Abstract
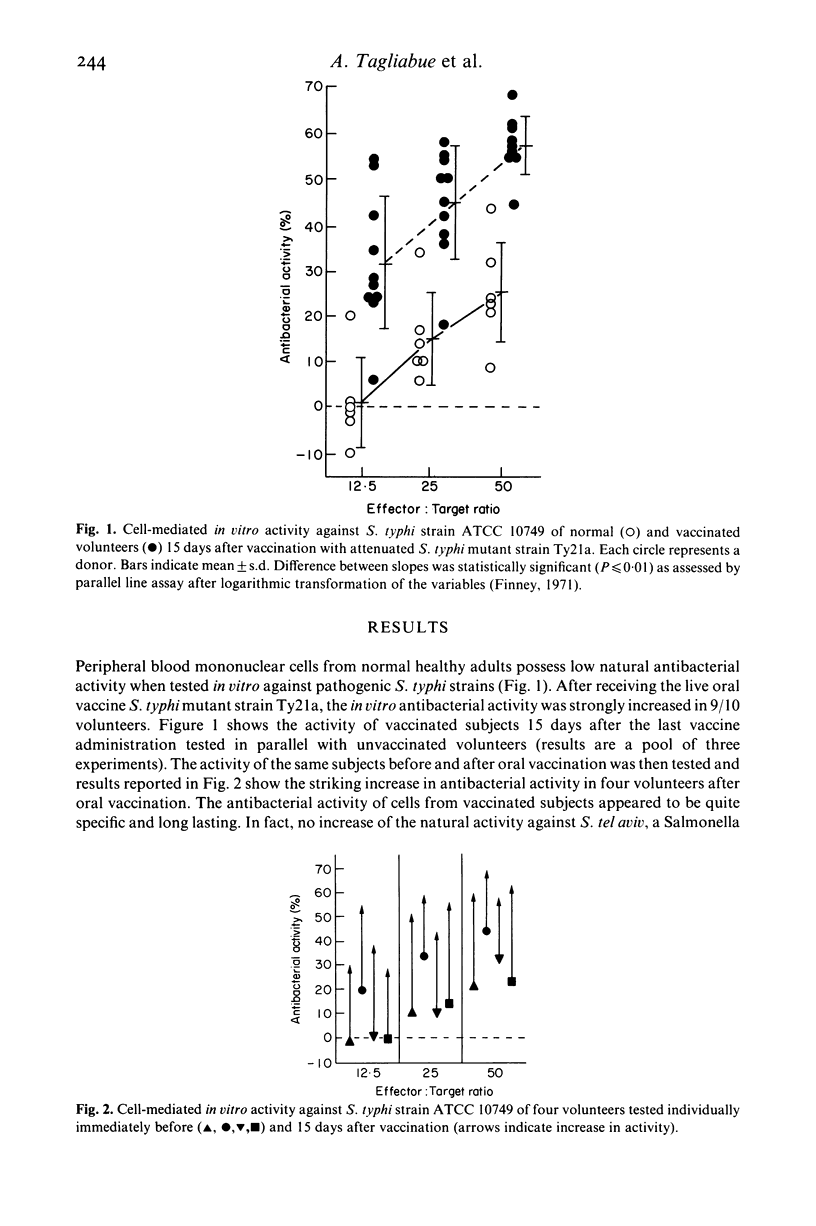
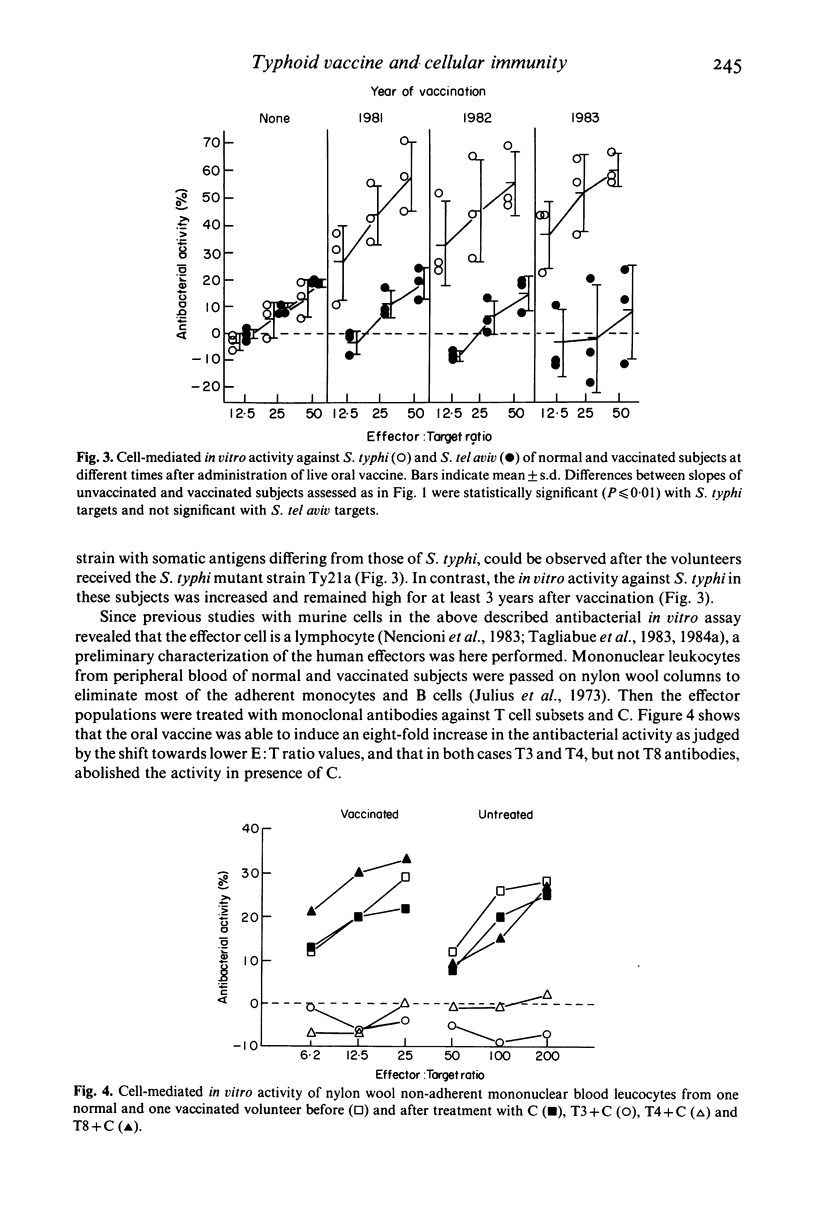
Seventeen adult volunteers were vaccinated orally with the live attenuated Salmonella typhi mutant strain Ty21a. Their peripheral blood mononuclear cells were tested at different times after vaccination for direct cell-mediated activity against bacteria, employing a simple short-term in vitro assay. It was observed that 16/17 of the vaccinated subjects acquired the capacity to express specific cellular immunity against S. typhi which lasted from 15 days to at least 3 years. The effector cell of the in vitro antibacterial activity was preliminarily characterized as a non-adherent T3+, T8-, T4+ lymphocyte. In parallel, mice immunized orally with S. typhimurium and proving resistant to reinfection were tested employing the same in vitro assay. Also in this case peripheral and, most important, intestinal lymphocytes were able to express cellular immunity against the agent of murine typhoid. It is concluded that administration of live oral vaccine against S. typhi results in the induction of specific cellular immunity which is expressed at the peripheral and, probably, also at the intestinal level.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Germanier R., Füer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975 May;131(5):553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- Hirschel B. Failures with oral typhoid vaccine Ty 21a. Lancet. 1983 Apr 9;1(8328):817–818. doi: 10.1016/s0140-6736(83)91870-6. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Nencioni L., Villa L., Boraschi D., Berti B., Tagliabue A. Natural and antibody-dependent cell-mediated activity against Salmonella typhimurium by peripheral and intestinal lymphoid cells in mice. J Immunol. 1983 Feb;130(2):903–907. [PubMed] [Google Scholar]
- Tagliabue A., Boraschi D., Villa L., Keren D. F., Lowell G. H., Rappuoli R., Nencioni L. IgA-dependent cell-mediated activity against enteropathogenic bacteria: distribution, specificity, and characterization of the effector cells. J Immunol. 1984 Aug;133(2):988–992. [PubMed] [Google Scholar]
- Tagliabue A., Nencioni L., Villa L., Boraschi D. Genetic control of in vitro natural cell-mediated activity against Salmonella typhimurium by intestinal and splenic lymphoid cells in mice. Clin Exp Immunol. 1984 Jun;56(3):531–536. [PMC free article] [PubMed] [Google Scholar]
- Tagliabue A., Nencioni L., Villa L., Keren D. F., Lowell G. H., Boraschi D. Antibody-dependent cell-mediated antibacterial activity of intestinal lymphocytes with secretory IgA. Nature. 1983 Nov 10;306(5939):184–186. doi: 10.1038/306184a0. [DOI] [PubMed] [Google Scholar]
- Wahdan M. H., Sérié C., Cerisier Y., Sallam S., Germanier R. A controlled field trial of live Salmonella typhi strain Ty 21a oral vaccine against typhoid: three-year results. J Infect Dis. 1982 Mar;145(3):292–295. doi: 10.1093/infdis/145.3.292. [DOI] [PubMed] [Google Scholar]


