Abstract
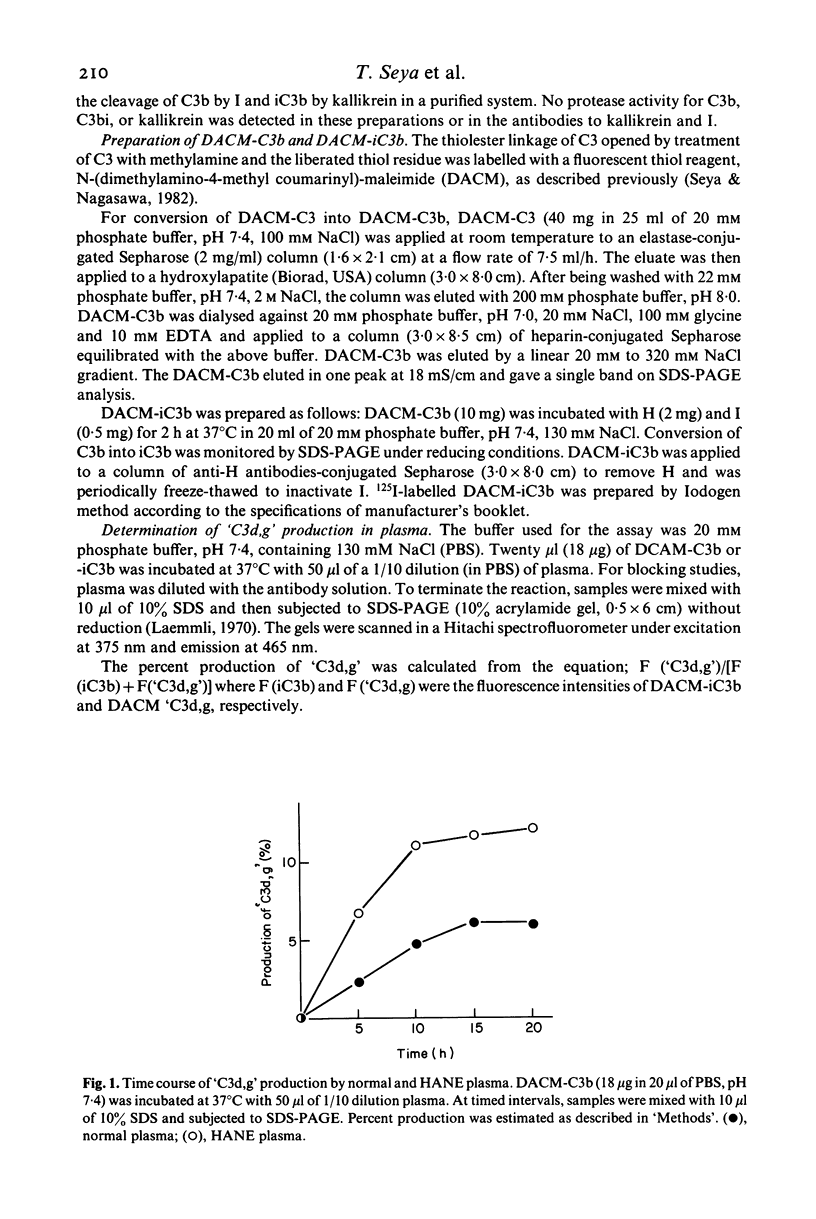
Recent studies have concluded that after complement activation the final physiologic degradation products of C3 are C3c and the fragment of relative molecular mass (Mr) 42,000 which contains the C3d and C3g domains and was therefore named C3d,g. Using fluorescent labelled C3b as a substrate, we have determined the putative C3d,g ('C3d,g') producing activity of both normal and hereditary angioneurotic oedema (HANE) plasmas. In normal plasmas, the rate of production of C3d,g was 1.0 +/- 0.2 X 10(-10) mol/ml/h and this activity was blocked by antibodies to I. In contrast, HANE, plasmas (deficient in C1INH) showed more than twice as much 'C3d,g' production as normal plasmas and both antibodies to I and kallikrein were required to inhibit this activity. Because of this result, a more sensitive gel system was employed to detect the Mr 42,000 peptide and two 'C3d,g' fragments of approximately equal intensity with Mr of 42,000 and 43,000 were defined. Incubation of purified kallikrein with labelled iC3b produced a C3d,g-like fragment, C3d-k, that aligned with the band of 43,000 Mr generated in HANE plasma. These results indicate that HANE plasma, in contrast to normal plasma, generates the bioactive C3d-k fragment. C1INH blocks the activities of kallikrein and C1s, and C3d-k generation in HANE plasma is probably secondary to the proteolytic activity of kallikrein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson J. P. Diagnosis and management of hereditary angioedema (HAE). Ann Allergy. 1979 Jun;42(6):348–352. [PubMed] [Google Scholar]
- Brandslund I., Siersted H. C., Svehag S. E., Teisner B. Double-decker rocket immunoelectrophoresis for direct quantitation of complement C3 split products with C3d specificities in plasma. J Immunol Methods. 1981;44(1):63–71. doi: 10.1016/0022-1759(81)90107-1. [DOI] [PubMed] [Google Scholar]
- Burdon K. L., Queng J. T., Thomas O. C., McGovern J. P. Observations on biochemical abnormalities in hereditary angioneurotic edema. J Allergy. 1965 Nov-Dec;36(6):546–557. doi: 10.1016/0021-8707(65)90192-9. [DOI] [PubMed] [Google Scholar]
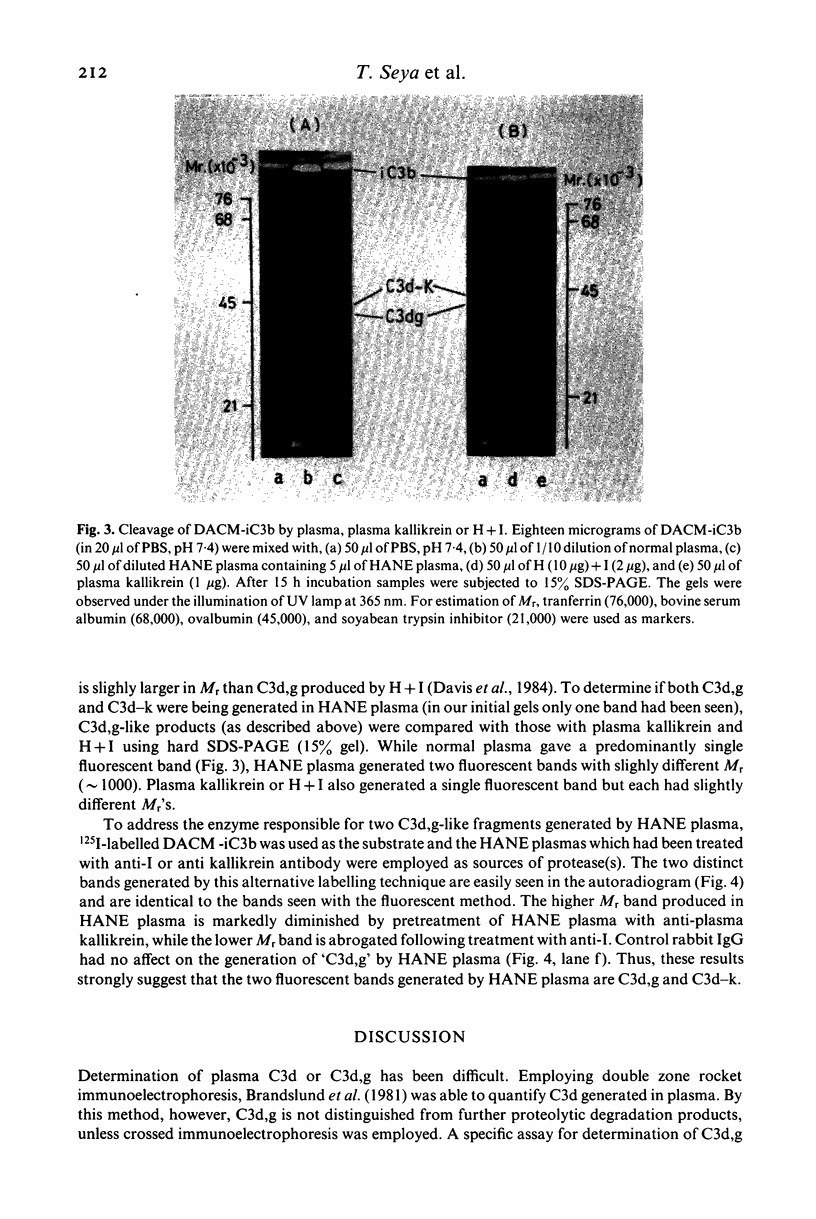
- Davis A. E., 3rd, Harrison R. A., Lachmann P. J. Physiologic inactivation of fluid phase C3b: isolation and structural analysis of C3c, C3d,g (alpha 2D), and C3g. J Immunol. 1984 Apr;132(4):1960–1966. [PubMed] [Google Scholar]
- Donaldson V. H., Ratnoff O. D., Dias Da Silva W., Rosen F. S. Permeability-increasing activity in hereditary angioneurotic edema plasma. II. Mechanism of formation and partial characterization. J Clin Invest. 1969 Apr;48(4):642–653. doi: 10.1172/JCI106022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykman T. R., Cole J. L., Iida K., Atkinson J. P. Polymorphism of human erythrocyte C3b/C4b receptor. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1698–1702. doi: 10.1073/pnas.80.6.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Initiation of C3 cleavage in the alternative complement pathway. J Immunol. 1975 Nov;115(5):1357–1361. [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Wong W. W. Complement ligand-receptor interactions that mediate biological responses. Annu Rev Immunol. 1983;1:243–271. doi: 10.1146/annurev.iy.01.040183.001331. [DOI] [PubMed] [Google Scholar]
- Fields T., Ghebrehiwet B., Kaplan A. P. Kinin formation in hereditary angioedema plasma: evidence against kinin derivation from C2 and in support of "spontaneous" formation of bradykinin. J Allergy Clin Immunol. 1983 Jul;72(1):54–60. doi: 10.1016/0091-6749(83)90052-0. [DOI] [PubMed] [Google Scholar]
- Frank M. M., Gelfand J. A., Atkinson J. P. Hereditary angioedema: the clinical syndrome and its management. Ann Intern Med. 1976 May;84(5):580–593. doi: 10.7326/0003-4819-84-5-580. [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B., Medicus R. G., Müller-Eberhard H. J. Potentiation of antiboty-dependent cell-mediated cytotoxicity by target cell-bound C3b. J Immunol. 1979 Sep;123(3):1285–1288. [PubMed] [Google Scholar]
- Granerus G., Hallberg L., Laurell A. B., Wetterquist H. Studies on the histamine metabolism and the complement system in hereditary angioneurotic edema. Acta Med Scand. 1967 Jul;182(1):11–22. doi: 10.1111/j.0954-6820.1967.tb11496.x. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr Effects of soluble immune complexes on Fc receptor- and C3b receptor-mediated phagocytosis by macrophages. J Exp Med. 1980 Oct 1;152(4):905–919. doi: 10.1084/jem.152.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDERMAN N. S., WEBSTER M. E., BECKER E. L., RATCLIFFE H. E. Hereditary angioneurotic edema. II. Deficiency of inhibitor for serum globulin permeability factor and/or plasma kallikrein. J Allergy. 1962 Jul-Aug;33:330–341. doi: 10.1016/0021-8707(62)90032-1. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Pangburn M. K., Oldroyd R. G. Breakdown of C3 after complement activation. Identification of a new fragment C3g, using monoclonal antibodies. J Exp Med. 1982 Jul 1;156(1):205–216. doi: 10.1084/jem.156.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Iida K., Mold C., Nussenzweig V. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J Exp Med. 1982 Dec 1;156(6):1739–1754. doi: 10.1084/jem.156.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth J. L., Morgan E. L., DiSipio R. G., Hugli T. E. Suppression of T lymphocyte functions by human C3 fragments. I. Inhibition of human T cell proliferative responses by a kallikrein cleavage fragment of human iC3b. J Immunol. 1983 Jun;130(6):2605–2611. [PubMed] [Google Scholar]
- Mori K., Nagasawa S. Studies on human high molecular weight (HMW) kininogen. II. Structural change of HMW kininogen by the action of human plasma kallikrein. J Biochem. 1981 May;89(5):1465–1473. doi: 10.1093/oxfordjournals.jbchem.a133339. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Ichihara C., Stroud R. M. Cleavage of C4b by C3b inactivator: production of a nicked form of C4b, C4b', as an intermediate cleavage product of C4b by C3b inactivator. J Immunol. 1980 Aug;125(2):578–582. [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Mechanism of action of the C3b inactivator: requirement for a high molecular weight cofactor (C3b-C4bINA cofactor) and production of a new C3b derivative (C3b'). Immunochemistry. 1977 Nov-Dec;14(11-12):749–756. doi: 10.1016/0019-2791(77)90345-7. [DOI] [PubMed] [Google Scholar]
- Nagase H., Barrett A. J. Human plasma kallikrein. A rapid purification method with high yield. Biochem J. 1981 Jan 1;193(1):187–192. doi: 10.1042/bj1930187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnoff O. D. The biology and pathology of the initial stages of blood coagulation. Prog Hematol. 1966;5:204–245. [PubMed] [Google Scholar]
- Ross G. D., Lambris J. D., Cain J. A., Newman S. L. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs CR1 cofactor activity. J Immunol. 1982 Nov;129(5):2051–2060. [PubMed] [Google Scholar]
- Schapira M., Scott C. F., Colman R. W. Contribution of plasma protease inhibitors to the inactivation of kallikrein in plasma. J Clin Invest. 1982 Feb;69(2):462–468. doi: 10.1172/JCI110470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T., Nagasawa S. A fluorometric method for determination of C3b inactivator. Clin Chim Acta. 1982 Mar 12;119(3):189–196. doi: 10.1016/0009-8981(82)90331-x. [DOI] [PubMed] [Google Scholar]
- Seya T., Nagasawa S. Limited proteolysis of complement protein C3b by regulatory enzyme C3b inactivator: isolation and characterization of a biologically active fragment, C3d,g. J Biochem. 1985 Jan;97(1):373–382. doi: 10.1093/oxfordjournals.jbchem.a135064. [DOI] [PubMed] [Google Scholar]
- Strunk R. C., Giclas P. C. Modulation of the activity of the classical complement pathway C3 convertase by surface-bound C3 or C5. J Immunol. 1980 Feb;124(2):520–526. [PubMed] [Google Scholar]
- Tack B. F., Morris S. C., Prahl J. W. Third component of human complement: structural analysis of the polypeptide chains of C3 and C3b. Biochemistry. 1979 Apr 17;18(8):1497–1503. doi: 10.1021/bi00575a017. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Nagasawa S., Koyama J. The NH-2-terminal sequences of a subunit of the first component of human complement, C1s, and its activated form, C1s. FEBS Lett. 1975 Feb 15;50(3):330–333. doi: 10.1016/0014-5793(75)80521-7. [DOI] [PubMed] [Google Scholar]
- Zeiger R. S., Twarog F. J., Colten H. R. Histaminase release from human granulocytes. J Exp Med. 1976 Oct 1;144(4):1049–1061. doi: 10.1084/jem.144.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaf F., Koedam J. A., Bouma B. N. Inactivation of kallikrein in human plasma. J Clin Invest. 1983 Jan;71(1):149–158. doi: 10.1172/JCI110743. [DOI] [PMC free article] [PubMed] [Google Scholar]