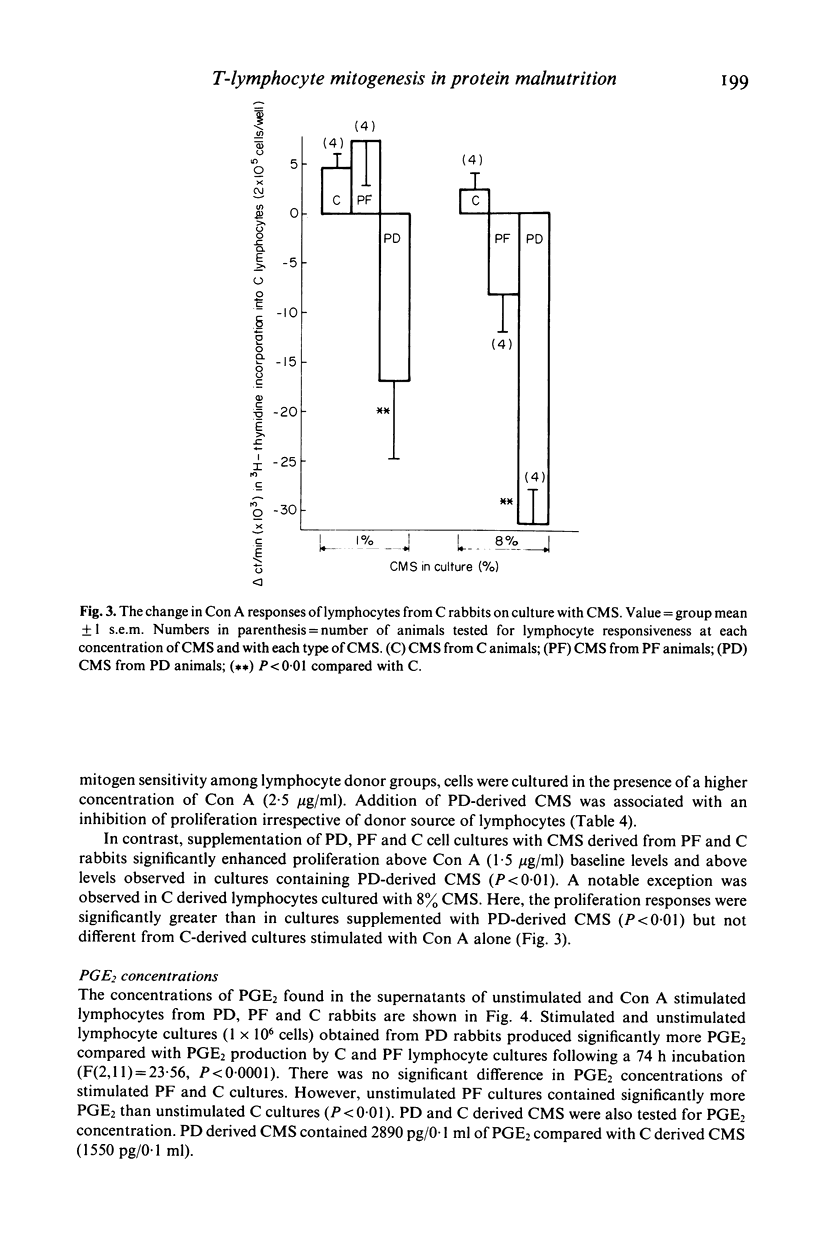
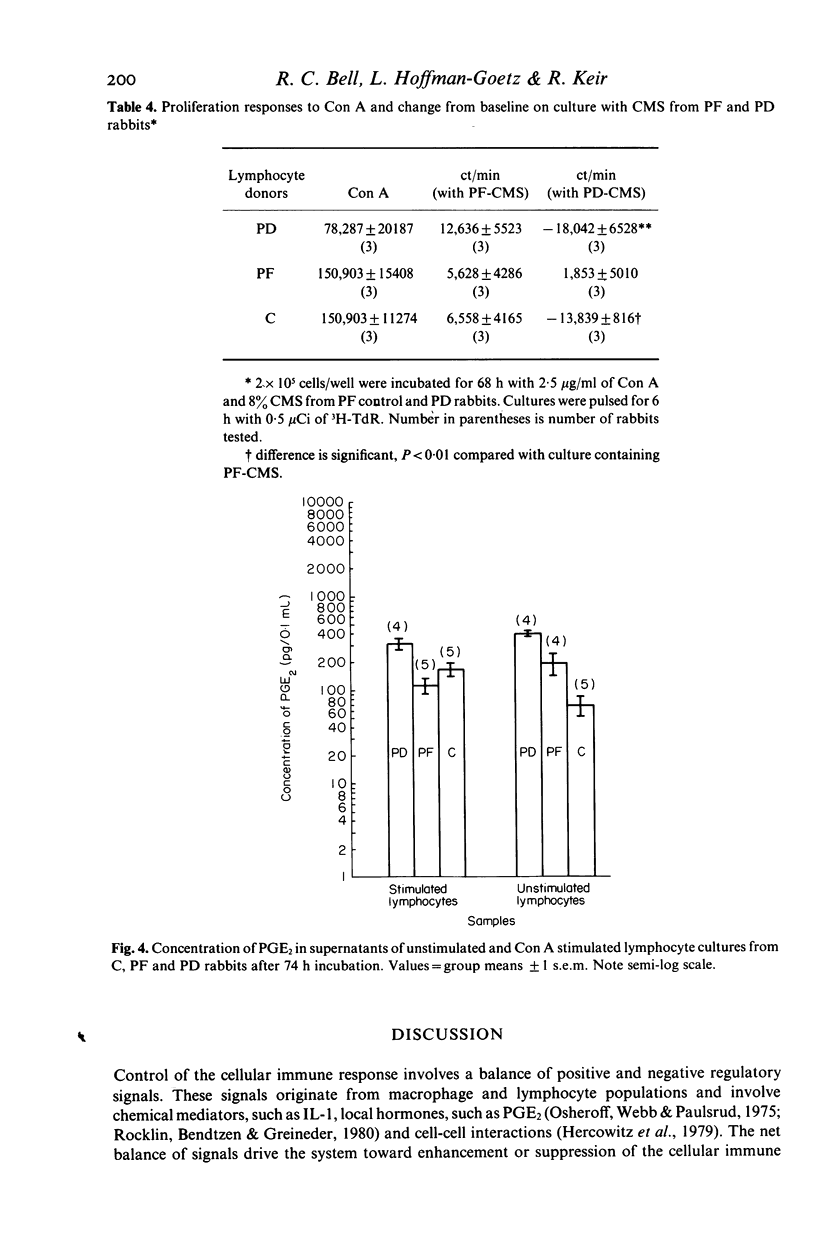
This study investigated changes in T-lymphocyte mitogenesis and immunoregulatory cytokines during protein malnutrition. In vitro T cell response to concanavalin A was compared among protein deprived (PD), energy restricted pair fed control (PF), and ad libitum control (C) rabbits. Cell cultures were supplemented with crude monocyte supernatants (CMS) from PD, PF or C animals at either 1% or 8% final concentration in culture. Prostaglandin E2 (PGE2) concentration of unstimulated or stimulated lymphocyte culture supernatants and CMS was determined. Lymphocyte cultures from PD, PF and C animals had enhanced 3H-thymidine incorporation when supplemented with C and/or PF derived CMS. Addition of 8% CMS from PD rabbits inhibited proliferation below levels observed in mitogen-only stimulated groups in all cultures. At the 1% concentration, inhibition was seen in PD and C derived cells cultures and modest enhancement was seen in PF cultures. PGE2 concentration in supernatants from stimulated and unstimulated lymphocyte cultures from PD rabbits were higher than in C and PF cell cultures. These results suggest (a) that under appropriate culture conditions lymphocytes from PD donors are capable of enhanced proliferation and (b) that depressed T cell mitogenesis observed in protein malnutrition may reflect alterations in immunoregulatory signals. The role of interleukin 1 (IL-1) and PGE2 in the modulation of this response is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandra R. K. Interactions of nutrition, infection and immune response. Immunocompetence in nutritional deficiency, methodological considerations and intervention strategies. Acta Paediatr Scand. 1979 Jan;68(1):137–144. doi: 10.1111/j.1651-2227.1979.tb04975.x. [DOI] [PubMed] [Google Scholar]
- Chandra R. K. Numerical and functional deficiency in T helper cells in protein energy malnutrition. Clin Exp Immunol. 1983 Jan;51(1):126–132. [PMC free article] [PubMed] [Google Scholar]
- Duff G. W., Durum S. K. The pyrogenic and mitogenic actions of interleukin-1 are related. Nature. 1983 Aug 4;304(5925):449–451. doi: 10.1038/304449a0. [DOI] [PubMed] [Google Scholar]
- Gross R. L., Newberne P. M. Role of nutrition in immunologic function. Physiol Rev. 1980 Jan;60(1):188–302. doi: 10.1152/physrev.1980.60.1.188. [DOI] [PubMed] [Google Scholar]
- Herman J., Rabson A. R. Prostaglandin E2 depresses natural cytotoxicity by inhibiting interleukin-1 production by large granular lymphocytes. Clin Exp Immunol. 1984 Aug;57(2):380–384. [PMC free article] [PubMed] [Google Scholar]
- Herscowitz H. B., Pennline K. J., Conrad R. E., Ullrich S. E., Gerber H. R. Macrophage-lymphocyte interactions mediated by soluble factors. Ann N Y Acad Sci. 1979;332:464–481. doi: 10.1111/j.1749-6632.1979.tb47141.x. [DOI] [PubMed] [Google Scholar]
- Hudspith B. N., Brostoff J., McNicol M. W., Johnson N. M. Anergy in sarcoidosis: the role of interleukin-1 and prostaglandins in the depressed in vitro lymphocyte response. Clin Exp Immunol. 1984 Aug;57(2):324–330. [PMC free article] [PubMed] [Google Scholar]
- Keenan R. A., Moldawer L. L., Yang R. D., Kawamura I., Blackburn G. L., Bistrian B. R. An altered response by peripheral leukocytes to synthesize or release leukocyte endogenous mediator in critically ill, protein-malnourished patients. J Lab Clin Med. 1982 Dec;100(6):844–857. [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W. Arachidonic acid metabolites regulate interleukin-1 production. Biochem Biophys Res Commun. 1985 Apr 30;128(2):892–897. doi: 10.1016/0006-291x(85)90130-5. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Koopman W. J., Wahl L. M., Dougherty S. F. Prostaglandin E2 rather than lymphocyte-activating factor produced by activated human mononuclear cells stimulates increases in murine thymocyte cAMP. Cell Immunol. 1980 Jan;49(1):64–73. doi: 10.1016/0008-8749(80)90056-8. [DOI] [PubMed] [Google Scholar]
- Osheroff P. L., Webb D. R., Paulsrud J. Induction of T-cell dependent splenic prostaglandin F2alpha by T-cell dependent antigen. Biochem Biophys Res Commun. 1975 Sep 2;66(1):425–429. doi: 10.1016/s0006-291x(75)80345-7. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Bendtzen K., Greineder D. Mediators of immunity: lymphokines and monokines. Adv Immunol. 1980;29:55–136. doi: 10.1016/s0065-2776(08)60043-7. [DOI] [PubMed] [Google Scholar]
- Rosenwasser L. J., Dinarello C. A. Ability of human leukocytic pyrogen to enhance phytohemagglutinin induced murine thymocyte proliferation. Cell Immunol. 1981 Sep 1;63(1):134–142. doi: 10.1016/0008-8749(81)90034-4. [DOI] [PubMed] [Google Scholar]
- Shellito J., Kaltreider H. B. Heterogeneity of immunologic function among subfractions of normal rat alveolar macrophages. Am Rev Respir Dis. 1984 May;129(5):747–753. doi: 10.1164/arrd.1984.129.5.747. [DOI] [PubMed] [Google Scholar]
- Watson R. R., Rister M., Baehner R. L. Superoxide dismutase activity in polymorphonuclear leukocytes and alveolar macrophages of protein malnourished rats and guinea pigs. J Nutr. 1976 Dec;106(12):1801–1808. doi: 10.1093/jn/106.12.1801. [DOI] [PubMed] [Google Scholar]
- Webb D. R., Rogers T. J., Nowowiejski I. Endogenous prostaglandin synthesis and the control of lymphocyte function. Ann N Y Acad Sci. 1979;332:262–270. doi: 10.1111/j.1749-6632.1979.tb47120.x. [DOI] [PubMed] [Google Scholar]


