Abstract
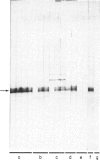
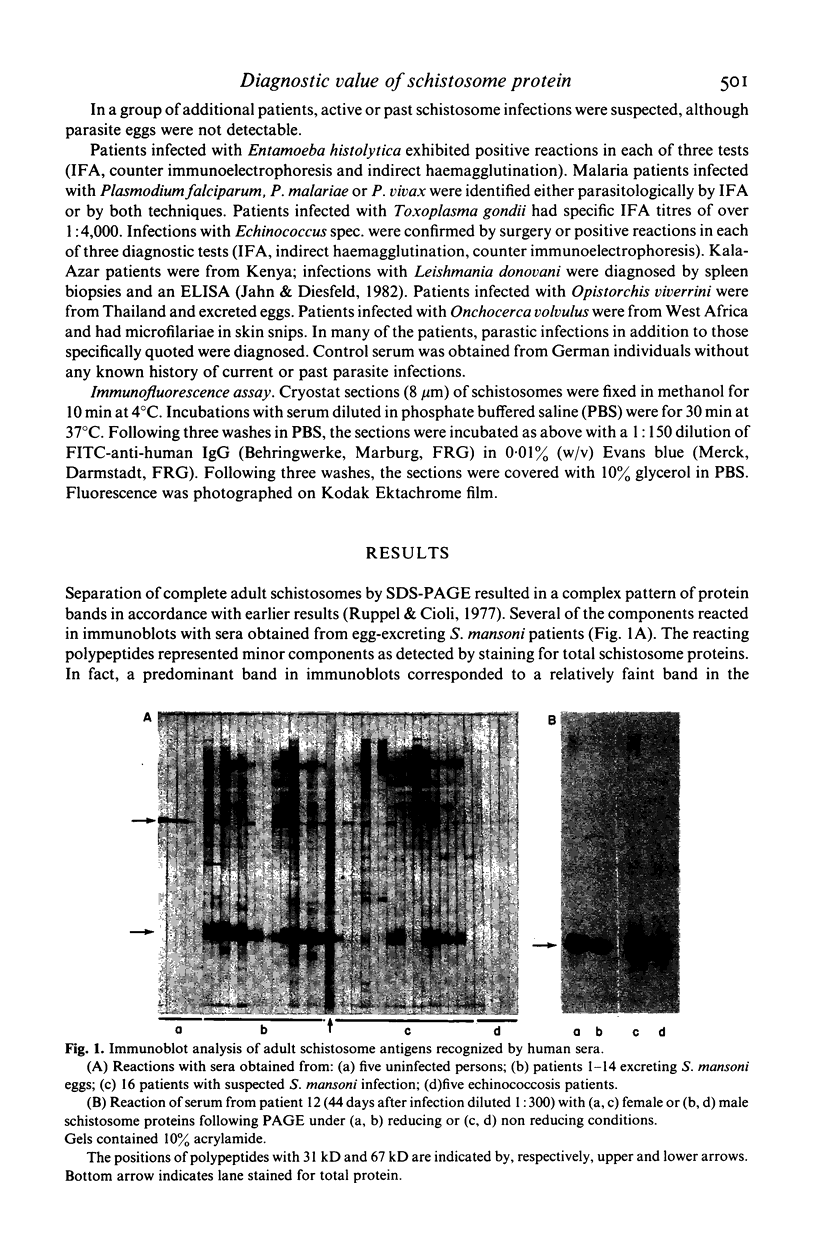
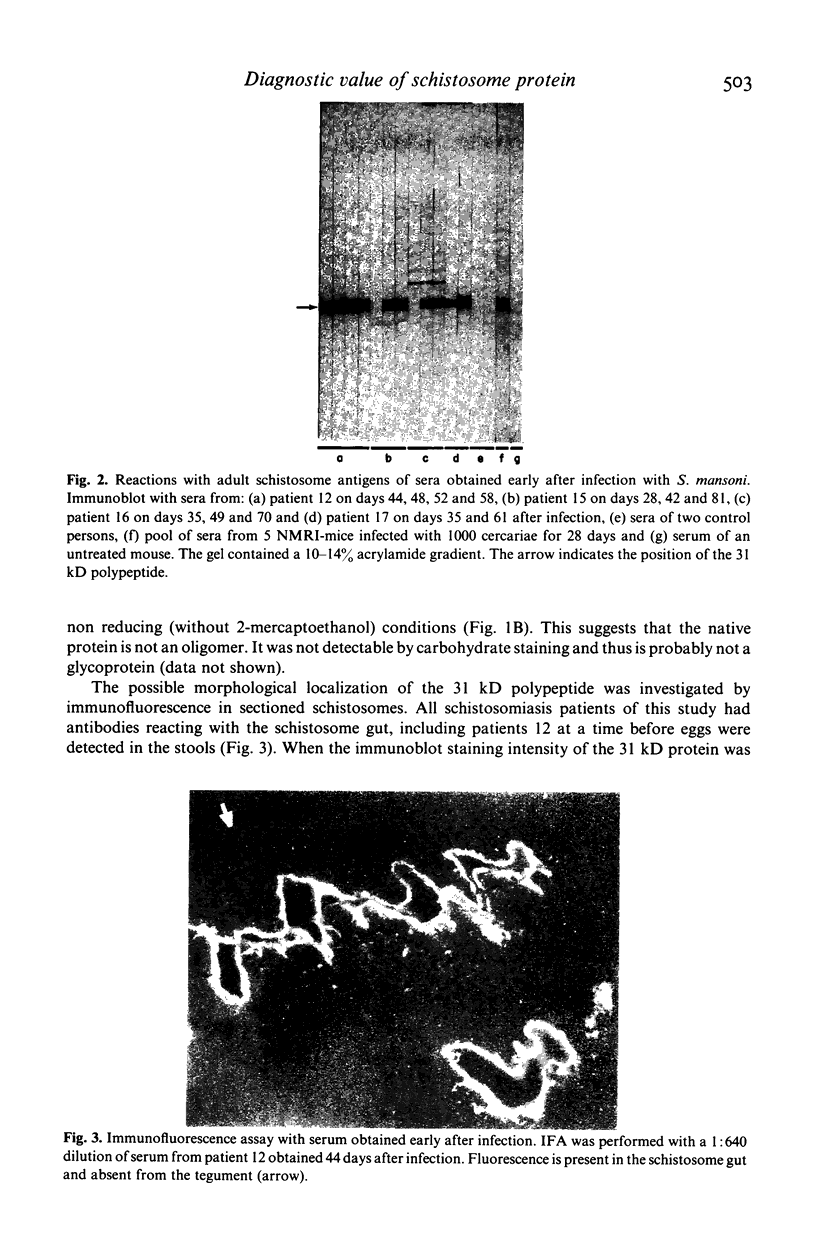
We compared the reaction in immunoblots of sera obtained from patients with parasitologically proven S. mansoni infections, with a suspected history of schistosomiasis infection, or with unrelated parasitic diseases. Several polypeptides from adult S. mansoni reacted with the schistosomiasis patients' sera in a heterogeneous manner. However, a component of approximately 31 kilo daltons (kD) reacted with all schistosomiasis sera and with several sera of suspected schistosomiasis cases. No reaction was ever observed with sera of patients harbouring other parasites. Thus, the polypeptide has potential diagnostic value. The use of sera of patients with recent infections demonstrated that: the earliest time of antibody formation against the 31 kD component was approximately 40 days post infection, the reaction with this polypeptide in immunoblots was exceptionally strong and antibodies directed against other schistosome proteins were barely detectable at this time. Identical results were obtained with sera of experimentally infected mice. The 31 kD component was present in parasites of either sex. It was apparently not a glycoprotein. Evidence suggests that the 31 kD polypeptide may originate from the schistosome gut.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlier Y., Bout D., Strecker G., Debray H., Capron A. Purification, immunochemical, and biologic characterization of the Schistosoma circulating M antigen. J Immunol. 1980 May;124(5):2442–2450. [PubMed] [Google Scholar]
- Cordingley J. S., Taylor D. W., Dunne D. W., Butterworth A. E. Clone banks of cDNA from the parasite Schistosoma mansoni: isolation of clones containing a potentially immunodiagnostic antigen gene. Gene. 1983 Dec;26(1):25–39. doi: 10.1016/0378-1119(83)90033-1. [DOI] [PubMed] [Google Scholar]
- Deelder A. M., Kornelis D., Van Marck E. A., Eveleigh P. C., Van Egmond J. G. Schistosoma mansoni: characterization of two circulating polysaccharide antigens and the immunological response to these antigens in mouse, hamster, and human infections. Exp Parasitol. 1980 Aug;50(1):16–32. doi: 10.1016/0014-4894(80)90004-1. [DOI] [PubMed] [Google Scholar]
- Deelder A. M., Reinders P. N., Rotmans J. P. Purification studies on an acidic protease from adult Schistosoma mansoni. Acta Leiden. 1977 Dec;45:91–103. [PubMed] [Google Scholar]
- Dunne D. W., Bain J., Lillywhite J., Doenhoff M. J. The stage-, strain- and species-specificity of a Schistosoma mansoni egg antigen fraction (CEF6) with serodiagnostic potential. Trans R Soc Trop Med Hyg. 1984;78(4):460–470. doi: 10.1016/0035-9203(84)90061-0. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Jahn A., Diesfeld H. J. Evaluation of a visually read ELISA for serodiagnosis and sero-epidemiological studies of kala-azar in the Baringo District, Kenya. Trans R Soc Trop Med Hyg. 1983;77(4):451–454. doi: 10.1016/0035-9203(83)90110-4. [DOI] [PubMed] [Google Scholar]
- Kagan I. G. Serologic diagnosis of schistosomiasis. Bull N Y Acad Med. 1968 Mar;44(3):262–277. [PMC free article] [PubMed] [Google Scholar]
- Kelsoe G. H., Weller T. H. Immunodiagnosis of infection with Schistosoma mansoni: enzyme-linked immunosorbent assay for detection of antibody to circulating antigen. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5715–5717. doi: 10.1073/pnas.75.11.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucius R., Rauterberg E. W., Diesfeld H. J. Identification of immunogenic proteins of Dipetalonema viteae (Filarioidea) by the "Western Blotting" technique. Tropenmed Parasitol. 1983 Jun;34(2):133–136. [PubMed] [Google Scholar]
- Mott K. E., Dixon H. Collaborative study on antigens for immunodiagnosis of schistosomiasis. Bull World Health Organ. 1982;60(5):729–753. [PMC free article] [PubMed] [Google Scholar]
- Nash T. E., Lunde M. N., Cheever A. W. Analysis and antigenic activity of a carbohydrate fraction derived from adult Schistosoma mansoni. J Immunol. 1981 Feb;126(2):805–810. [PubMed] [Google Scholar]
- Norden A. P., Strand M. Schistosoma mansoni, S. haematobium, and S. japonicum: identification of genus-, species-, and gender-specific antigenic worm glycoproteins. Exp Parasitol. 1984 Feb;57(1):110–123. doi: 10.1016/0014-4894(84)90070-5. [DOI] [PubMed] [Google Scholar]
- Pelley R. P., Warren K. S., Jordan P. Purified antigen radioimmunoassay in serological diagnosis of schistosomiasis mansoni. Lancet. 1977 Oct 15;2(8042):781–785. doi: 10.1016/s0140-6736(77)90722-x. [DOI] [PubMed] [Google Scholar]
- Rotmans J. P. Schistosoma mansoni: antigenic characterization of malate dehydrogenase isoenzymes and use in the defined antigen substrate spheres (DASS) system. Exp Parasitol. 1978 Nov;46(1):49–58. doi: 10.1016/0014-4894(78)90155-8. [DOI] [PubMed] [Google Scholar]
- Ruppel A., Cioli D. A comparative analysis of various developmental stages of Schistosoma mansoni with respect to their protein composition. Parasitology. 1977 Dec;75(3):339–343. doi: 10.1017/s003118200005188x. [DOI] [PubMed] [Google Scholar]
- Ruppel A., Rother U., Diesfeld H. J. Schistosoma mansoni: development of primary infections in mice genetically deficient or intact in the fifth component of complement. Parasitology. 1982 Oct;85(Pt 2):315–323. doi: 10.1017/s0031182000055293. [DOI] [PubMed] [Google Scholar]
- Ruppel A., Rother U., Vongerichten H., Diesfeld H. J. Schistosoma mansoni: complement activation in human and rodent sera by living parasites of various developmental stages. Parasitology. 1983 Aug;87(Pt 1):75–86. doi: 10.1017/s0031182000052434. [DOI] [PubMed] [Google Scholar]
- Sauer M. C., Senft A. W. Properties of a proteolytic enzyme from Schistosoma mansoni. Comp Biochem Physiol B. 1972 Jun 15;42(2):205–220. doi: 10.1016/0305-0491(72)90267-2. [DOI] [PubMed] [Google Scholar]
- Senft A. W., Goldberg M. W., Byram J. E. Hemoglobinolytic activity of serum in mice infected with Schistosoma mansoni. Am J Trop Med Hyg. 1981 Jan;30(1):96–101. doi: 10.4269/ajtmh.1981.30.96. [DOI] [PubMed] [Google Scholar]
- Senft A. W., Maddison S. E. Hypersensitivity to parasite proteolytic enzyme in schistosomiasis. Am J Trop Med Hyg. 1975 Jan;24(1):83–89. doi: 10.4269/ajtmh.1975.24.83. [DOI] [PubMed] [Google Scholar]
- Senft A. W., Weltman J. K., Goldgraber M. B., Egyud L. G., Kuntz R. E. Species specificity of the immediate hypersensitivity to schistosomal proteolytic enzyme. Parasite Immunol. 1979 Spring;1(1):79–89. doi: 10.1111/j.1365-3024.1979.tb00697.x. [DOI] [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965 Nov;55(4):695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- TIMMS A. R., BUEDING E. Studies of a proteolytic enzyme from Schistosoma mansoni. Br J Pharmacol Chemother. 1959 Mar;14(1):68–73. doi: 10.1111/j.1476-5381.1959.tb00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]