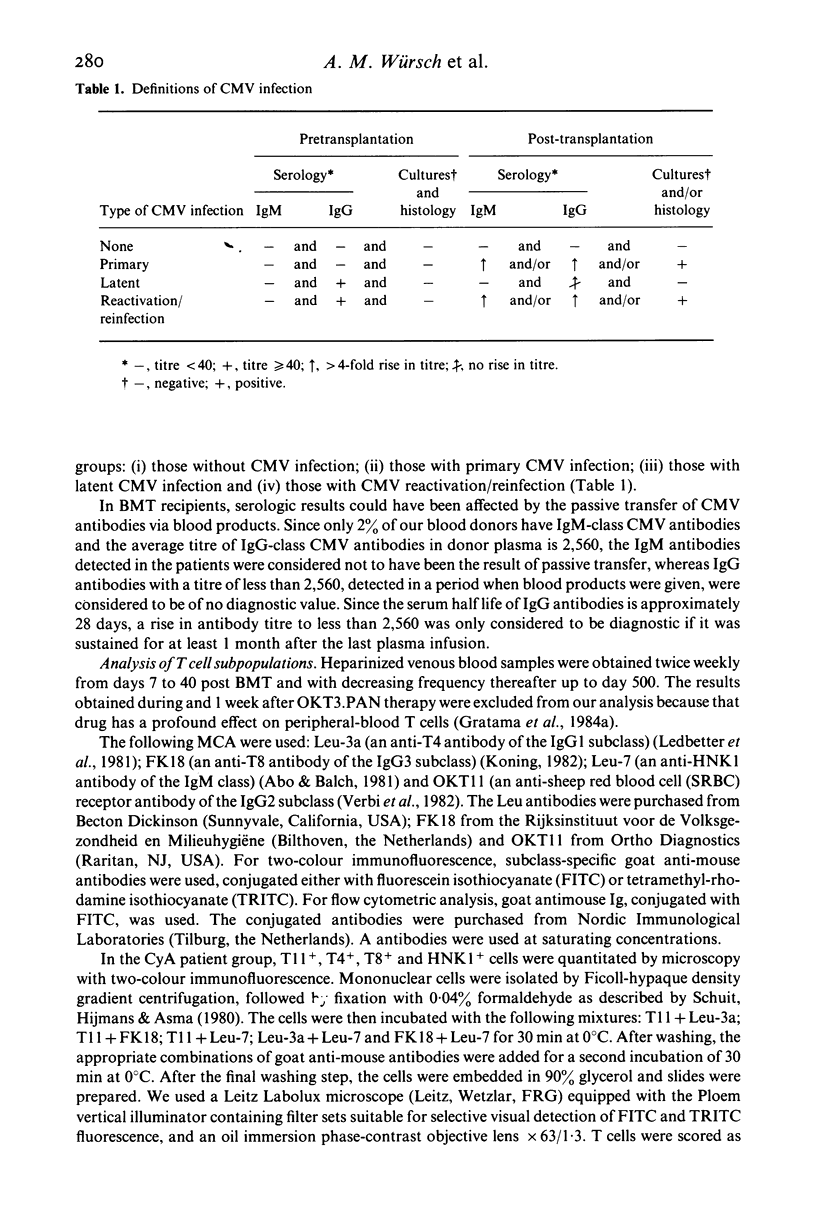
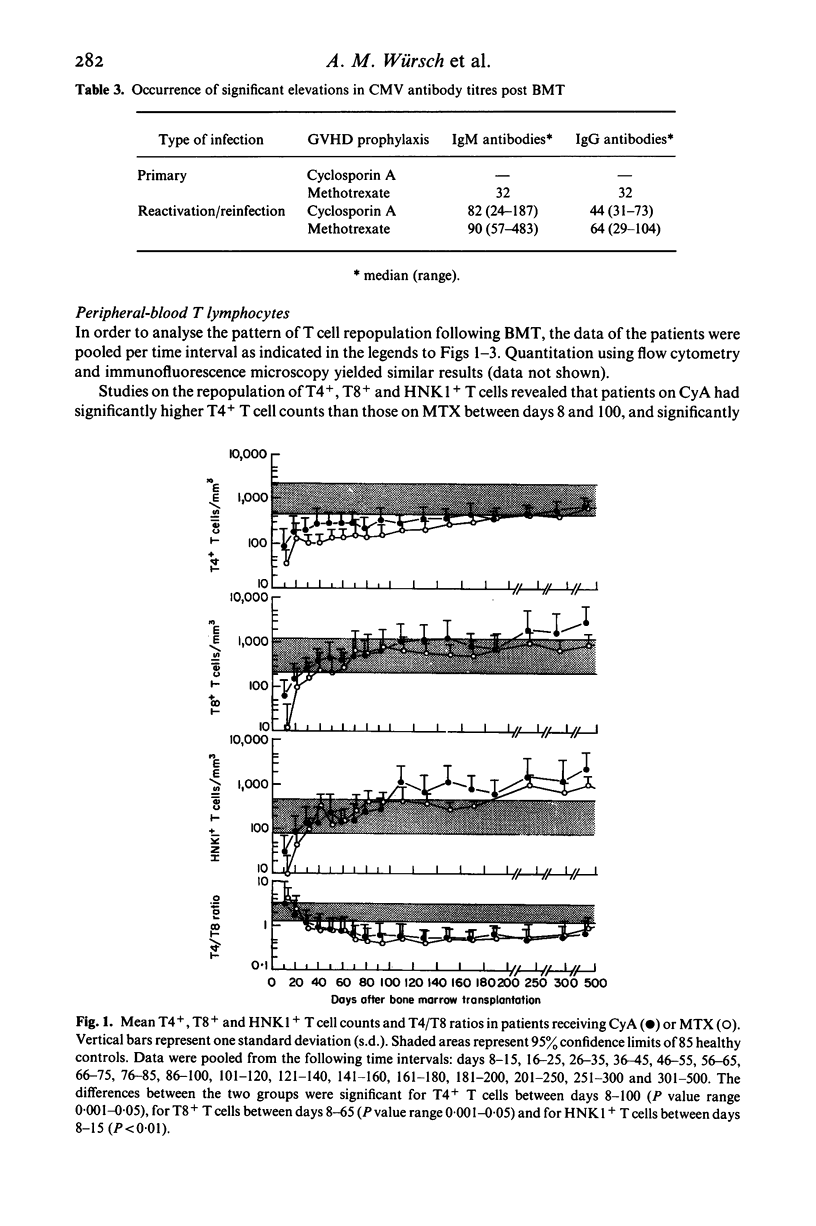
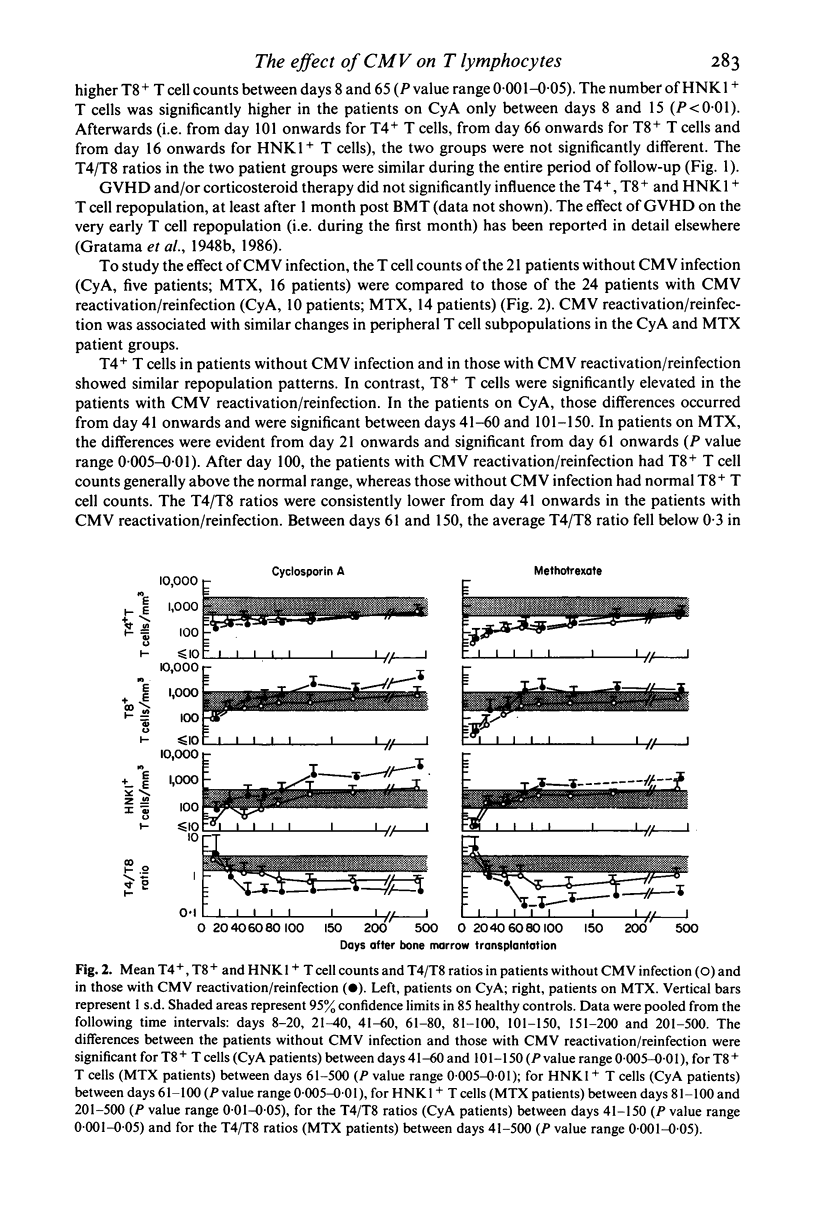
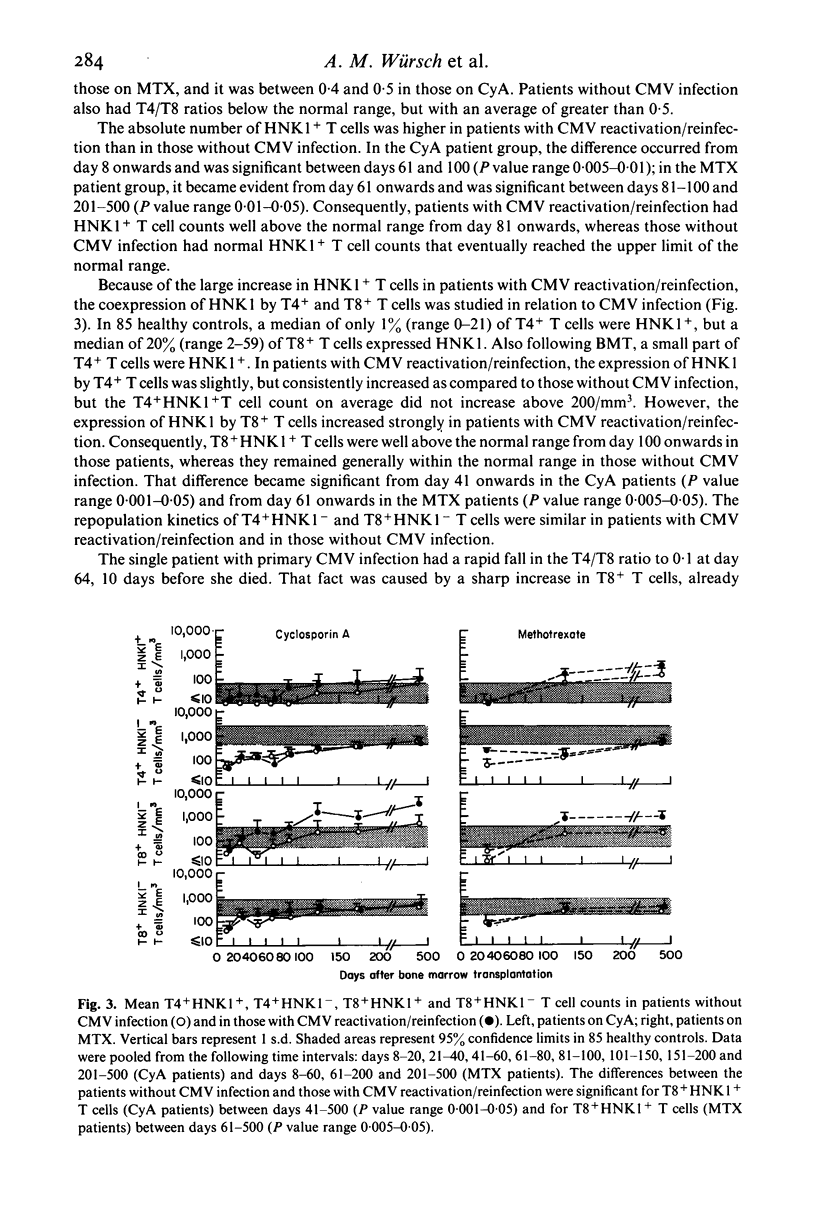
Abstract
The influence of cytomegalovirus (CMV) infection on peripheral T lymphocyte repopulation was studied in 59 bone marrow transplant (BMT) recipients who received either cyclosporin A (CyA) or methotrexate (MTX) as prophylaxis for acute graft-versus-host disease. We used monoclonal antibodies and single- or double-marker immunofluorescence for the quantitation of T4+, T8+ and HNK1+ T cell subpopulations. CMV infection was serologically diagnosed by an enzyme-linked immunosorbent assay (ELISA), and by viral cultures and histological studies. Among the 52 patients who were evaluable for CMV infection, one had a primary infection and 24 had CMV reactivation/reinfection after BMT. In the latter patients, increases to supranormal levels were observed in T8+ T cells and HNK1+ T cells, both in patients on CyA and in patients on MTX. Double-marker immunofluorescence revealed that the two markers were largely expressed by the same cells, which therefore had the T8+ HNK1+ phenotype. In addition, the very small subset of T4+ HNK+ T cells was slightly, but consistently, increased in the patients with CMV reactivation/reinfection. CMV infection did not influence the numbers of T4+ HNK1- and T8+ HNK1- T cells. The long-lasting presence of large numbers of T8+ HNK1+ T cells in patients who had CMV reactivation/reinfection suggests a continuing interaction between the virus and the immune system of its host.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Abo T., Cooper M. D., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. I. Two distinct phenotypes of human NK cells with different cytotoxic capability. J Immunol. 1982 Oct;129(4):1752–1757. [PubMed] [Google Scholar]
- Buckner C. D., Clift R. A. Marrow transplantation for acute lymphoblastic leukemia. Semin Hematol. 1984 Jan;21(1):43–47. [PubMed] [Google Scholar]
- Carney W. P., Iacoviello V., Hirsch M. S. Functional properties of T lymphocytes and their subsets in cytomegalovirus mononucleosis. J Immunol. 1983 Jan;130(1):390–393. [PubMed] [Google Scholar]
- Carney W. P., Rubin R. H., Hoffman R. A., Hansen W. P., Healey K., Hirsch M. S. Analysis of T lymphocyte subsets in cytomegalovirus mononucleosis. J Immunol. 1981 Jun;126(6):2114–2116. [PubMed] [Google Scholar]
- Favrot M., Janossy G., Tidman N., Blacklock H., Lopez E., Bofill M., Lampert I., Morgenstein G., Powles R., Prentice H. G. T cell regeneration after allogeneic bone marrow transplantation. Clin Exp Immunol. 1983 Oct;54(1):59–72. [PMC free article] [PubMed] [Google Scholar]
- Gratama J. W., Jansen J., Lipovich R. A., Tanke H. J., Goldstein G., Zwaan F. E. Treatment of acute graft-versus-host disease with monoclonal antibody OKT3. Clinical results and effect on circulating T lymphocytes. Transplantation. 1984 Nov;38(5):469–474. doi: 10.1097/00007890-198411000-00005. [DOI] [PubMed] [Google Scholar]
- Gratama J. W., Naipal A., Oljans P., Zwaan F. E., Verdonck L. F., de Witte T., Vossen J. M., Bolhuis R. L., de Gast G. C., Jansen J. T lymphocyte repopulation and differentiation after bone marrow transplantation. Early shifts in the ratio between T4+ and T8+ T lymphocytes correlate with the occurrence of acute graft-versus-host disease. Blood. 1984 Jun;63(6):1416–1423. [PubMed] [Google Scholar]
- Gratama J. W., Schuurman R. K., Van Leeuwen A., Jansen J., Oljans P., Tanke H. J., Van Rood J. J. Comparison of complement-dependent cytotoxicity and indirect immunofluorescence for enumeration of T-cell subpopulations in human peripheral blood. J Immunol Methods. 1983 Nov 11;64(1-2):99–108. doi: 10.1016/0022-1759(83)90388-5. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. J., Rinaldo C. R., Jr, Leary P. L., Zaia J. A., Hirsch M. S. Immune response to herpesvirus antigens in adults with acute cytomegaloviral mononucleosis. J Infect Dis. 1979 Dec;140(6):851–857. doi: 10.1093/infdis/140.6.851. [DOI] [PubMed] [Google Scholar]
- Meyers J. D., Flournoy N., Thomas E. D. Cytomegalovirus infection and specific cell-mediated immunity after marrow transplant. J Infect Dis. 1980 Dec;142(6):816–824. doi: 10.1093/infdis/142.6.816. [DOI] [PubMed] [Google Scholar]
- Middeldorp J. M., Jongsma J., ter Haar A., Schirm J., The T. H. Detection of immunoglobulin M and G antibodies against cytomegalovirus early and late antigens by enzyme-linked immunosorbent assay. J Clin Microbiol. 1984 Oct;20(4):763–771. doi: 10.1128/jcm.20.4.763-771.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzolo G., Semenzato G., Chilosi M., Morittu L., Ambrosetti A., Warner N., Bofill M., Janossy G. Distribution and heterogeneity of cells detected by HNK-1 monoclonal antibody in blood and tissues in normal, reactive and neoplastic conditions. Clin Exp Immunol. 1984 Jul;57(1):195–206. [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Burns W. H., Kirmani N., Rook A. H., Manischewitz J., Jackson L., Santos G. W., Saral R. HLA-restricted cytotoxic T lymphocytes are an early immune response and important defense mechanism in cytomegalovirus infections. Rev Infect Dis. 1984 Mar-Apr;6(2):156–163. doi: 10.1093/clinids/6.2.156. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Schroff R. W., Gale R. P., Fahey J. L. Regeneration of T cell subpopulations after bone marrow transplantation: cytomegalovirus infection and lymphoid subset imbalance. J Immunol. 1982 Nov;129(5):1926–1930. [PubMed] [Google Scholar]
- Speck B., Gratwohl A., Osterwalder B., Nissen C. Bone marrow transplantation for chronic myeloid leukemia. Semin Hematol. 1984 Jan;21(1):48–52. [PubMed] [Google Scholar]
- Verbi W., Greaves M. F., Schneider C., Koubek K., Janossy G., Stein H., Kung P., Goldstein G. Monoclonal antibodies OKT 11 and OKT 11A have pan-T reactivity and block sheep erythrocyte "receptors". Eur J Immunol. 1982 Jan;12(1):81–86. doi: 10.1002/eji.1830120115. [DOI] [PubMed] [Google Scholar]
- Verdonck L. F., de Gast G. C. Is cytomegalovirus infection a major cause of T cell alterations after (autologous) bone-marrow transplantation? Lancet. 1984 Apr 28;1(8383):932–935. doi: 10.1016/s0140-6736(84)92391-2. [DOI] [PubMed] [Google Scholar]
- ten Napel C. H., The T. H. Acute cytomegalovirus infection and the host immune response. II. Relationship of suppressed in vitro lymphocyte reactivity to bacterial recall antigens and mitogens with the development of cytomegalovirus-induced lymphocyte reactivity. Clin Exp Immunol. 1980 Feb;39(2):272–278. [PMC free article] [PubMed] [Google Scholar]



