Abstract
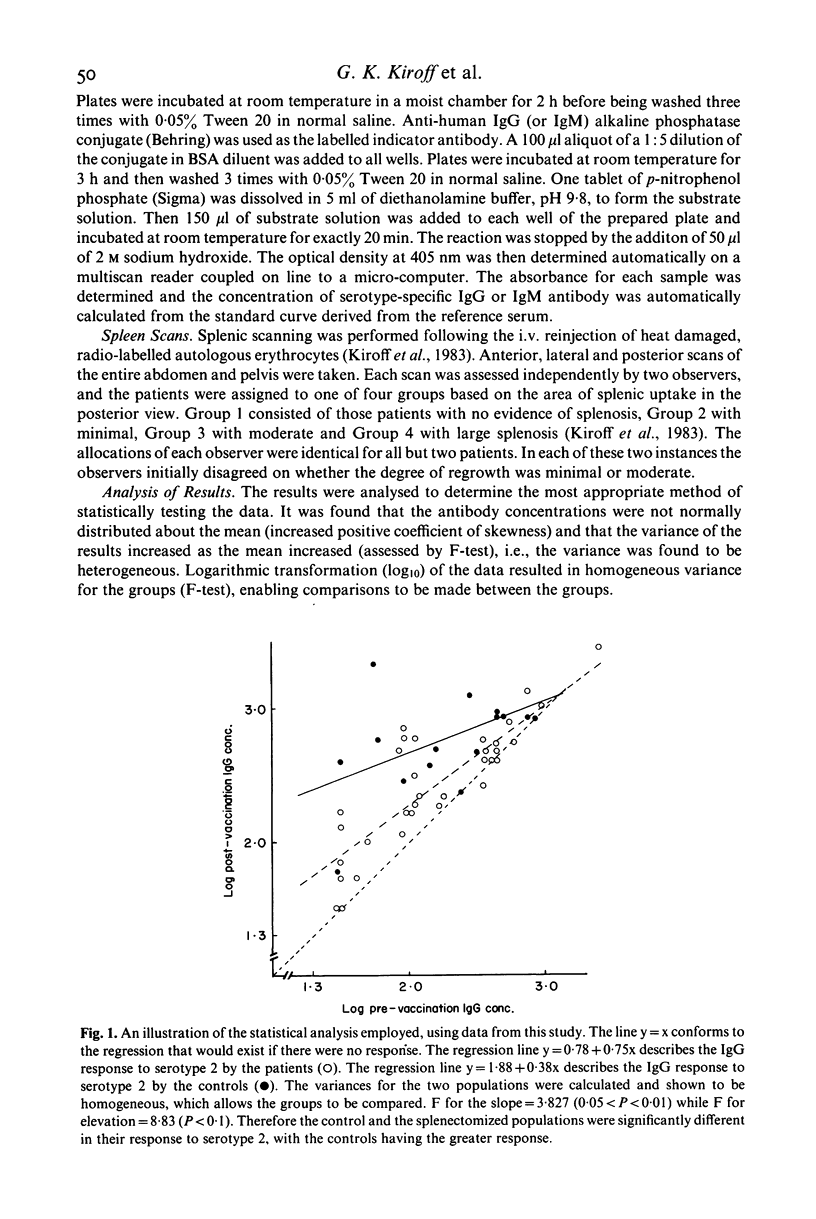
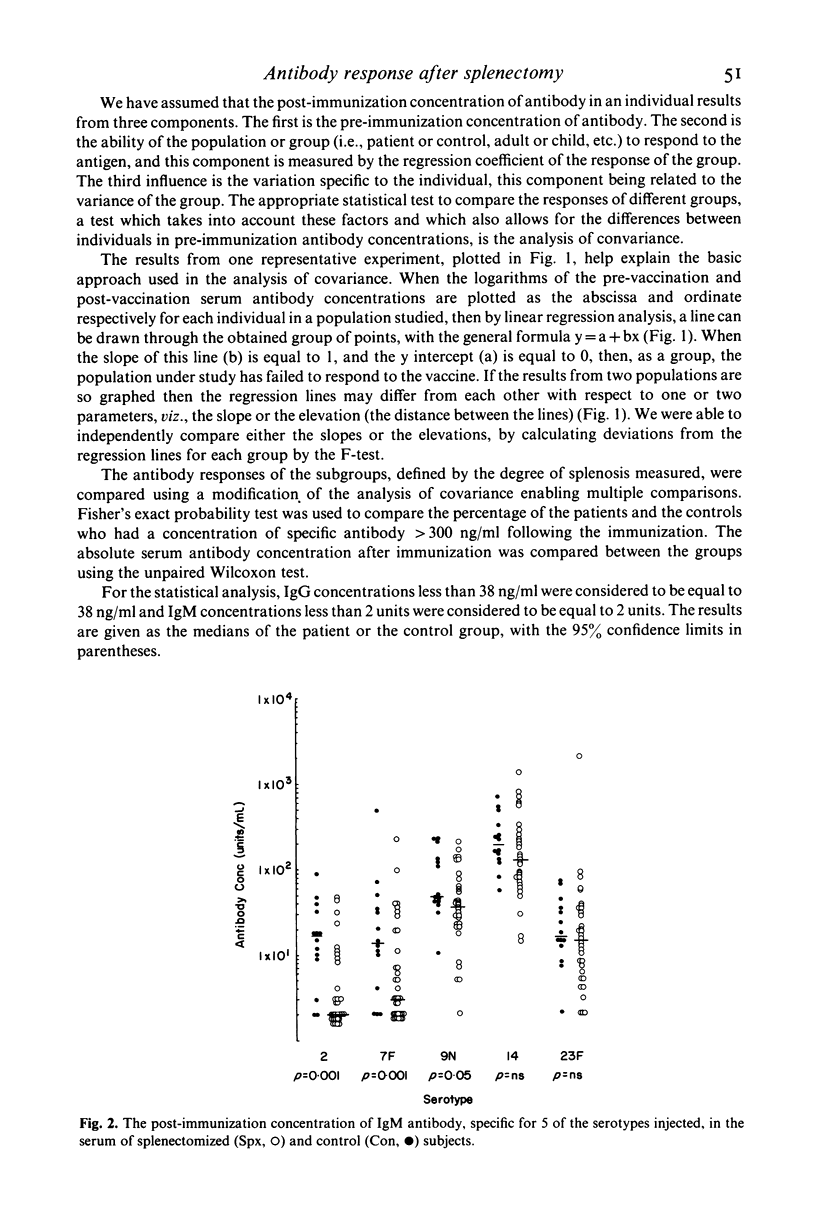
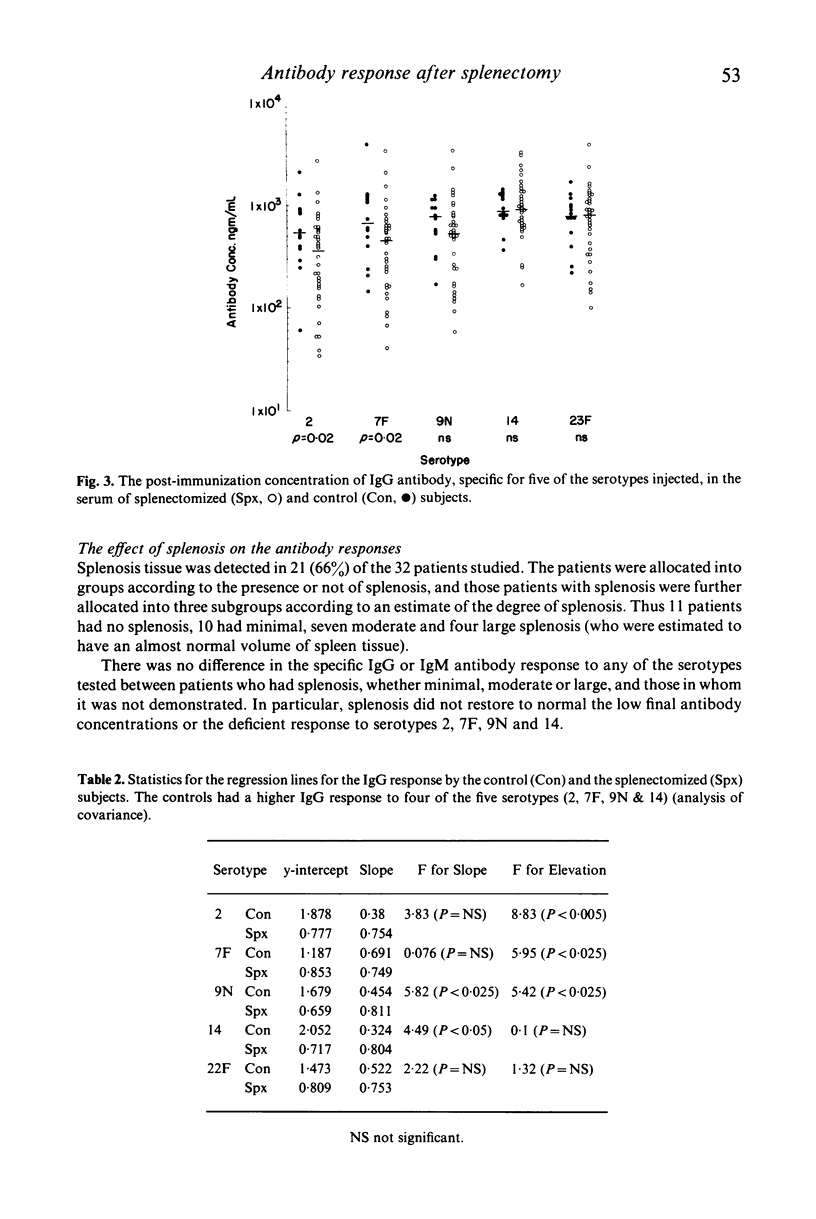
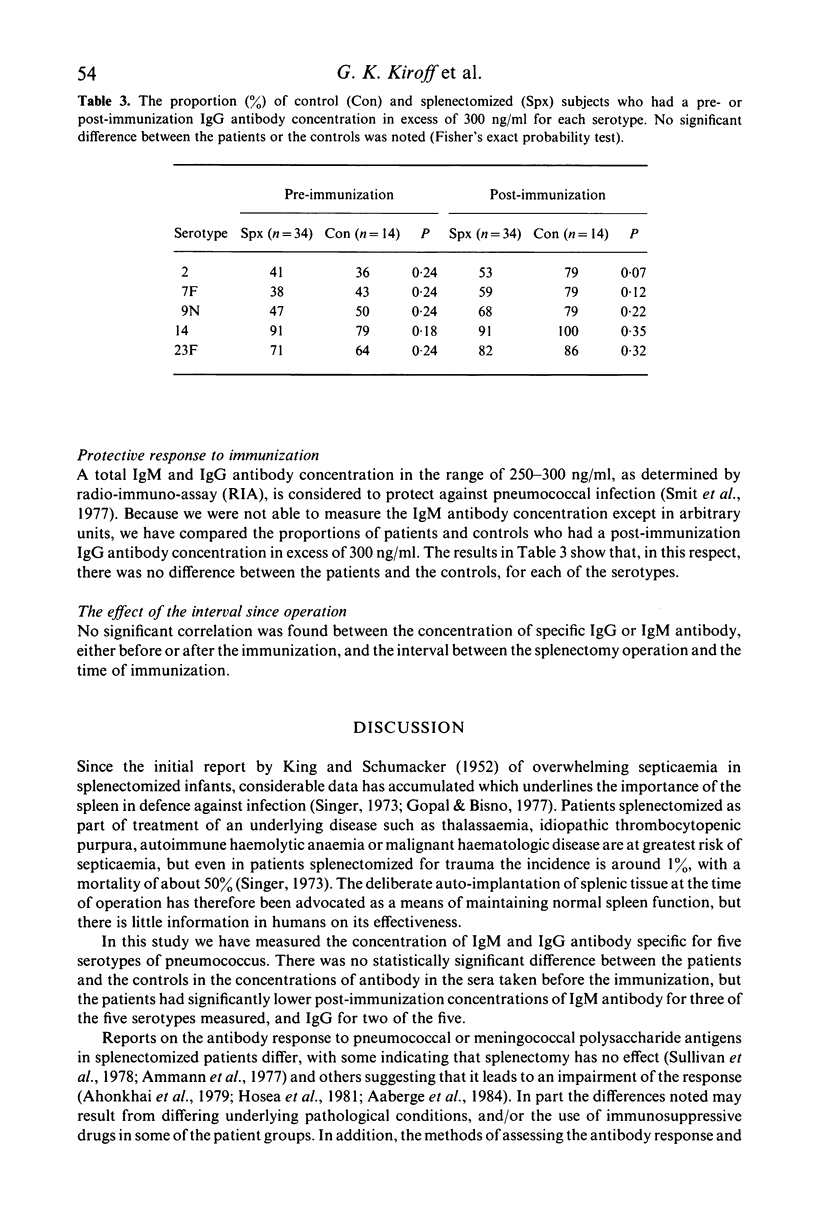
The experiments were to determine if ectopic splenic tissue in humans would restore to normal those antibody responses which are reduced in patients who have been splenectomized. The IgM and IgG antibody response to subcutaneous injection of polyvalent pneumococcal polysaccharide vaccine (PNEUMOVAX) was determined in 34 patients who had been splenectomized for trauma and 14 controls, by measuring the concentration of antibody specific for five of the serotypes in the vaccine in serum samples taken before and 1 month after the immunization. The patients had significantly lower post-immunization concentrations of IgM antibody for three of the five serotypes measured, and IgG for two of the five. The antibody response to the immunization was assessed by comparing the post- to the pre-immunization concentration of antibody by analysis of covariance. The patients had a significantly lower IgM response to three of the five serotypes measured and IgG response to four of the five. It is concluded that in adult humans the spleen is important in the maintenance of normal humoral immune responses. The presence and degree of ectopic splenic regrowth (splenosis) in the splenectomized patients was assessed by a spleen-specific radio-isotopic scan. There was no difference between patients with splenosis and those without, or between those with different degrees of splenosis, in any of the parameters of the antibody response measured. This is in vivo evidence indicating that ectopic splenic tissue in humans does not normalize the altered antibody responses observed following splenectomy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammann A. J., Addiego J., Wara D. W., Lubin B., Smith W. B., Mentzer W. C. Polyvalent pneumococcal-polysaccharide immunization of patients with sickle-cell anemia and patients with splenectomy. N Engl J Med. 1977 Oct 27;297(17):897–900. doi: 10.1056/NEJM197710272971701. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Frank M. M. The role of the spleen in experimental pneumococcal bacteremia. J Clin Invest. 1981 Apr;67(4):975–982. doi: 10.1172/JCI110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew P. A., Kiroff G. K., Ferrante A., Cohen R. C. Alterations in immunoglobulin synthesis by peripheral blood mononuclear cells from splenectomized patients with and without splenic regrowth. J Immunol. 1984 Jan;132(1):191–196. [PubMed] [Google Scholar]
- Ferrante A., Kiroff G. K., Goh D. H., Drew P. A. Elevated natural killer (NK) cell activity: a possible role in resistance to infection and malignancy in immunodeficient splenectomized patients. Med Hypotheses. 1985 Feb;16(2):133–146. doi: 10.1016/0306-9877(85)90067-2. [DOI] [PubMed] [Google Scholar]
- Gopal V., Bisno A. L. Fulminant pneumococcal infections in 'normal' asplenic hosts. Arch Intern Med. 1977 Nov;137(11):1526–1530. [PubMed] [Google Scholar]
- Hosea S. W., Burch C. G., Brown E. J., Berg R. A., Frank M. M. Impaired immune response of splenectomised patients to polyvalent pneumococcal vaccine. Lancet. 1981 Apr 11;1(8224):804–807. doi: 10.1016/s0140-6736(81)92681-7. [DOI] [PubMed] [Google Scholar]
- KING H., SHUMACKER H. B., Jr Splenic studies. I. Susceptibility to infection after splenectomy performed in infancy. Ann Surg. 1952 Aug;136(2):239–242. doi: 10.1097/00000658-195208000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiroff G. K., Mangos A., Cohen R., Chatterton B. E., Jamieson G. G. Splenic regeneration following splenectomy for traumatic rupture. Aust N Z J Surg. 1983 Oct;53(5):431–434. doi: 10.1111/j.1445-2197.1983.tb02479.x. [DOI] [PubMed] [Google Scholar]
- Kusminsky R. E., Chang H. H., Hossino H., Zekan S. M., Boland J. P. An omental implantation technique for salvage of the spleen. Surg Gynecol Obstet. 1982 Sep;155(3):407–408. [PubMed] [Google Scholar]
- Likhite V. V. Protection against fulminant sepsis in splenectomized mice by implantation of autochthonous splenic tissue. Exp Hematol. 1978 May;6(5):433–439. [PubMed] [Google Scholar]
- Moore G. E., Stevens R. E., Moore E. E., Aragon G. E. Failure of splenic implants to protect against fatal postsplenectomy infection. Am J Surg. 1983 Sep;146(3):413–414. doi: 10.1016/0002-9610(83)90430-0. [DOI] [PubMed] [Google Scholar]
- Rice H. M., James P. D. Ectopic splenic tissue failed to prevent fatal pneumococcal septicaemia after splenectomy for trauma. Lancet. 1980 Mar 15;1(8168 Pt 1):565–566. doi: 10.1016/s0140-6736(80)91056-9. [DOI] [PubMed] [Google Scholar]
- Schwartz A. D., Dadash-Zadeh M., Goldstein R., Luck S., Conway J. J. Antibody response to intravenous immunization following splenic tissue autotransplantation in Sprague-Dawley rats. Blood. 1977 May;49(5):779–783. [PubMed] [Google Scholar]
- Schwartz A. D., Goldthorn J. F., Winkelstein J. A., Swift A. J. Lack of protective effect of autotransplanted splenic tissue to pneumococcal challenge. Blood. 1978 Mar;51(3):475–478. [PubMed] [Google Scholar]
- Singer D. B. Postsplenectomy sepsis. Perspect Pediatr Pathol. 1973;1:285–311. [PubMed] [Google Scholar]
- Smit P., Oberholzer D., Hayden-Smith S., Koornhof H. J., Hilleman M. R. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA. 1977 Dec 12;238(24):2613–2616. [PubMed] [Google Scholar]
- Sullivan J. L., Ochs H. D., Schiffman G., Hammerschlag M. R., Miser J., Vichinsky E., Wedgwood R. J. Immune response after splenectomy. Lancet. 1978 Jan 28;1(8057):178–181. doi: 10.1016/s0140-6736(78)90612-8. [DOI] [PubMed] [Google Scholar]


