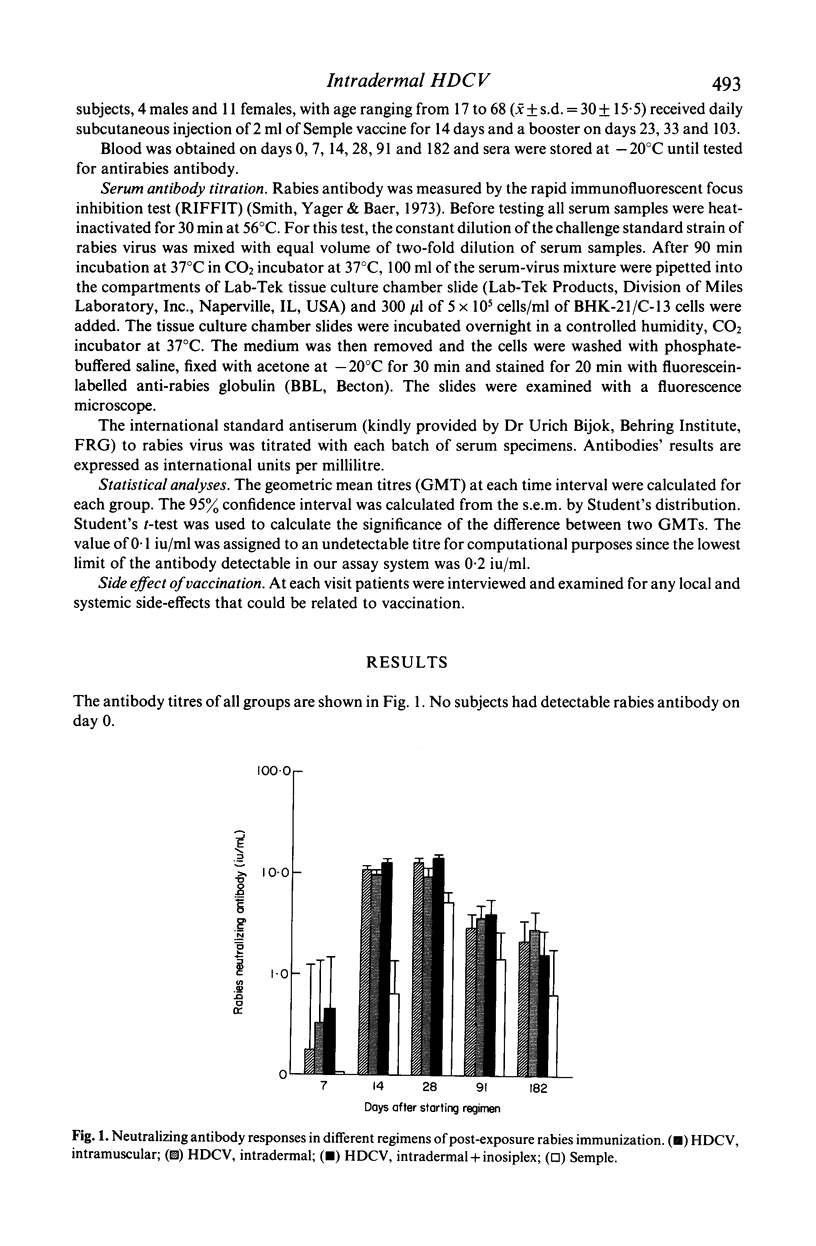
Abstract
A closely-spaced multisite intradermal regimen of human diploid cell rabies vaccine (HDCV) was evaluated in 39 patients after low-risk exposure to rabies, in comparison to full-dose intramuscular HDCV and sheep brain-derived rabies (Semple) vaccine. The regimen consisted of four intradermal injections, 0.1 ml each of HDCV on days 0, 3 and 7, followed by two booster doses of only 0.1 ml each on days 28 and 91 administered intradermally. Although the total amount of HDCV used in this intradermal regimen was 1.4 ml or one-quarter of the conventional intramuscular regimen, a higher proportion of the recipients of this economical intradermal regimen, as compared to the full-dose intramuscular regimen, developed neutralizing antibodies above the hypothetical protective level of 0.5 iu/ml 7 days after starting immunization. Besides the earlier antibody response, the peak antibody level of the intradermal regimen was also satisfactorily high and not significantly different from that after the intramuscular regimen. Simultaneous administration of inosiplex, an antiviral and immunopotentiating agent, during the first 10 days of intradermal immunization resulted in an even higher antibody response for as long as 91 days. In contrast, but not unexpectedly, Semple vaccine evoked lower, more sluggish and inconsistent antibody responses. The side-effects of intradermal HDCV were mild, mainly local and self-remitting. We therefore recommend our intensive intradermal regimen of HDCV vaccination for safe, effective and economical use in post-exposure rabies immunization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajjan N., Soulebot J. P., Triau R., Biron G. Intradermal immunization with rabies vaccine. Inactivated Wistar strain cultivated in human diploid cells. JAMA. 1980 Dec 5;244(22):2528–2531. doi: 10.1001/jama.244.22.2528. [DOI] [PubMed] [Google Scholar]
- Aoki F. Y., Tyrrell D. A., Hill L. E. Immunogenicity and acceptability of a human diploid-cell culture rabies vaccine in volunteers. Lancet. 1975 Mar 22;1(7908):660–662. doi: 10.1016/s0140-6736(75)91761-4. [DOI] [PubMed] [Google Scholar]
- Bernard K. W., Roberts M. A., Sumner J., Winkler W. G., Mallonee J., Baer G. M., Chaney R. Human diploid cell rabies vaccine. Effectiveness of immunization with small intradermal or subcutaneous doses. JAMA. 1982 Feb 26;247(8):1138–1142. doi: 10.1001/jama.247.8.1138. [DOI] [PubMed] [Google Scholar]
- Cox J. H., Schneider L. G. Prophylactic immunization of humans against rabies by intradermal inoculation of human diploid cell culture vaccine. J Clin Microbiol. 1976 Feb;3(2):96–101. doi: 10.1128/jcm.3.2.96-101.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith G. M., Thiers B. H., Fudenberg H. H. An open-label trial of immunomodulation therapy with inosiplex (Isoprinosine) in patients with alopecia totalis and cell-mediated immunodeficiency. J Am Acad Dermatol. 1984 Aug;11(2 Pt 1):224–230. doi: 10.1016/s0190-9622(84)70153-8. [DOI] [PubMed] [Google Scholar]
- Lemon S. M., Miller R. N., Pang L. W., Prier R. E., Bernard K. W. Failure to achieve predicted antibody responses with intradermal and intramuscular human diploid cell rabies vaccine. Lancet. 1984 May 19;1(8386):1098–1100. doi: 10.1016/s0140-6736(84)92509-1. [DOI] [PubMed] [Google Scholar]
- Ratanavongsiri J., Sriwanthana B., Ubol S., Phanuphak P. Cell-mediated immune response following intracutaneous immunisation with human diploid cell rabies vaccine. Asian Pac J Allergy Immunol. 1985 Dec;3(2):187–190. [PubMed] [Google Scholar]
- Smith J. S., Yager P. A., Baer G. M. A rapid reproducible test for determining rabies neutralizing antibody. Bull World Health Organ. 1973 May;48(5):535–541. [PMC free article] [PubMed] [Google Scholar]
- Trivedi C. R. Profile of dog bites, rabies and default in antirabies immunisation at V.S.G. Hospital, Ahmedabad. J Indian Med Assoc. 1981 Apr;76(7-8):134–136. [PubMed] [Google Scholar]
- Warrell M. J., Warrell D. A., Suntharasamai P., Viravan C., Sinhaseni A., Udomsakdi D., Phanfung R., Xueref C., Vincent-Falquet J. C., Nicholson K. G. An economical regimen of human diploid cell strain anti-rabies vaccine for post-exposure prophylaxis. Lancet. 1983 Aug 6;2(8345):301–304. doi: 10.1016/s0140-6736(83)90288-x. [DOI] [PubMed] [Google Scholar]


