Abstract
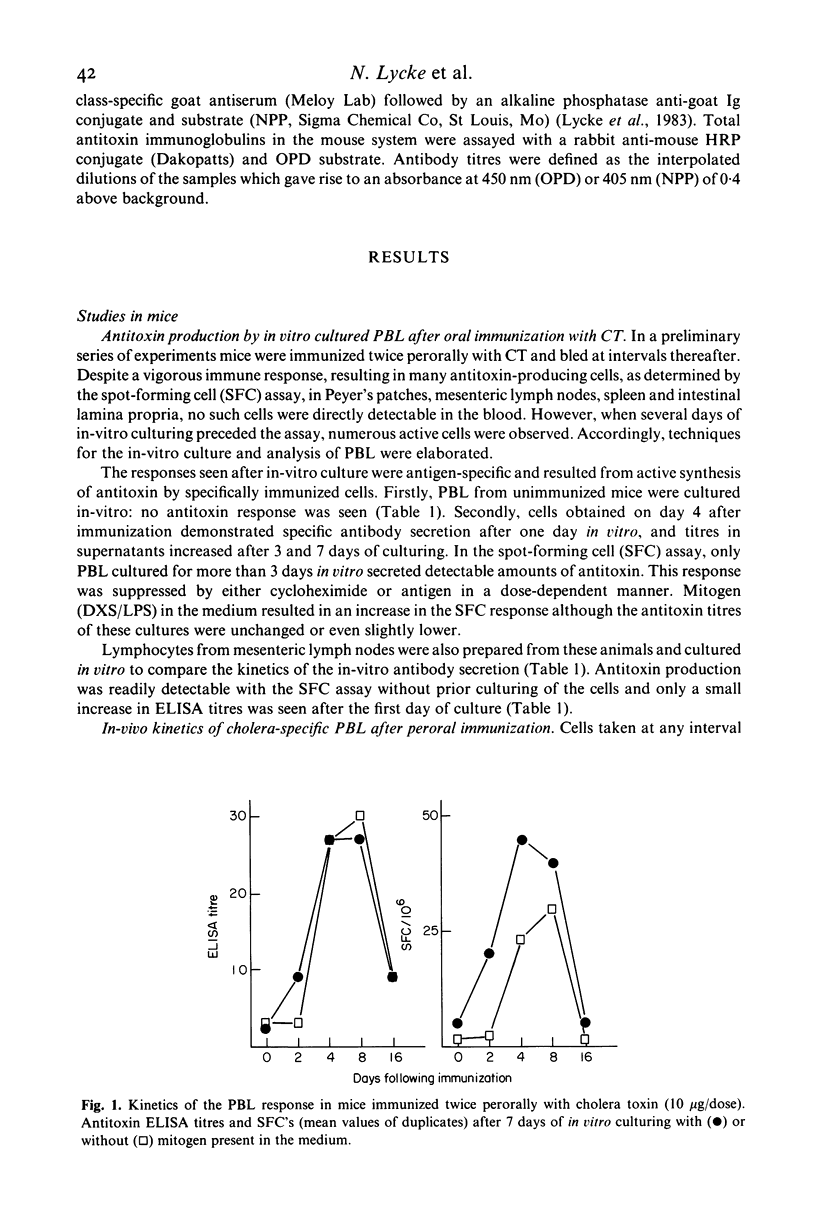
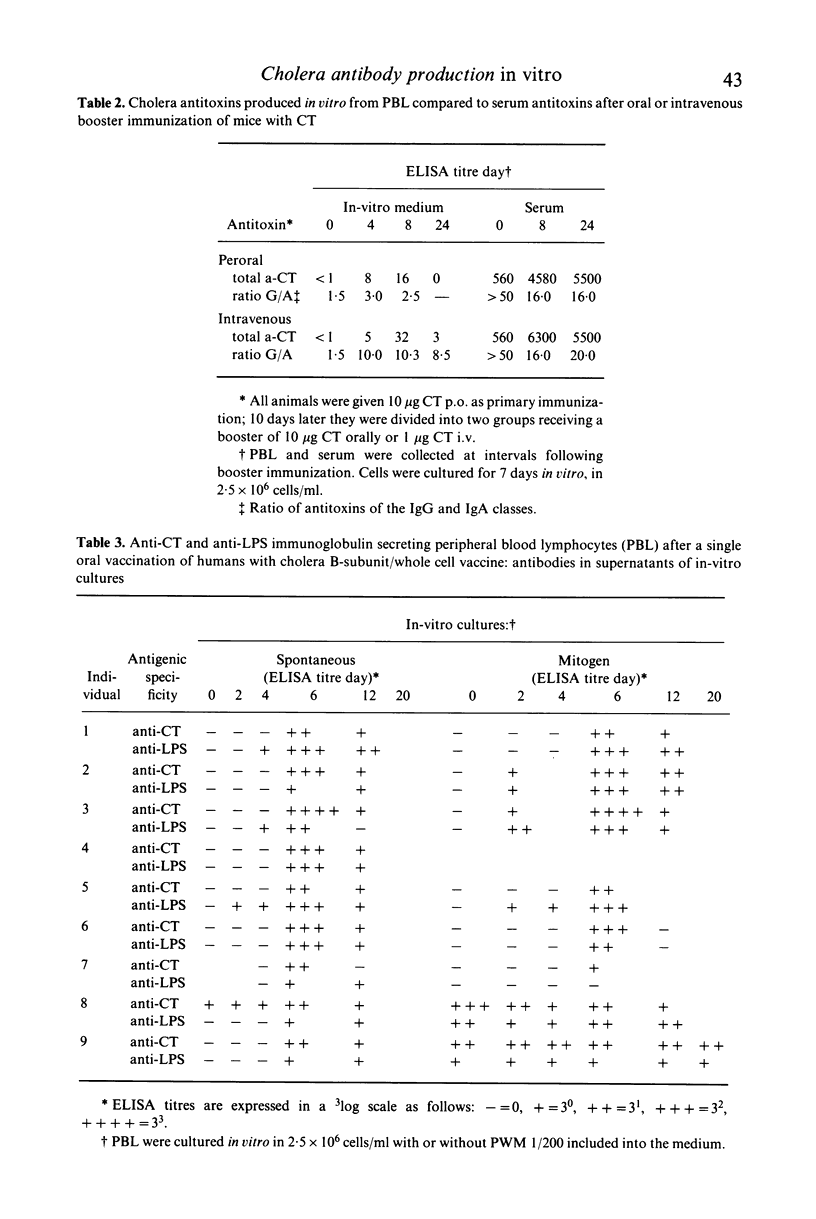
We have studied specific antibody production from peripheral blood lymphocytes (PBL) after oral cholera immunization of humans and mice. Two oral immunizations with cholera toxin (CT) in mice or a single dose of the combined cholera B-subunit/whole cell vaccine in humans gave rise to PBL which spontaneously secreted cholera-specific antibodies when cultured in vitro. A high proportion of IgA antibodies was seen in contrast to antibodies produced by PBL after parenteral immunization which were predominantly IgG. Cultured PBL produced antitoxin as well as anti-lipopolysaccharide antibodies after oral immunization, whereas serum only revealed titre rises for anti-CT. Antibody-secreting PBL appeared in the blood 2-4 days after immunization and persisted for about two weeks with a peak after 6-8 days. Mitogen stimulation in vitro of PBL from multiply-orally vaccinated humans activated a population of specific IgM antibody-secreting cells which persisted for several months following immunization, suggesting the presence of long-lived memory cells. The analysis of IgA antibody production from in-vitro cultured PBL seems to be a promising technique to assess the local immunogenicity of oral vaccines.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cash R. A., Music S. I., Libonati J. P., Craig J. P., Pierce N. F., Hornick R. B. Response of man to infection with Vibrio cholerae. II. Protection from illness afforded by previous disease and vaccine. J Infect Dis. 1974 Oct;130(4):325–333. doi: 10.1093/infdis/130.4.325. [DOI] [PubMed] [Google Scholar]
- Cupps T. R., Goldsmith P. K., Volkman D. J., Gerin J. L., Purcell R. H., Fauci A. S. Activation of human peripheral blood B cells following immunization with hepatitis B surface antigen vaccine. Cell Immunol. 1984 Jun;86(1):145–154. doi: 10.1016/0008-8749(84)90367-8. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C. C., Nilsson L. A., Nygren H., Ouchterlony O., Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Dec 16;65(1-2):109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- Gerrard T. L., Fauci A. S. Activation and immunoregulation of antigen-specific human b lymphocyte responses: multifaceted role of the monocyte. J Immunol. 1982 May;128(5):2367–2372. [PubMed] [Google Scholar]
- Kawanishi H., Strober W. T cell regulation of IgA immunoglobulin production in gut-associated lymphoid tissues. Mol Immunol. 1983 Sep;20(9):917–930. doi: 10.1016/0161-5890(83)90034-2. [DOI] [PubMed] [Google Scholar]
- Kettman J., Wetzel M. Antibody synthesis in vitro, a marker of B cell differentiation. J Immunol Methods. 1980;39(3):203–222. doi: 10.1016/0022-1759(80)90056-3. [DOI] [PubMed] [Google Scholar]
- Lange S., Holmgren J. Protective antitoxic cholera immunity in mice: influence of route and number of immunizations and mode of action of protective antibodies. Acta Pathol Microbiol Scand C. 1978 Aug;86C(4):145–152. doi: 10.1111/j.1699-0463.1978.tb02572.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Lindholm L., Holmgren J. IgA isotype restriction in the mucosal but not in the extramucosal immune response after oral immunizations with cholera toxin or cholera B subunit. Int Arch Allergy Appl Immunol. 1983;72(2):119–127. doi: 10.1159/000234853. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mitchell D. M., Fitzharris P., Knight R. A., Schild G. C. Kinetics of specific in vitro antibody production following influenza immunization. Clin Exp Immunol. 1982 May;48(2):491–498. [PMC free article] [PubMed] [Google Scholar]
- Peters M., Fauci A. S. Antigen-specific human B-cell responses: modulation by immunoregulatory T-cell subsets. Cell Immunol. 1983 Dec;82(2):223–231. doi: 10.1016/0008-8749(83)90157-0. [DOI] [PubMed] [Google Scholar]
- Peters M., Fauci A. S. Selective activation of antigen-specific human B cells in recently immunized individuals by nonspecific factors in the absence of antigen. J Immunol. 1983 Feb;130(2):678–680. [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr Determinants of the localization, magnitude, and duration of a specific mucosal IgA plasma cell response in enterically immunized rats. J Immunol. 1982 Mar;128(3):1311–1315. [PubMed] [Google Scholar]
- Stevens R. H., Macy E., Morrow C., Saxon A. Characterization of a circulating subpopulation of spontaneous antitetanus toxoid antibody producing B cells following in vivo booster immunization. J Immunol. 1979 Jun;122(6):2498–2504. [PubMed] [Google Scholar]
- Stevens R. H., Saxon A. Differential synthesis of IgM and IgA anti-tetanus toxoid antibody in vitro following in vivo booster immunization of humans. Cell Immunol. 1979 Jun;45(1):142–150. doi: 10.1016/0008-8749(79)90369-1. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Jertborn M., Gothefors L., Karim A. M., Sack D. A., Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984 Jun;149(6):884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- Thomson P. D., Harris N. S. Detection of plaque-forming cells in the peripheral blood of actively immunized humans. J Immunol. 1977 Apr;118(4):1480–1482. [PubMed] [Google Scholar]
- Yarchoan R., Murphy B. R., Strober W., Clements M. L., Nelson D. L. In vitro production of anti-influenza virus antibody after intranasal inoculation with cold-adapted influenza virus. J Immunol. 1981 Nov;127(5):1958–1963. [PubMed] [Google Scholar]


