Abstract
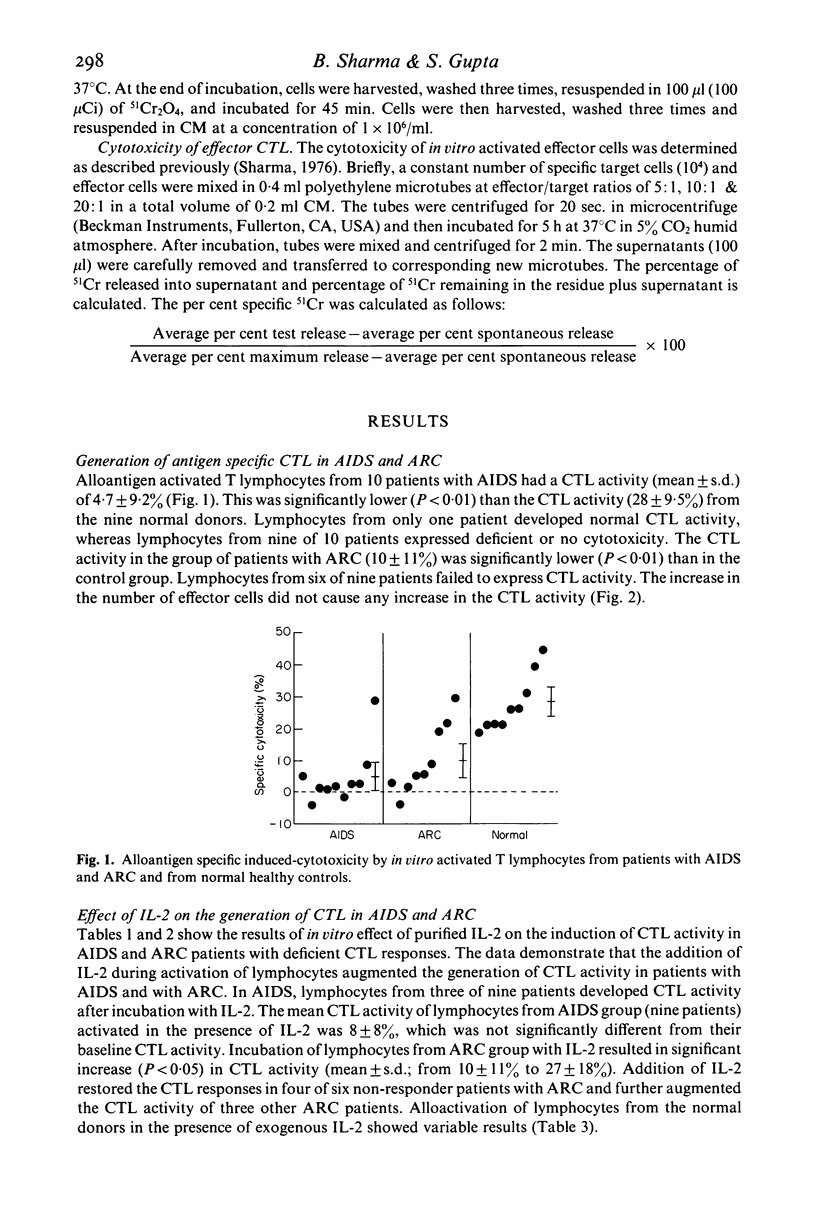
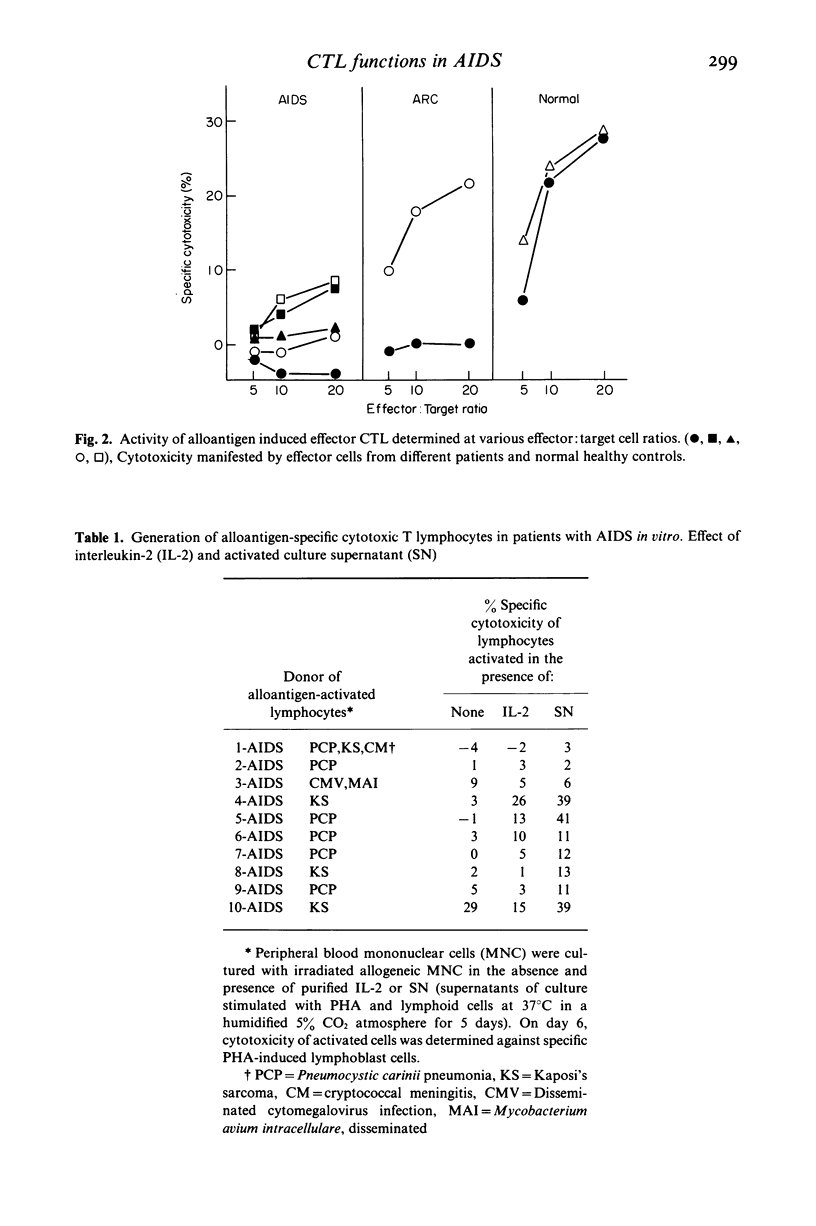
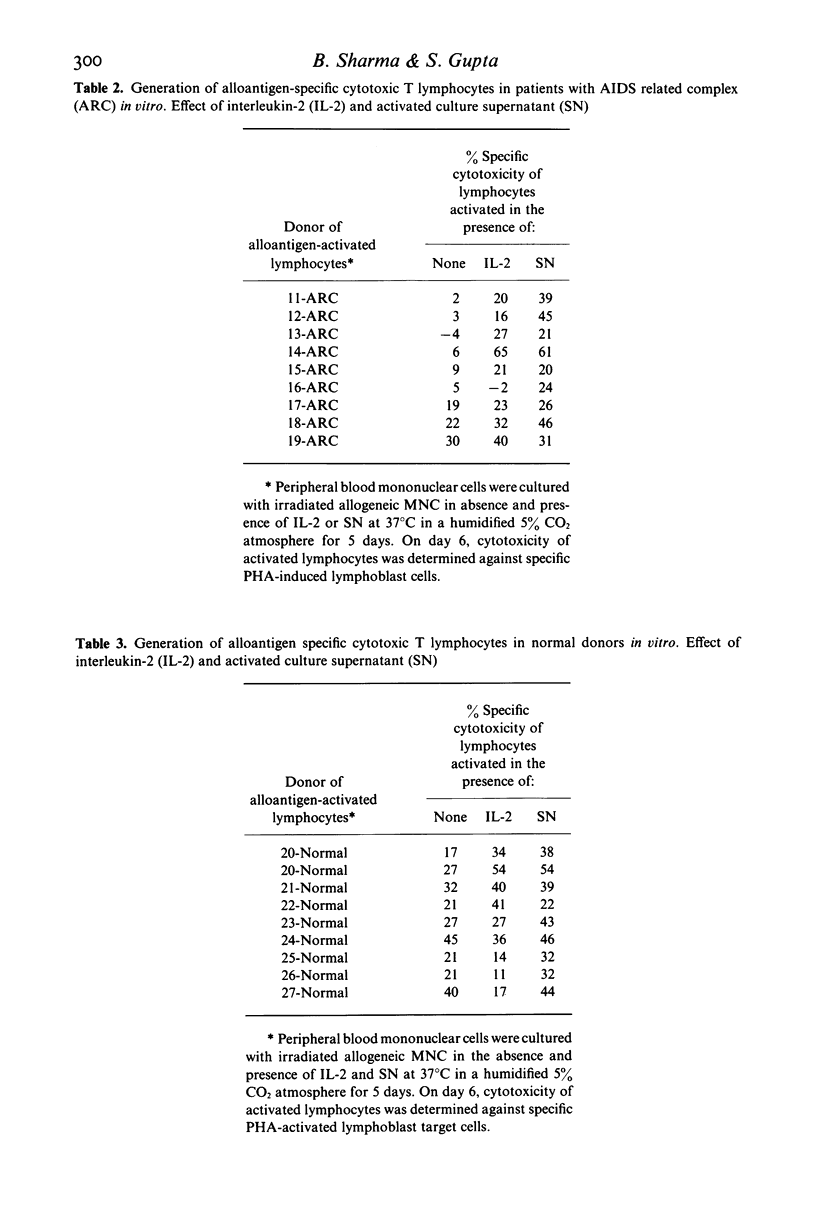
Alloantigen specific primary cytotoxic T lymphocyte (CTL) responses were examined in vitro in 10 patients with AIDS and nine with AIDS-related complex (ARC). The lymphocytes from patients with AIDS and ARC expressed significantly less (P less than 0.01) CTL activity (mean +/- s.d.; 4.7 +/- 9% and 10 +/- 11% respectively) when compared with CTL activity in normal healthy heterosexual controls (28 +/- 9.5%). When data were analysed for individual patients, lymphocytes from nine of 10 patients with AIDS and six of nine with ARC had deficient or no CTL activity. In vitro addition of purified human interleukin-2 (IL-2) during the generation of CTL resulted in significant enhancement (P less than 0.05) of CTL activity in ARC group (mean +/- s.d.; 27 +/- 18) but not in AIDS group (mean +/- s.d.; 8 +/- 8%). The presence of IL-2 augmented the induction of CTL activity in three of nine patients in AIDS group and in five of six in ARC group. In vitro addition of lectin-free supernatant (SN) obtained from cultures stimulated with PHA as well as with lymphoid cell restored the CTL functions in three of six AIDS patients and in one patient with ARC who did not respond to exogenous IL-2. The CTL activity developed in the presence of SN was higher than that manifested in the presence of IL-2 in both AIDS (SN versus IL-2; mean +/- s.d., 18 +/- 15.6% versus 8 +/- 8%) and in the ARC group (SN versus IL-2; mean +/- s.d., 35 +/- 13.9% versus 27 +/- 18.3%). Lymphocytes from three AIDS patients, however, failed to develop any CTL activity in the presence of either IL-2 or SN. These results demonstrate that: (i) the lymphocytes from majority of patients with AIDS and with ARC have deficient ability to develop into alloantigen specific primary CTL effectors, and (ii) the defective CTL functions are restored by the addition of purified IL-2 or SN in all patients with ARC and only in a subset of patients with AIDS, suggesting heterogeneity of pre-CTL to respond to IL-2 and some differentiation factor in order to differentiate in CTL effectors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantor H., Boyse E. A. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J Exp Med. 1975 Jun 1;141(6):1390–1399. doi: 10.1084/jem.141.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever M. A., Greenberg P. D., Fefer A., Gillis S. Augmentation of the anti-tumor therapeutic efficacy of long-term cultured T lymphocytes by in vivo administration of purified interleukin 2. J Exp Med. 1982 Apr 1;155(4):968–980. doi: 10.1084/jem.155.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey J. L., Prince H., Weaver M., Groopman J., Visscher B., Schwartz K., Detels R. Quantitative changes in T helper or T suppressor/cytotoxic lymphocyte subsets that distinguish acquired immune deficiency syndrome from other immune subset disorders. Am J Med. 1984 Jan;76(1):95–100. doi: 10.1016/0002-9343(84)90756-3. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Mizel S. B., Farrar J. J. Participation of lymphocyte activating factor (Interleukin 1) in the induction of cytotoxic T cell responses. J Immunol. 1980 Mar;124(3):1371–1377. [PubMed] [Google Scholar]
- Fernandez-Cruz E., Woda B. A., Feldman J. D. Elimination of syngeneic sarcomas in rats by a subset of T lymphocytes. J Exp Med. 1980 Oct 1;152(4):823–841. doi: 10.1084/jem.152.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S. Interleukin 2: biology and biochemistry. J Clin Immunol. 1983 Jan;3(1):1–13. doi: 10.1007/BF00919133. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. S., Schroff R., Schanker H. M., Weisman J. D., Fan P. T., Wolf R. A., Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981 Dec 10;305(24):1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- Gupta S., Gillis S., Thornton M., Goldberg M. Autologous mixed lymphocyte reaction in man. XIV. Deficiency of the autologous mixed lymphocyte reaction in acquired immune deficiency syndrome (AIDS) and AIDS related complex (ARC). In vitro effect of purified interleukin-1 and interleukin-2. Clin Exp Immunol. 1984 Nov;58(2):395–401. [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Safai B. Deficient autologous mixed lymphocyte reaction in Kaposi's sarcoma associated with deficiency of Leu-3+ responder T cells. J Clin Invest. 1983 Feb;71(2):296–300. doi: 10.1172/JCI110769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson E. L., Coutinho A. The role of mitogenic lectins in T-cell triggering. Nature. 1979 Jul 19;280(5719):239–241. doi: 10.1038/280239a0. [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Askonas B. A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981 Aug 1;154(2):225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C. T., Fitch F. W. Accessory cell requirements for the generation of cytolytic T lymphocytes. J Immunol. 1979 Jun;122(6):2598–2604. [PubMed] [Google Scholar]
- Miller R. G., Shcilling R. M., Phillips R. A. Requirement for non T-cells in the generation of cytotoxic T lymphocytes (CTL) in vitro. II. Characterization of the active cells in the spleen of nude mice. J Immunol. 1977 Jan;118(1):166–174. [PubMed] [Google Scholar]
- Okada M., Henney C. S. The differentiation of cytotoxic T cells in vitro. III. The role of helper T cells and their products in the differentiation of cytotoxic cells from "memory" cell populations. J Immunol. 1980 Aug;125(2):850–857. [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Bevan M. J. A differentiation factor required for the expression of cytotoxic T-cell function. Nature. 1982 Apr 22;296(5859):754–757. doi: 10.1038/296754a0. [DOI] [PubMed] [Google Scholar]
- Reddehase M., Suessmuth W., Moyers C., Falk W., Droege W. Interleukin 2 is not sufficient as helper component for the activation of cytotoxic T lymphocytes but synergizes with a late helper effect that is provided by irradiated I-region-incompatible stimulator cells. J Immunol. 1982 Jan;128(1):61–68. [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Selik R. M., Haverkos H. W., Curran J. W. Acquired immune deficiency syndrome (AIDS) trends in the United States, 1978-1982. Am J Med. 1984 Mar;76(3):493–500. doi: 10.1016/0002-9343(84)90669-7. [DOI] [PubMed] [Google Scholar]
- Sharma B., Terasaki P. I. In vitro immunization to cultured human tumor cells. Cancer Res. 1974 Jan;34(1):115–118. [PubMed] [Google Scholar]
- Siegel R. L., Fox R. W. A longitudinal study of a patient with acquired immunodeficiency syndrome using T cell subset analysis. Adv Exp Med Biol. 1983;166:295–303. doi: 10.1007/978-1-4757-1410-4_25. [DOI] [PubMed] [Google Scholar]
- Stern A. S., Pan Y. C., Urdal D. L., Mochizuki D. Y., DeChiara S., Blacher R., Wideman J., Gillis S. Purification to homogeneity and partial characterization of interleukin 2 from a human T-cell leukemia. Proc Natl Acad Sci U S A. 1984 Feb;81(3):871–875. doi: 10.1073/pnas.81.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Hardt C., Rouse B. T., Röllinghoff M., Scheurich P., Pfizenmaier K. Dissection of the proliferative and differentiative signals controlling murine cytotoxic T lymphocyte responses. J Exp Med. 1982 Jun 1;155(6):1876–1881. doi: 10.1084/jem.155.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Röllinghoff M. T-T-cell interactions during the vitro cytotoxic allograft responses. I. Soluble products from activated Lyl+ T cells trigger autonomously antigen-primed Ly23+ T cells to cell proliferation and cytolytic activity. J Exp Med. 1978 Dec 1;148(6):1523–1538. doi: 10.1084/jem.148.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells M. A., Daniel S., Djeu J. Y., Kiley S. C., Ennis F. A. Recovery from a viral respiratory tract infection. IV. Specificity of protection by cytotoxic T lymphocytes. J Immunol. 1983 Jun;130(6):2908–2914. [PubMed] [Google Scholar]
- Woodward J. G., Fernandez P. A., Daynes R. A. Cell-mediated immune response to syngeneic UV-induced tumors. III. Requirement for an Ia+ macrophage in the in vitro differentiation of cytotoxic T lymphocytes. J Immunol. 1979 Apr;122(4):1196–1202. [PubMed] [Google Scholar]
- Yap K. L., Ada G. L., McKenzie I. F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978 May 18;273(5659):238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]


