Abstract
Integrin α1β1 is a collagen receptor abundantly expressed on microvascular endothelial cells. As well as being the only collagen receptor able to activate the Ras/Shc/mitogen-activated protein kinase pathway promoting fibroblast cell proliferation, it also acts to inhibit collagen and metalloproteinase (MMP) synthesis. We have observed that in integrin α1-null mice synthesis of MMP7 and MMP9 was markedly increased compared with that of their wild-type counterparts. As MMP7 and MMP9 have been shown to generate angiostatin from circulating plasminogen, and angiostatin acts as a potent inhibitor of endothelial cell proliferation, we determined whether tumor vascularization was altered in the α1-null mice. Tumors implanted into α1-null mice showed markedly decreased vascularization, with a reduction in capillary number and size, which was accompanied by an increase in plasma levels of angiostatin due to the action of MMP7 and MMP9 on circulating plasminogen. In vitro analysis of α1-null endothelial cells revealed a marked reduction of their proliferation on both integrin α1-dependent (collagenous) and independent (noncollagenous) substrata. This reduction was prevented by culturing α1-null cells with plasma derived from plasminogen-null animals, thus omitting the source from which to generate angiostatin. Plasma from tumor-bearing α1-null animals uniquely inhibited endothelial cell growth, and this inhibition was relieved by the coaddition of either MMP inhibitors, or antibody to angiostatin. Integrin α1-deficient mice thus provide a genetically characterized model for enhanced angiostatin production and serve to reveal an unwanted potential side effect of MMP inhibition, increased tumor angiogenesis.
Endothelial cell proliferation has been shown to be inhibited by a variety of factors, the best known of which is angiostatin, a cleavage product of plasminogen (1). Angiostatin can be generated from circulating plasminogen from different enzymes, including the matrix metalloproteases (MMPs) MMP2, MMP7, MMP9, and MMP12 (2–6). Whereas MMP12 is mainly secreted by macrophages (3), MMP2, MMP7, and MMP9 can be produced by different cell types, including endothelial cells (7–9). Endothelial synthesis of MMPs seems to have opposite effects on tumor angiogenesis, on the one hand facilitating extracellular matrix degradation and new blood vessel formation (10), and on the other hand blocking angiogenesis by producing inhibitors of endothelial cell growth, including angiostatin (5, 11).
The integrins are an important group of physiological regulators of MMP expression (12–14). One of these is α1β1, which is unique among the collagen receptors able to actitivate the Ras/Shc/mitogen-activated protein kinase (MAPK) pathway promoting cell proliferation (15). It also inhibits collagen and collagenase synthesis (13, 16). α1-null mouse (17) fibroblasts fail to grow on collagenous substrata (15) and show increased expression of MMPs, including MMP13 (18), MMP2, MMP7, and MMP9. This finding, together with the observation that integrin α1 is normally abundant on microvascular endothelium (19), prompted us to analyze tumor vascularisation and endothelial growth in the α1 null host.
Materials and Methods
Primary Tumor Growth.
All experiments were performed according to institutional animal care guidelines. Four- to 6-month-old 129Sv/Jae male wild-type and α1-null mice were given four dorsal s.c. injections, one in each quadrant, of a syngeneic large T antigen/ras/myc-transformed fibroblast line, 60.5 (20), which is tumorigenic in the 129Sv/Jae host. Tumors initiated with 105 cells in 200 μl of PBS were permitted to grow for 10–14 days before harvest. The proportion of injections giving rise to tumors was noted for each animal, and tumor dimensions were measured with a caliper. Tumors were frozen for histology.
Immunostaining and Quantification.
Seven-micrometer frozen sections were stained with anti-mouse CD31 (PharMingen) and Vactastain ABC and counterstained with hematoxylin. Sections, with labels coded to blind the observer, were imaged with a digital camera (Pixera, Los Gatos, CA), and the images were processed by using Scion Image (Frederick, MD) software. CD31-positive structures in each section were automatically counted and their areas were measured. Differences in tumor vascularity (number of and area occupied by CD31-positive structures per tumor microscopic field) were determined for each section. Data were transferred to a spreadsheet and then sorted by genotype.
Zymography.
Gelatin and casein zymograms of skin explants were performed as described (18). For assay of MMP secretion by primary endothelial cells, 105 cells were plated onto 10-cm dishes coated with 10 μg/ml of collagen I and incubated in complete microvascular endothelial growth media (EGM-2-MV) containing 5% FCS (Clonetics, San Diego). Cells then were washed with PBS and incubated with 5 ml of serum-free medium for 48 hr. The medium was recovered and processed as described (18). One hundred micrograms of total proteins was loaded for zymography. For plasma MMPs, blood was collected with 1/10 vol of 3 mM EDTA/0.1% sodium citrate and spun for 30 min at 1,000 × g. Plasma was precleared with a mixture of protein A and G Sepharose beads. Normalization by protein (Micro-BCA, Pierce) was done to correct for minor (±5%) differences in plasma dilution by anticoagulant. Thirty micrograms of total proteins was loaded for zymography. For sponges, gelfoam sterile sponges (Upjohn) were implanted s.c. in the dorsum of 2- to 4-month-old SV129/Jae mice, recovered after 7 days, dissected free of adherent tissue, pulverized in liquid nitrogen, and resuspended in Laemmli's sample buffer. One hundred micrograms of total proteins was loaded for zymography. Normalization by protein was done to correct for small variations in sponge recovery caused by breakage. MMP9 bands were quantified by densitometry using an Alpha Imager 2000 (Alpha Innotech, San Leandro, CA).
Western Blot.
One hundred micrograms of total plasma proteins from untreated, tumor-bearing, and sponge-bearing Sv129/Jae mice was loaded on 10% SDS/PAGE and run under nonreducing conditions (21, 22). Gels were either stained to confirm equal loading of proteins or transferred onto Immobilon-P (Millipore). Membranes then were incubated with a purified mouse mAb (final concentration 5 μg/ml) reacting with an elastase degradation product of human and mouse plasminogen containing kringle 1–3/angiostatin, (anti-K1–3). No band was detected in elastase-treated plasma from plasminogen null mice (not shown). The signal was visualized with a peroxidase-conjugated goat anti-mouse and an ECL kit (Pierce). For the digestion experiment, 20 μl of 2% mouse serum in digestion buffer with 60 mM CaCl2 (2) was incubated, with or without 2.5 μM BB94 (Batimastat) (gift from V. Quaranta, The Scripps Research Institute), with 7.5 μg plasma from tumor-bearing mice. This amount of plasma did not by itself yield visible angiostatin fragments. Samples were incubated at 37°C for 16–18 hr and heated at 95°C for 5 min in Laemmli's sample buffer before SDS/PAGE.
Immunoprecipitation Assay.
Cells were trypsinized, washed, and labeled with NHS LC biotin (Pierce), according to the manufacturer's instructions. Cell lysates and immunoprecipitations were prepared as described (23). Immunoprecipitations with either rabbit anti-β1 (gift of R. O. Hynes, Massachusetts Institute of Technology) or anti-αv (PharMingen) were separated on 6% SDS/PAGE under nonreducing conditions, transferred onto Immobilon-P (Millipore), and visualized with the Vectastain elite ABC kit (Vector Laboratories).
Recovery of Mouse Lung Endothelial Cells.
SV129/Jae wild-type and α1-null mice (2 months old) were anesthetized, and the lung vasculature was perfused with PBS/2 mM EDTA followed by 0.25% trypsin/2 mM EDTA via the right ventricle. Heart and lungs were removed en bloc and incubated at 37°C for 20 min. The visceral pleura then was trimmed away, and the perfusion was repeated. Primary endothelial cells > 90% pure by immunostaining with anti-CD31 were recovered and grown on tissue culture plastic for 3 days in EGM-2-MV containing 5% FCS (Clonetics). For proliferation assays, 104 primary endothelial cells were plated in the same medium on 96-well plates coated with 20 μg/ml bovine collagen I (Vitrogen 100) or 10 μg/ml human fibrinogen (gift of Z. Ruggeri, The Scripps Research Institute). After 2 days cells were pulsed for an additional 48 hr with 3H-thymidine (1 μCi/well). In the “change” experiment, complete medium was changed every 12 hr, including the labeling period. In the “swap” experiment, wild-type cells were grown in media previously conditioned from α1-null cells and vice versa. Conditioned medium was made by growing cells on collagen I for 3 days in complete medium containing 5% FCS. In some experiments cells were grown in EGM-2-MV containing 5% normal or plasminogen null mouse plasma (24) instead of the FCS. The cells then were harvested, and trichloroacetic acid-precipitated lysates were measured with a β-counter. Cell counts paralleled the results of 3H-thymidine incorporation (not shown).
Human Umbilical Vein Endothelial Cells (HUVEC) and Bovine Artery Endothelial Cells (BAEC) Proliferation.
HUVEC or BAEC (5 × 103) were plated on uncoated 96-well plates in the presence of basal endothelial growth medium (Clonetics) containing 2% FCS and plasma from tumor-bearing mice (0.25, 0.5, and 1 mg/ml final concentration of mouse plasma proteins). After 2 days in culture, cells were pulsed for an additional 48 hr with 3H-thymidine (1 μCi/well), and then harvested and counted as indicated above. For the growth rescue experiment, 5 × 103 HUVEC or BAEC were plated with plasma from tumor-bearing wild-type or α1-null mice (1 mg/ml) in the presence of anti-K1–3 or TIMP-1 (Chemicon) or BB94. After 2 days in culture, cells were pulsed and harvested as above.
Results
Tumor-Bearing α1-Null Mice Show Reduced Angiogenesis.
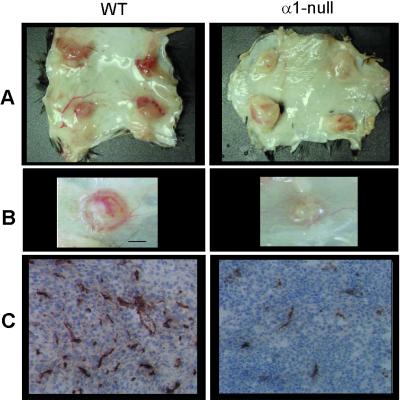
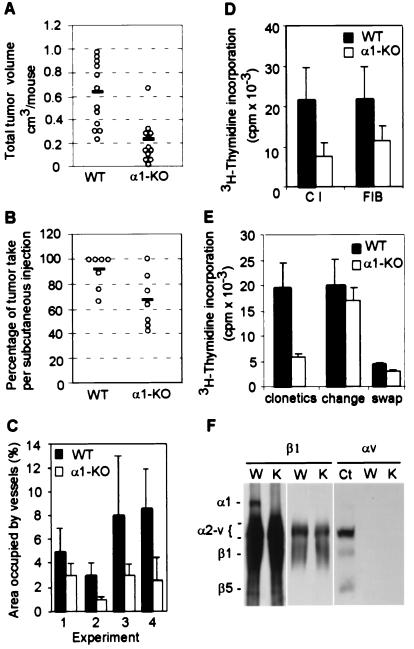
To determine whether tumor vascularization was altered in integrin α1-null mice, tumors were initiated with 105 cells (20) and permitted to grow for 10–14 days before harvest. Tumors from α1-null animals were remarkably paler, less vascularized (Fig. 1 A and B), and smaller (Fig. 2A) than those from wild-type animals, and a smaller proportion of tumors from α1-null hosts grew from the injected initiating cell bolus (Fig. 2B). Tumors vessels from wild-type hosts were positive for integrin α1 and α2 subunits, confirming their localization in microvascular endothelium (19, 25). To quantitate the difference in tumor vascularity, frozen sections of the tumors were immunostained for the endothelial marker CD31 (Fig. 1C). The total area of tumor sections occupied by vascular structures was reduced approximately 50% in the α1-null hosts (Fig. 2C), and the vessels tended to be both smaller and fewer in number. Tumor histology was otherwise similar with minimal inflammation or lymphocyte infiltration.
Figure 1.
Morphological analysis of tumor-bearing wild-type (WT) and α1-null mice. (A and B) Typical gross appearance of tumors grown from four quadrant bolus injections of 105 cells in the subcutis of wild-type and α1-null animals. (A) Complete dissection of dorsal skin after 14 days. (B) Close-up of tumors after 10 days (scale bar = 1 mm). In the wild-type host, tumors appeared strikingly more vascular than those grown in α1-null mice. This difference was apparent regardless of the size of the tumors. (C) Blood vessel staining of tumor sections from wild-type and α1-null mice showing a reduction in size and number in the latter. (Objective magnification: ×20.)
Figure 2.
(A) Tumor volume in wild-type (WT) and α1-null (α1-KO) hosts at 14 days. ○ indicate the total tumor volume for each mouse and the solid line the mean of samples. Four independent experiments were performed, each with a minimum of three animals per genotype. α1-null hosts show a significant reduction in tumor volume (P < 0.05) (B) Proportion of tumors growing from the initiating cell bolus after 10–14 days. The number of tumors grown from the injected initiating cell bolus of 105 cells was counted for each animal. ○ indicate the mean result for each experiment, and the solid line the mean of means. Seven independent experiments were performed, each with a minimum of three animals per genotype. α1-null hosts show a significant reduction in tumor take (P < 0.05). Although tumor size increased with growth time, percentage take did not change with growth time. (C) Tumors from α1-null hosts show reduced vascularity as measured by extent of CD31 positive structures (P < 0.05). Bars and errors indicate the mean and SD (five tumors per genotype were examined in experiment 1, three per genotype in experiment 2, seven per genotype in experiment 3, five per genotype in experiment 4). (D) α1-Null primary lung endothelial cells show reduced proliferation on collagen and fibrinogen as compared with wild-type cella (P < 0.05). The y axis indicates absolute cpm incorporation of primary endothelial cells grown as described in Materials and Methods. Bars and errors indicate the mean and SD of three different experiments (with a total of six animals for each group). (E) Inhibition of proliferation of α1-null endothelial cells is the result of a soluble factor. The y axis is the same as in D. Means and SD are of triplicate samples of endothelial cells from three wild-type and α1-deficient mice. α1-Null cells grow less than their wild-type counterparts when cultured in complete medium (“clonetics”). Changing the medium at 12 hourly intervals (“change”) rescues α1-null cell growth, returning growth to wild-type levels; while adding conditioned media from α1-null cells (“swap”) prevents wild-type cell growth. (F) Immunoprecipitation from lysates of wild-type (W) and α1-null (K) endothelial cells with anti-β1 or anti-αv antibody. The designation α2-v indicates the positions of subunits α2, 3, 4, 5, and v, which are incompletely resolved from one another. Anti-β1 antibody failed to precipitate α1 integrin in α1-null endothelial cells (Left). A light exposure of the same membrane is shown to reveal no differences in α2-v bands between wild-type and α1-null endothelial cells (Middle). Anti-αv antibody failed to precipitate αv integrin in lysates of endothelial cells of both genotype, unlike in those of control embryonic fibroblasts (Ct) (Right).
α1-Null Endothelial Cells Show Reduced Growth on Both Collagen and Fibrinogen.
The reduction in tumor vasculature in α1-null mice might be caused by an intrinsic reduction in proliferation of endothelial cells themselves, similar to that noted for fibroblasts (15). Therefore pulmonary microvascular endothelial cells were obtained from wild-type and α1-null animals. Cells were cultured on 20 μg/ml collagen type I or 10 μg/ml fibrinogen, and their proliferation was assessed by measuring 3H-thymidine incorporation. After 4 days of culture, a 3-fold reduction in cell proliferation was observed in the α1-null endothelial cells, which remained as well attached to the dish as wild-type cells (Fig. 2D). Unexpectedly, this reduction in proliferation was apparent when cells were plated on fibrinogen as well as collagen (Fig. 2D), in marked contrast to our previously observed results for fibroblasts (15). The reduced proliferation on fibrinogen, which would be expected to be independent of α1β1-mediated activation of erk1/2, suggested that absence of the collagen/α1β1/MAPK pathway could not be the sole cause for reduced α1-null endothelial cell proliferation. To determine whether α1-null endothelial cells were making a soluble growth inhibitory factor, endothelial cells were grown for 3 days in the presence of EGM-2-MV/5% FCS, which was replaced every 12 hr. This procedure returned α1-null cell growth to wild-type levels both on collagen (Fig. 2E) and fibrinogen (not shown). Secondly, wild-type endothelial cells cultured in the presence of medium previously conditioned by α1-null cells showed markedly reduced proliferation on both collagen (Fig. 2E) and fibrinogen (not shown). Thus the inhibition of α1-null endothelial cell proliferation was not influenced by the substrate present at the time of plating, but depended on the generation of antiproliferative factor(s) in the culture medium. The fact that α1-null cells proliferated at wild-type levels on collagen when the medium was replaced might suggest that the collagen/α1β1/MAPK proliferative pathway is not as important for endothelial cells as it is for fibroblasts; however, it seems more likely that, in the long time course of the assay, the cells have themselves made significant modification to the matrix, making the effect of the collagen/α1β1/MAPK pathway relatively unimportant.
To ensure that absence of integrin α1 did not lead to alteration of other endothelial integrins, we analyzed endothelial lysates by immunoprecipitation using anti-β1 and anti-αv polyclonal antibodies (Fig. 2F). Apart from the absence of α1 itself, other β1 partners appeared normal in the α1-null cells. αv was absent in both genotypes, consistent with prior reports of quiescent endothelial cells (26).
α1-Null Mice Produce Excess MMPs in Endothelial Cells and Other Tissues.
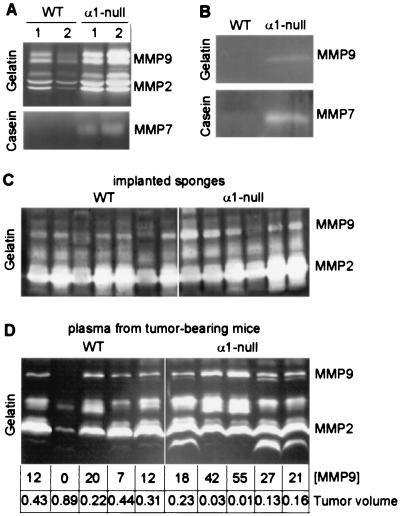
We previously have shown that native collagenase MMP13 transcript is increased in the skin of α1-null animals (18). Zymographic analyses of skin explants revealed increased synthesis of MMP13 (18) as well as gelatinases MMP2 and MMP9 and matrilysin MMP7 in the α1-null skins (Fig. 3A). α1-Null endothelial cells also showed increased production of MMP9 and MMP7 (Fig. 3B), whereas MMP2 levels were comparable between the two genotypes (not shown). To determine whether alterations in MMP expression also could be seen during tissue activation in vivo, we implanted gelfoam sponges s.c. into wild-type and α1-null animals to allow ingrowth of granulation tissue. Like the tumors, sponges in the α1-null hosts appeared less well vascularized than those in the wild-type animals, while the overall histology was otherwise similar, dominated by infiltrating fibroblasts and endothelial cells (not shown). Abundant MMP2 was detected in all the sponges (Fig. 3C) but MMP9 was markedly increased in most α1-null samples compared with the wild type (Fig. 3C). Increased expression of MMP9 also was observed in plasma derived from tumor-bearing α1-null mice and exceeded that seen in wild-type animals (Fig. 3D). Quantification of the MMP9 bands by densitometry revealed a striking inverse correlation between plasma MMP9 levels and total tumor volume, which persisted through all of the wild-type and α1-null samples (Fig. 3D). In the wild-type sample where MMP9 expression was lowest, the tumor volume was the largest. In contrast, the highest expression of MMP9, seen in the α1-null mice, corresponded to the lowest tumor volumes.
Figure 3.
MMP synthesis in wild-type (WT) and α1-null mice. (A) Gelatin and casein zymograms of conditioned media from full thickness skin explants from two wild-type and two α1-null mice. Increased levels of MMP2 (72- to 74-kDa pair), MMP9 (92- to 94-kDa pair), and MMP7 (21–28 kDa) are seen in the two α1-null samples as compared with the wild type. (B) Gelatin and casein zymograms of conditioned medium from wild-type and α1-null primary endothelial cells showing increased level of MMP9 and MMP7 in the α1-null cells. (C) Gelatin zymogram of gelfoam sponges from wild-type and α1-null mice. In most samples, MMP9 levels are increased in α1-null samples relative to the wild-type samples. (D) Gelatin zymogram of plasma from tumor-bearing wild-type and α1-null mice showing similarly increased levels of MMP9 in the plasma from α1-null samples. MMP9 bands were quantified by densitometry and compared with the measured tumor volume (cm3) for each animal. There is a striking inverse correlation between MMP9 expression and tumor size within the two groups.
Tumor-Bearing α1-Null Mice Have Raised Plasma Angiostatin.
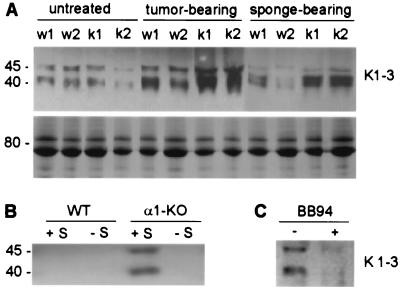
One of the substrates of MMP9 and MMP7 is plasminogen, which is cleaved to yield a variety of closely related molecular species of ≈40–45 kDa containing Kringle 1–4 or 1–3 of plasminogen, collectively termed angiostatin (2, 4, 5). As angiostatin is known to be antiangiogenic in its ability to inhibit endothelial cell proliferation (1), and MMP7 and MMP9 are increased in α1-null mice, we examined whether angiostatin levels in α1-null mice were increased as compared with their wild-type counterparts. Plasma angiostatin from wild-type and α1-deficient mice, which were either untreated, had undergone sponge placement, or were tumor-bearing, was visualized by Western blot with a mouse mAb reacting with both plasminogen and angiostatin. Whereas similarly low levels of angiostatin were detected in the plasma of untreated animals of both genotypes, in the α1-null mice with tumors or sponge implants plasma angiostatin was markedly increased compared with tumor- or sponge-bearing wild-type animals (Fig. 4A). Such a band was not seen in plasma derived from plasminogen-null animals (24) (not shown).
Figure 4.
(A) Angiostatin synthesis in wild-type and α1-null mice. (Upper) Western blot of plasma angiostatin. Increased levels of angiostatin (40- to 45-kDa fragments of plasminogen) are evident in the plasma of α1-null mice (K) with tumors or sponge implants as compared with their wild-type (W) counterparts. Membranes incubated with secondary antibody alone or irrelevant IgG were used as negative controls (not shown). (Lower) Portion of a Coomassie staining of an equal loading of the plasma used for Western blot analysis, showing integrity of the samples. (B) Plasma from tumor-bearing α1-null mice generates angiostatin from mouse serum. Two percent mouse serum (S) was incubated with 7.5 μg total plasma proteins from tumor-bearing wild-type (WT) or α1-null (α1-KO) mice. This amount of total proteins did not yield visible angiostatin bands in plasma from tumor-bearing wild-type (WT −S) and α1-null (α1-KO −S) mice incubated in absence of mouse plasma. In contrast, angiostatin fragments were detected in mouse serum incubated with plasma from tumor-bearing α1-null mice (α1-KO +S), but not in the sample incubated with plasma from tumor-bearing wild-type mice (WT +S). (C) BB94 prevents the generation of angiostatin from mouse serum. Two percent mouse serum was incubated with 7.5 μg total plasma proteins from tumor-bearing α1-null mice in the presence or absence of 2.5 μM BB94. The presence of 2.5 μM BB94 (+ BB94) prevents angiostatin formation.
Angiostatin Is Responsible for Inhibition of Endothelial Cell Growth.
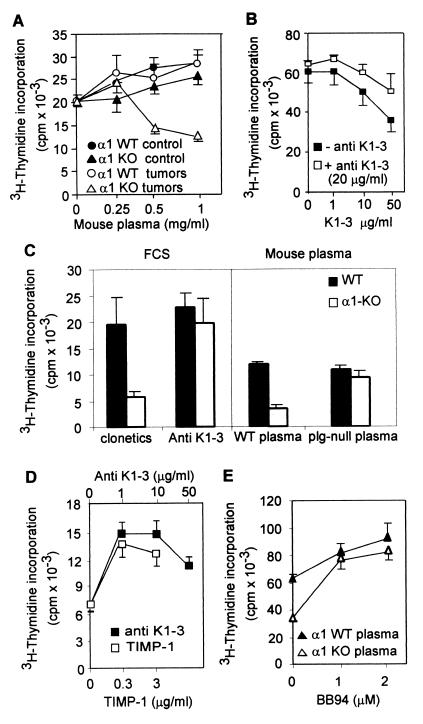
To determine whether the increased angiostatin detected in the plasma of tumor-bearing α1-null mice might contribute to their deficient tumor angiogenesis, we examined 3H-thymidine incorporation of HUVEC grown in the presence of 2% FCS and different concentrations of plasma derived from untreated or tumor-bearing wild-type or α1-null hosts. HUVEC showed similar 3H-thymidine incorporation when treated with plasma from untreated mice of both genotypes or plasma from tumor-bearing wild-type mice (Fig. 5A). In contrast, in the presence of plasma from tumor-bearing α1-null mice, HUVEC showed a marked reduction in proliferation (Fig. 5A). Similar results were obtained by using BAEC (not shown). BAEC grown in the presence of 50 μg/ml K1–3 showed a 50% reduction in proliferation as compared with untreated cells (Fig. 5B), consistent with previous reports (27) and were rescued by coaddition of anti-K1–3 (20 μg/ml) (Fig. 5B), confirming the efficacy of both angiostatin and the antibody. To determine whether angiostatin was the factor causing inhibition of primary α1-null endothelial cell growth, endothelial cells were grown on fibrinogen or collagen in EGM-2-MV/5%FCS containing anti-K1–3 antibody, or in EGM-2-MV with the FCS replaced by plasma derived from plasminogen-null mice (24), thus omitting the substrate from which to generate angiostatin. Either the presence of anti-K1–3 or the absence of plasminogen in the culture medium returned α1-null endothelial cell growth to wild-type levels (Fig. 5C). Anti-K1–3 did not augment the growth of wild-type endothelial cells and thus was not of itself mitogenic.
Figure 5.
(A) Effect of plasma from untreated and tumor-bearing mice on HUVEC proliferation. The y axis indicates absolute incorporation of 3H-thymidine. Means and SDs are of triplicate samples of plasma from three untreated or tumor-bearing animals of each genotype. Only plasma from tumor-bearing α1-null mice reduces HUVEC proliferation (P < 0.05). (B) BAEC proliferation in the presence or absence of antiangiostatin. Angiostatin inhibits BAEC proliferation (− anti-K1–3), whereas addition of anti-K1–3 rescues their proliferation (+ anti-K1–3) (P < 0.05). (C) Angiostatin is responsible for the growth inhibition of α1-null cells. (Left) Cell growth in endothelial growth medium containing 5% FCS (clonetics). Addition of antiangiostatin antibody (Anti-K1–3) at 20 μg/ml rescues growth of α1-null cells. (Right) Growth in media containing 5% mouse plasma instead of FCS. Growth of α1-null cells is inhibited in presence of normal mouse plasma (WT plasma), but is not inhibited in the presence of plasma from plasminogen null mice (plg-null plasma). Means and SDs are of triplicate samples of endothelial cells from three wild-type and α1-null mice. (D) Effect of coaddition of antiangiostatin or TIMP-1 on HUVEC proliferation in the presence of plasma from tumor-bearing α1-null mice. The y axis is as in A. HUVEC were grown for 96 hr with plasma from tumor-bearing α1-null mice (1 mg/ml) in the presence of anti-K1–3 or TIMP-1 at concentrations indicated. Treatment with either antibody or TIMP-1 rescued HUVEC proliferation (P < 0.05). (E) Effects of addition of BB94, at concentrations indicated, on BAEC proliferation in the presence of 1 mg/ml plasma from tumor-bearing wild-type or α1-null mice. The y axis is as in A. Inhibition of BAEC growth by plasma from α1-null tumor-bearing mice is rescued by BB94 (P < 0.05).
MMPs Present in Mouse Plasma Generate Angiostatin.
We hypothesized that the inhibition of HUVEC proliferation observed in the presence of plasma from tumor-bearing α1-null mice (Fig. 5A) was caused not only by the angiostatin present in the mouse plasma, but also by the excess levels of plasma MMPs able to generate angiostatin from the serum in the HUVEC growth medium. To test this hypothesis, HUVEC and BAEC were treated with plasma derived from tumor-bearing α1-null mice in the presence of different concentrations of anti-K1–3 or the well-known inhibitors of gelatinases TIMP-1 and BB94 (28, 29). Treatment with antibody, TIMP-1, or BB94 rescued endothelial cell proliferation from the inhibitory effects of added plasma from tumor-bearing α1-null mice (Fig. 5 D and E), indicating that angiostatin is responsible for the inhibition of cell growth and that the generation of angiostatin is caused by the action of MMP in the added mouse plasma. To confirm this inference, mouse serum (used as a source of plasminogen) was incubated with small amounts of plasma from tumor-bearing wild-type or α1-null animals. Western blot analysis then was performed to detect angiostatin generated from serum. The small amount of tumor-bearing mouse plasma used in the assay (Fig. 4B), or the untreated mouse serum used as its substrate (not shown), did not show any detectable angiostatin. However, when plasma from an α1-null tumor-bearing mouse was added to the substrate serum, detectable angiostatin was generated, indicating that the α1-null tumor-bearing plasma contained catalytic activity to generated angiostatin, which was not observed in the wild-type plasma (Fig. 4B). Furthermore, addition of BB94 prevented the angiostatin generation from mouse serum (Fig. 4C).
Discussion
In this study we have shown that in α1-null mice tumor angiogenesis is markedly reduced compared with that of wild-type animals. This reduction is caused by overexpression of MMPs in the α1-null and consequent generation of angiostatin from circulating plasminogen. Increased MMP in the α1-null mouse is most probably the result of a deficiency in the activation of the Ras/Shc/MAPK pathway (15). Direct inhibition of MAPK strongly enhances expression of fibronectin-induced MMP2 and MMP9 in T lymphocytes (30) and induces an up-regulation of MMP9 synthesis in tumor cells (16) consistent with the increased expression of this MMP in the α1-null animals. This finding suggests that activation of MAPK via α1 integrin is an inhibitory pathway for MMP synthesis. Integrins α2β1, α4β1, and α5β1, normally expressed by endothelial cells (31, 32), are able to up-regulate expression of different MMPs in various cell systems (13, 30, 33), and it is likely that this up-regulation is enhanced in the absence of integrin α1. A second possibility is that loss of α1 integrin leads to an up-regulation of other endothelial integrins able to induce MMPs and consequent angiostatin synthesis. However, the immunoprecipitation data suggests that this is not the case. As integrin α1 is a collagen receptor the question arises as to why wild-type cells do not overproduce MMPs and do not make angiostatin when plated on fibrinogen, which is not a ligand for α1. We can think of two possibilities: either, by the time of assay, the cells have synthesized enough collagen (34–36) to activate the receptor, or integrin α1 in fact acts to inhibit MMP synthesis or release when not bound to ligand (37). Our findings that absence of α1 integrin leads to reduced angiogenesis via the induction of MMP synthesis and consequent angiostatin generation provide a mechanism for the results of Senger et al. (25) showing that inhibition of α1 integrin with blocking antibody markedly reduces vascular endothelial growth factor-driven angiogenesis in vivo. Our results also support the paradigm that secretion of excess MMPs by endothelial cells promotes autoinhibition of angiogenesis by generating angiostatin (5, 11). Indeed, it was observed recently that expression of MMP2 during blood vessel formation can prevent and block angiogenesis by generating angiostatin from circulating plasminogen (6). This finding contrasts the well-documented induction of endothelial cell growth and migration via degradation of extracellular matrix components (10) where, for example, binding of MMP2 to integrin αvβ3 on the tip of sprouting vessels seems to facilitate extracellular matrix degradation and consequent new blood vessel formation (38). Taken together these results suggest that although synthesis of MMPs by endothelial cells is essential for the process of new blood vessel formation by allowing remodeling and invasion, increased production MMPs seems to have a strong role in endothelial cell homeostasis by increasing synthesis of angiostatin. We have shown that in vitro angiostatin is the key inhibitor of endothelial cell proliferation in α1 null cells. It is possible, however, that in vivo other antiangiogenic molecules such as endostatin, which also may be generated by MMP cleavage, also can contribute to the inhibition of tumor vascularisation.
We see inverse correlation between plasma MMP9 and tumor growth not only when comparing wild-type to α1-null animals, as groups, but also between individual wild-type and α1-null animals. Also, although less prominent than in the α1-null, an increase in plasma angiostatin is evident in tumor-bearing wild-type mice. Thus, we would argue that integrin α1-null animals and endothelial cells serve to reveal a mechanism of endothelial homeostasis that is relevant even in normal animals.
In summary we have, unexpectedly, identified a genetic model for regulated, but increased, angiostatin production. In the α1-null mouse loss of a Ras/MAPK pathway leads to production of excess MMPs, including MMP9 and MMP7. The increase in these MMPs, evident in circumstances of tissue activation, generates increased amounts of angiostatin from plasminogen. This increase, in turn, causes a reduction in tumor angiogenesis. This unexpected finding also suggests that MMP inhibitors that inhibit MMP9 or MMP7 may have the unwanted effect, in treating metastatic disease, of preventing angiostatin production and thus causing a paradoxical increase in tumor angiogenesis.
Acknowledgments
This work is dedicated to the memory of George Pardy. We thank Vito Quaranta and Judah Folkman for valuable suggestions and Michael Green and William Kiosses for technical assistance. This work was supported by grants to H.A.G., P.S., and L.A.M. by the National Institutes of Health and to H.A.G. by the Scleroderma Foundation.
Abbreviations
- MAPK
mitogen-activated protein kinase
- MMP
metalloproteinase
- EGM-2-MV
microvascular endothelial growth media
- HUVEC
human umbilical vein endothelial cells
- BAEC
bovine artery endothelial cells
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040378497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040378497
References
- 1.O'Reilly M S, Holmgren L, Shing Y, Chen C, Rosenthal R A, Moses M, Lane W S, Cao Y, Sage E H, Folkman J. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 2.Cornelius L A, Nehring L C, Harding E, Bolanowski M, Welgus H G, Kobayashi D K, Pierce R A, Shapiro S D. J Immunol. 1998;161:6845–6852. [PubMed] [Google Scholar]
- 3.Dong Z, Kumar R, Yang X, Fidler I J. Cell. 1997;88:801–810. doi: 10.1016/s0092-8674(00)81926-1. [DOI] [PubMed] [Google Scholar]
- 4.Patterson B C, Sang Q A. J Biol Chem. 1997;272:28823–28825. doi: 10.1074/jbc.272.46.28823. [DOI] [PubMed] [Google Scholar]
- 5.Sang Q X. Cell Res. 1998;8:171–177. doi: 10.1038/cr.1998.17. [DOI] [PubMed] [Google Scholar]
- 6.O'Reilly M S, Wiederschain D, Stetler-Stevenson W G, Folkman J, Moses M A. J Biol Chem. 1999;274:29568–29571. doi: 10.1074/jbc.274.41.29568. [DOI] [PubMed] [Google Scholar]
- 7.Hanemaaijer R, Koolwijk P, le Clercq L, de Vree W J, van Hinsbergh V W. Biochem J. 1993;296:803–809. doi: 10.1042/bj2960803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson C J, Nguyen M. Int J Biochem Cell Biol. 1997;29:1167–1177. doi: 10.1016/s1357-2725(97)00061-7. [DOI] [PubMed] [Google Scholar]
- 9.Nagashima Y, Hasegawa S, Koshikawa N, Taki A, Ichikawa Y, Kitamura H, Misugi K, Kihira Y, Matuo Y, Yasumitsu H, Miyazaki K. Int J Cancer. 1997;72:441–445. doi: 10.1002/(sici)1097-0215(19970729)72:3<441::aid-ijc11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Hiraoka N, Allen E, Apel I J, Gyetko M R, Weiss S J. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- 11.Kleiner D E, Stetler-Stevenson W G. Cancer Chemother Pharmacol. 1999;43:S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 12.Broberg A, Heino J. Exp Cell Res. 1996;228:29–35. doi: 10.1006/excr.1996.0295. [DOI] [PubMed] [Google Scholar]
- 13.Ravanti L, Heino J, Lopez-Otin C, Kahari V M. J Biol Chem. 1999;274:2446–2455. doi: 10.1074/jbc.274.4.2446. [DOI] [PubMed] [Google Scholar]
- 14.Riikonen T, Westermarck J, Koivisto L, Broberg A, Kahari V M, Heino J. J Biol Chem. 1995;270:13548–13552. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- 15.Pozzi A, Wary K K, Giancotti F G, Gardner H A. J Cell Biol. 1998;142:587–594. doi: 10.1083/jcb.142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vo H P, Lee M K, Crowe D L. Int J Oncol. 1998;13:1127–1134. doi: 10.3892/ijo.13.6.1127. [DOI] [PubMed] [Google Scholar]
- 17.Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- 18.Gardner H, Broberg A, Pozzi A, Laato M, Heino J. J Cell Sci. 1999;112:263–272. doi: 10.1242/jcs.112.3.263. [DOI] [PubMed] [Google Scholar]
- 19.Defilippi P, van Hinsbergh V, Bertolotto A, Rossino P, Silengo L, Tarone G. J Cell Biol. 1991;114:855–863. doi: 10.1083/jcb.114.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soloway P D, Alexander C M, Werb Z, Jaenisch R. Oncogene. 1996;13:2307–2314. [PubMed] [Google Scholar]
- 21.Standker L, Schrader M, Kanse S M, Jurgens M, Forssmann W G, Preissner K T. FEBS Lett. 1997;420:129–133. doi: 10.1016/s0014-5793(97)01503-2. [DOI] [PubMed] [Google Scholar]
- 22.Rivas M J, Arii S, Furutani M, Harada T, Mizumoto M, Nishiyama H, Fujita J, Imamura M. Hepatology. 1998;28:986–993. doi: 10.1002/hep.510280413. [DOI] [PubMed] [Google Scholar]
- 23.Yang J T, Rayburn H, Hynes R O. Development (Cambridge, UK) 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 24.Ploplis V A, Carmeliet P, Vazirzadeh S, Van Vlaenderen I, Moons L, Plow E F, Collen D. Circulation. 1995;92:2585–2593. doi: 10.1161/01.cir.92.9.2585. [DOI] [PubMed] [Google Scholar]
- 25.Senger D R, Claffey K P, Benes J E, Perruzzi C A, Sergiou A P, Detmar M. Proc Natl Acad Sci USA. 1997;94:13612–13617. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks P C, Clark R A, Cheresh D A. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 27.Lucas R, Holmgren L, Garcia I, Jimenez B, Mandriota S J, Borlat F, Sim B K, Wu Z, Grau G E, Shing Y, et al. Blood. 1998;92:4730–4741. [PubMed] [Google Scholar]
- 28.Gomez D E, Alonso D F, Yoshiji H, Thorgeirsson U P. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- 29.Wojtowicz-Praga S M, Dickson R B, Hawkins M J. Invest New Drugs. 1997;15:61–75. doi: 10.1023/a:1005722729132. [DOI] [PubMed] [Google Scholar]
- 30.Esparza J, Vilardell C, Calvo J, Juan M, Vives J, Urbano-Marquez A, Yague J, Cid M C. Blood. 1999;94:2754–2766. [PubMed] [Google Scholar]
- 31.Luscinskas F W, Lawler J. FASEB J. 1994;8:929–938. doi: 10.1096/fasebj.8.12.7522194. [DOI] [PubMed] [Google Scholar]
- 32.Polverini P J. Am J Pathol. 1996;148:1023–1029. [PMC free article] [PubMed] [Google Scholar]
- 33.Huhtala P, Humphries M J, McCarthy J B, Tremble P M, Werb Z, Damsky C H. J Cell Biol. 1995;129:867–879. doi: 10.1083/jcb.129.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko M K, Kay E P. Mol Vision. 1999;5:17. [PubMed] [Google Scholar]
- 35.Gu X, Ko M K, Kay E P. Invest Ophthalmol Visual Sci. 1999;40:289–295. [PubMed] [Google Scholar]
- 36.Lenz O, Striker L J, Jacot T A, Elliot S J, Killen P D, Striker G E. J Am Soc Nephrol. 1998;9:2040–2047. doi: 10.1681/ASN.V9112040. [DOI] [PubMed] [Google Scholar]
- 37.Gardner H A. Curr Rheum Rep. 1999;1:28–33. doi: 10.1007/s11926-999-0021-5. [DOI] [PubMed] [Google Scholar]
- 38.Brooks P C, Stromblad S, Sanders L C, von Schalscha T L, Aimes R T, Stetler-Stevenson W G, Quigley J P, Cheresh D A. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]