Abstract
Genetically engineered, neuroattenuated herpes simplex viruses (HSVs) expressing various cytokines can improve survival when used in the treatment of experimental brain tumors. These attenuated viruses have both copies of γ134.5 deleted. Recently, we demonstrated increased survival of C57BL/6 mice bearing syngeneic GL-261 gliomas when treated with an engineered HSV expressing IL-4, as compared with treatment with the parent construct (γ134.5−) alone or with a virus expressing IL-10. Herein, we report construction of a conditionally replication-competent mutant expressing both subunits of mIL-12 (M002) and its evaluation in a syngeneic neuroblastoma murine model. IL-12 induces a helper T cell subset type 1 response, which may induce more durable antitumor effects. In vitro studies showed that, when infected with M002, both Vero cells and murine Neuro-2a neuroblastoma cells produced physiologically relevant levels of IL-12 heterodimers, as determined by ELISA. M002 was cytotoxic for Neuro-2a cells and human glioma cell lines U251MG and D54MG. Neurotoxicity studies, as defined by plaque-forming units/LD50, performed in HSV-1-sensitive A/J strain mice found that M002 was not toxic even at high doses. When evaluated in an intracranial syngeneic neuroblastoma murine model, median survival of M002-treated animals was significantly longer than the median survival of animals treated with R3659, the parent γ134.5− mutant lacking any cytokine gene insert. Immunohistochemical analysis of M002-treated tumors identified a pronounced influx of CD4+ T cells and macrophages as well as CD8+ cells when compared with an analysis of R3659-treated tumors. We conclude that M002 produced a survival benefit via oncolytic effects combined with immunologic effects meditated by helper T cells of subset type 1.
Eradication of malignancies arising in the brain has proven to be a formidable task. As an example, gliomas, the most common primary brain tumor, are almost always fatal despite aggressive surgical resection, radiotherapy, and chemotherapy; the overall 5-year survival rate for glioblastoma (GBM), the most malignant glioma, is less than 5.5%, and the median survival is approximately 1 year.
Because of the grim prognosis for such patients, alternative therapeutic approaches, most notably viral and gene therapy, have been investigated (for reviews, see refs. 1–3). We and others have reported on the use of neuroattenuated replication-competent herpes simplex viruses (HSVs) for the treatment of primary brain tumors. These viruses typically contain one or more mutations within the viral genome, including thymidine kinase (tk; ref. 4), ribonucleotide reductase (5, 6), UTPase (7), or γ134.5 (8, 9). Mutations within the γ134.5 gene, located within the inverted repeats of the unique long segment, have been shown to cause reduction in neurovirulence of HSV (10). Moderate increases in long-term survival for engineered HSV-treated versus untreated animals have been reported in both syngeneic and xenogeneic murine tumor models of GBM (1, 4, 8, 9, 11–15). In addition, phase I studies in humans with malignant glioma suggest that a multiply mutated HSV (G207) at doses up to 3 × 109 plaque-forming units (pfu) is safe for intracranial inoculation (J.M.M., M. D. Medlock, S. D. Rabkin, G.Y.G., F. Feigenbaum, W. D. Hunter, T. Todo, C. Tornatore, and R. L. Martuza, unpublished data).
Despite these advantages, it seems likely that multiple modalities of therapy will be necessary to eradicate malignant tumors of the central nervous system as well as those originating outside the brain. To increase the efficacy of antineoplastic therapy, we are studying mutant HSVs as vectors for gene therapy. These HSVs, which contain identical deletions within both copies of the γ134.5 gene, retain the ability to replicate in and to lyse rapidly dividing cells, such as those found in tumors, but are unable to replicate in postmitotic cells, such as those found in the normal adult central nervous system. We will refer to such HSVs as conditionally replication-competent. Additionally, they have been engineered to express foreign genes designed to augment their antitumor effects. Initially, we studied conditionally replicating mutants expressing IL-4 and IL-10 (13). These viruses were evaluated in an orthotopic model of murine GBM by using syngeneic GL-261 tumors implanted into immunocompetent C57BL/6 mice. In this model, treatment with IL-4-expressing HSV increased survival over treatment with HSV alone, suggesting that cytokine gene therapy may mediate enhanced tumor-specific killing. IL-4 gene therapy has been shown to enhance antiglioma effects in several gene therapy models (16–18). Such effects are mediated by helper T cells of subset 2 (TH-2) and have been attributed to CD4+ lymphocytes and other effector cells such as eosinophils (19). Although IL-4 was effective in these animal models, generation of a TH-1 response, including the induction of a memory response against tumor cells, might be hypothesized to have a more durable antitumor effect.
We therefore constructed a virus expressing a cytokine with increased potential for a tumor-specific response. IL-12 is a cytokine with potent antitumor properties. It is produced by antigen-presenting cells, including B lymphocytes, dendritic cells, and monocytes. It acts to enhance the cytolytic activity of natural killer cells and cytotoxic T lymphocytes as well as the development of a TH-1-type immune response (20–26). IL-12 also possesses antiangiogenic properties, which may represent a second potential mechanism for its antitumor activity (27, 28). IL-12 has been shown to produce antiglioma immune activity in two different rodent models (29, 30). Although experimental models that use IL-12 for gene therapy have been promising, none have used IL-12 expressed from a replication-competent vector (20, 29, 31–34). Notably, phase I human studies that use systemic IL-12 therapy have shown the toxicity of this cytokine, presumably caused by its pleitropic effects (35).
We have developed a conditionally replication-competent γ134.5− mutant, which expresses mIL (mIL)-12 (M002), to evaluate treatment of experimental murine brain tumors. Our results are summarized as follows. (i) M002 retains its ability to replicate in murine tumor cells, thus mediating direct tumor cell oncolysis. (ii) M002 produces IL-12 at physiologically relevant amounts (≈1 ng/ml), allowing for direct expression of the cytokine within the tumor cells after inoculation. (iii) Survival of immunocompetent A/J mice, a murine strain more sensitive to HSV infection, that were implanted with the syngeneic Neuro-2a clone of C1300 neuroblastoma tumor cells was significantly longer (median = 52 days) after treatment with M002 than it was after treatment with the non-cytokine-expressing parent virus R3659 (median = 24 days). (iv) Immunohistochemical analysis of brain tissue identified significantly more immune-related inflammatory infiltration by CD4+ T cells, macrophages, and, to a lesser extent, CD8+ T cells in the M002-treated tumors than in the R3659-treated tumors. We conclude that the oncolytic effect of M002 is enhanced by an IL-12-induced antitumor immune response.
Materials and Methods
Cells.
Vero cells (American Type Culture Collection) were grown and maintained in MEM (Cellgro, Mediatech, Washington, DC) containing 7% (vol/vol) FBS. The human 143 tk− cells (143tk−, American Type Culture Collection) were grown in DMEM (Cellgro) supplemented with 10% (vol/vol) FBS. Rabbit skin cells (originally acquired from J. McClaren, University of New Mexico, Albuquerque, NM) were maintained in DMEM supplemented with 5% (vol/vol) FBS. The human malignant glioma cell lines U251MG and D54MG were obtained from D. Bigner (Duke University, Durham, NC), whereas the murine neuroblastoma cell line Neuro-2a (derived from strain A/J mice) was purchased from the American Type Culture Collection (CCL 131, passage 171). These latter three cell lines were maintained in a 50:50 (vol/vol) mixture of DMEM and Ham's Nutrient Mixture F-12 (DMEM/F12) supplemented with 2.6 mM l-glutamine and 7% (vol/vol) FBS.
Plasmids and Viruses.
HSV-1 (F) strain is a low passage clinical isolate used as the prototype HSV-1 strain in our series (36, 37). Virus R3659 has been described (38). Construction of M002, which expresses mIL-12 under the transcriptional control of the murine early-growth response-1 promoter (Egr-1), is described below. This strategy is identical to that used to construct the cytokine-expressing viruses R8306 (mIL-4) and R8308 (mIL-10; ref. 13). The plasmids containing the p40 and p35 subunits of mIL-12 in pBluescript SK(+) (Stratagene; ref. 39) were kindly provided by Ueli Gubler (Hoffmann–LaRoche). The coding region for the p40 subunit was removed by digestion with HindIII (5′ end) and BamHI (3′ end), whereas the coding region for the p35 subunit was removed by digestion with NcoI (5′ end) and EcoRI (3′ end). The internal ribosome entry site (IRES) sequence was amplified from the vector pCITE-4a+ (Novagen) by using PCR and primers 5′-CITE (5′-CGCGGATCCTTATTTTCCACCATATTGCC-3′), which has a BamHI site, and 3′-CITE (5′-GGAGCCATGGATTATCATCGTGTTTTTC-3′), which has an NcoI site that retains the translational start sequence. Plasmid pBS-IL-12 was constructed by three-way ligation of the murine p40, murine p35, and IRES sequences into the HindIII and EcoRI sites of pBS-SK+, such that the IRES sequence separates the p40 and p35 coding sequences. This arrangement effectively duplicates a strategy previously reported for expression of the mIL-12 subunits (33). The IL-12 genes were entirely sequenced by the University of Alabama at Birmingham Cancer Center DNA Sequencing Facility.
The HSV shuttle plasmid pRB4878 has been described (ref. 13; kindly provided by Bernard Roizman). Plasmid 4878-IL-12 was constructed as follows. pBS-mIL-12 was digested with XhoI and SpeI to remove a 2.2-kilobase (kb) fragment containing the entire IL-12 subunit coding regions, including the IRES; the ends were filled in with the Klenow fragment, and the construct was ligated into a blunted KpnI site located between the Egr-1 promoter and hepatitis poly(A) sequences within pRB4878. M001 (tk−) and M002 (tk repaired at the native locus) were constructed via homologous recombination as described (13). Two tk-repaired viruses, M002.29 and M002.211, were confirmed by Southern blot hybridization of restriction enzyme-digested viral DNAs that were electrophoretically separated on a 1% agarose, 1× Tris-phosphate EDTA gel and transferred to a Zeta-Probe membrane (Bio-Rad). The blot was hybridized with the appropriate DNA probe labeled with alkaline phosphatase by using the Gene Images AlkPhos Direct DNA labeling system (Amersham Pharmacia). IL-12 production was established by ELISA.
ELISA.
Production of mIL-12 by the recombinant viruses was confirmed and quantified by using a murine p70 ELISA kit (R & D Systems). Six-well plates were seeded at a confluence of 4 × 105 cells per well 1 day before infection with M002 or control virus at a multiplicity of infection of 1 in a total volume of 0.5 ml. After 2 h, inoculum was removed; 1 ml of growth medium was overlaid onto infected wells; and plates were incubated for 24 h at 37°C. The supernatant was removed, and either undiluted or 10-fold dilutions of supernatants were analyzed by ELISA, according to the manufacturer's protocol. Experiments were performed at least three times to determine average level of cytokine production.
In Vitro Characterization of M001/M002.
In vitro replication of M001 in subconfluent cultures of U251MG and D54MG cells was determined as described (13) at 12, 24, 48, and 72 h after infection. For cytotoxicity assays, the various cell lines were infected with M002.29 and M002.211 at a multiplicity of infection of 1. The dose producing 50% cell kill was determined by alamarBlue (Accumed International, West Lake, OH) assay as described (12). Dye conversion values were obtained by reading plates on a Bio-Tek EL310 plate reader (Winooski, VT) with the OD value at 590 nm subtracted from the OD at 562 nm. The decrease in OD relative to uninfected cells was plotted against number of virus pfu/ml to determine the number of pfu needed to produce a 50% reduction in OD.
Animals.
Specific pathogen-free female A/J strain mice were obtained from Charles River Breeding Laboratories and used at approximately 8 weeks of age. All animal studies were conducted in accordance with guidelines for animal use and care established by the University of Alabama at Birmingham Animal Resource Program and the Institutional Animal Care and Use Committee (protocol 97K03985).
In Vivo Characterization of M002.
For determination of M002 neurovirulence in A/J strain mice, graded numbers of virus pfu were prepared in sterile milk, and 5 μl of each dilution was inoculated into the right cerebral hemisphere of three to five mice as described (9). For survival studies, A/J strain mice were stereotactically inoculated with 104 Neuro-2a cells in the right cerebral hemisphere. After 5 days, mice were randomly divided into three cohorts of 10 mice each, and 5 × 106 to 1 × 107 pfu of M002, R3659, or vehicle were stereotactically inoculated at the same site of tumor inoculation. Mice were assessed daily; moribund mice were killed, and the date of death was recorded as described (9).
Histopathology.
Three sets of three mice each were injected with Neuro-2a and then treated with M002, R3659, or vehicle as described above. At days 3, 5, 6, 7, 9, 10, and 14, one mouse from each group was killed, and its brain was harvested and frozen in Tissue-Tek OCT compound (Miles, Elkhart, IN). Sections that were 10–12 μm thick were cut through the injection site in each brain and mounted on 3-aminopropyltriethoxysilane-coated slides, fixed in 95% (vol/vol) ethanol, and blocked in PBS-2% (wt/vol) BSA. Sections were stained with standard hematoxylin and eosin to determine the degree of residual tumor, the presence of neurotoxicity, and the extent of any inflammatory response. To characterize the nature of the inflammatory infiltrate, serial sections were reacted with rat monoclonal antibodies specific for mouse CD4, CD8, and macrophage markers, and the antibody binding was detected by using biotinylated rabbit anti-rat Ig followed successively with an avidin-biotin-horseradish peroxidase complex and 1% diaminobenzidine (13).
Results
Construction of a Recombinant HSV-1 Expressing mIL-12.
Previously, we demonstrated that recombinant HSVs that express mIL-4 could significantly improve survival when injected into tumors implanted in brains of immunocompetent mice in a syngeneic murine model (13). To extend these initial studies, we wanted to evaluate recombinant HSVs that would express the well described antitumor cytokine IL-12.
Biologically active mIL-12 consists of a heterodimer of the p40 and p35 subunits. We therefore constructed recombinant HSV M001(tk−) and M002 (tk+) to express both mIL-12 subunits within a single expression cassette, separated by the IRES from the 5′ untranslated region of equine encephalomyocarditis virus (Fig. 1). Recombinant virus M001 was obtained by cotransfection of plasmid DNAs with R3659 viral DNA and selection of tk(−) viruses on 143tk− cells overlaid with medium containing 100 μg/ml bromodeoxyuridine. The recombinant tk− mIL-12-expressing virus M001 was confirmed by Southern blot hybridization (data not shown). Recombinant virus M002 was obtained by cotransfection of M001 viral DNA with pRB4867, a plasmid used to repair the 501-bp deletion within the tk gene in its native locus (UL23), and by subsequent selection in HAT (hypoxanthine/aminopterin/thymidine) medium. These recombinant viruses contain two copies of the IL-12 construct replacing both copies of the γ134.5 gene. To confirm the presence of the mIL-12 insert in M002, viral DNAs were isolated, digested with NcoI, and evaluated by Southern blot hybridization as described in Materials and Methods and shown in Fig. 2. Repair of the tk gene was also verified by hybridization to a probe specific for the tk gene insert (data not shown).
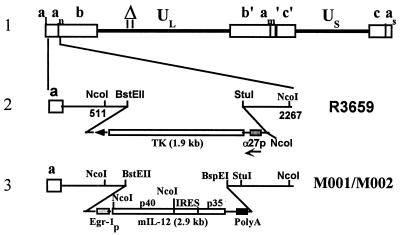
Figure 1.
Schematic representation of mIL-12-expressing HSV (M002). Line 1 illustrates the HSV-1 (F) Δ305 genome, which contains a 501-bp deletion within the tk gene, as indicated by the Δ symbol. UL and US represent the unique long and unique short sequences, respectively. The inverted repeat sequences are indicated by a, b, and c, with subscripts n and m representing variable numbers of a sequences. a1 and as represent the a sequences flanking the UL and US terminal repeats. Line 2 shows the sequence arrangement of the recombinant HSV R3659. The BstEII–StuI fragment within the γ134.5 gene was replaced by the chimeric α27-tk gene in the inverted sequences ab (shown above) and b′a′ (not shown) flanking the UL sequence. Line 3 shows the sequence arrangements of the relevant regions in the recombinant mIL-12-expressing HSV M001 (tk−) or M002 (tk+). NcoI restriction sites are indicated.
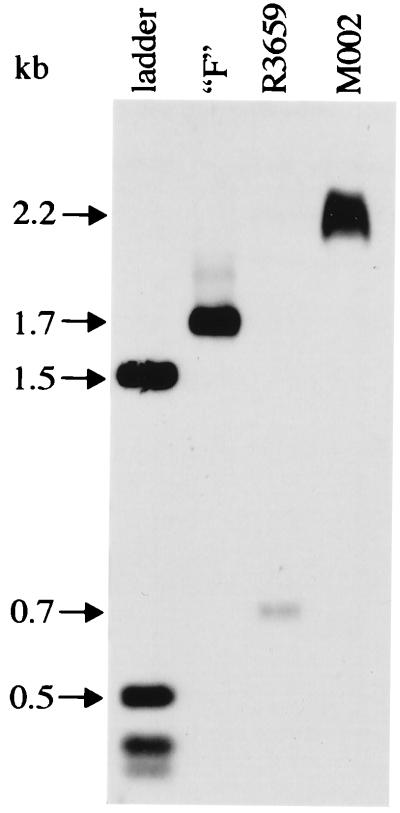
Figure 2.
Southern blot hybridization confirming the presence of mIL-12 in M002. Viral DNAs were isolated, digested with NcoI, and electrophoretically separated, and Southern blot hybridization was performed as described in Materials and Methods. The predicted fragment sizes for each viral DNA (1.76 kb for HSV-1 (F), 0.7 kb for R3659, and 2.2 kb for M002) are indicated by arrows. The 1.6-kb, 0.52-kb, and all smaller bands of the 1-kb DNA ladder (Life Technologies, Grand Island, NY) will also hybridize to the probe, which has the same vector backbone as the ladder.
Expression of mIL-12 by M002.
To determine whether the recombinant viruses expressed physiologically relevant levels of mIL-12, we quantified culture supernatants from M002- or R3659-infected Vero and Neuro-2a cells by using a commercially available ELISA kit specific for mIL-12 p70 heterodimers. We evaluated IL-12 production from two genetically identical subclones of M002, clone 29 and clone 211. The averaged values are indicated in Table 1. The highest production of mIL-12 by M002 was seen in Vero and Neuro-2a cells, which produced 3–4 ng per 5 × 105 cells per 24 h after infection at a multiplicity of infection of 1. Production was slightly lower in D54MG and U251MG cell lines, at 1.8 and 0.8 ng per 5 × 105 cells per 24 h. Such levels have been shown to produce antitumor responses in other models (29, 40).
Table 1.
IL-12 production by M002 in normal and tumor cell lines
| Cell type | Cytokine production, pg per ml per 24 h* | |
|---|---|---|
| Vero | 3,400 | |
| D54MG | 1,780 | |
| U251MG | 820 | |
| Neuro-2A | 3,240 | |
Values represent only mIL-12 heterodimers.
Growth of Wild-Type and Recombinant Viruses in Tumor Cell Lines.
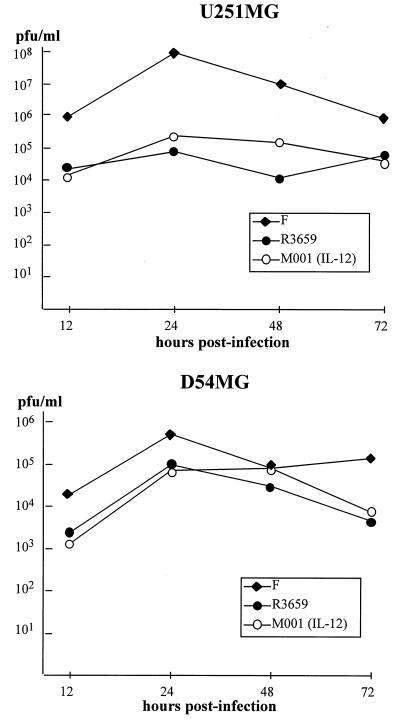
Before repairing the tk gene to create M002, we first established the replication competence of our tk(−) IL-12-expressing HSV (M001) as compared with wild-type F or the backbone virus R3659 in the human glioma cell lines D54MG and U251MG. As indicated in Fig. 3, M001 replicated as well as R3659 in both glioma cell lines and as well as the wild-type F strain in D54MG. This finding confirmed replication competence of the IL-12-expressing virus, allowing comparisons with other cytokine-expressing viruses.
Figure 3.
In vitro replication of M001 in human glioma cells. Replicating monolayers of U251MG (Upper) or D54MG (Lower) human malignant glioma cell lines were infected at 1 pfu per cell with HSV-1 (F) (closed diamonds), R3659 (closed circles), or M001 (open circles). Replicate cultures were harvested at 12, 24, 48, and 72 h after infection, and virus titers were determined on Vero cell monolayers.
Viruses containing mutations or deletions within the γ134.5 locus have been shown to have a direct cytolytic effect on D54MG and U251MG (12). We quantitatively measured the cytolytic activity of M002 on Neuro-2a cells as well as D54MG and U251MG cells by alamarBlue assay and compared the results with R3659 cytolytic activity. As shown in Table 2, the cytolytic activity of M002 was slightly higher than that of R3659 in all cell lines tested. Thus, this virus is at least as cytotoxic in both human glioma cells and in murine Neuro-2a cells as its parent virus and may even have a slight growth advantage.
Table 2.
Viral cytotoxicity of tumor cell lines
| Cells | Tumor/cell origin | Cytotoxicity, pfu/TD50
|
||
|---|---|---|---|---|
| R3659 | M002.29 | M002.211 | ||
| U251MG | GBM | 1.9 | 1 | 1.1 |
| D54MG | GBM | 14.4 | 1.6 | 7.8 |
| Neuro-2A | Neuroblastoma | 3 | 2.6 | 5.6 |
Values were obtained 3 days after virus infection and a 3-h incubation with alamarBlue dye.
A Syngeneic Model for Neuroblastoma.
Previously, we have used the GL-261 glioma cell line, derived from C57BL/6 mice, which are relatively resistant to HSV-1 infection (41). This property is less than ideal from the standpoint of evaluating recombinant cytokine-expressing HSV therapeutically. Thus, we used the Neuro-2a spontaneous neuroblastoma of A/J mouse origin because of the known sensitivity of these cells to HSV-1 (41). Neuro-2a, one of several clonal derivatives of the C-1300 spontaneous neuroblastoma of A/J mice, has been transplanted in various sites, including intracerebrally, for evaluating multiple therapeutic modalities. Macklis and Madison (42) implanted C-1300 in the brains of mice, and we have modified this approach for our purposes. We determined the optimal tumor cell dose for evaluating M002 in intracranial Neuro-2a tumors by inoculating 103, 104, or 105 cells stereotactically into the caudate and monitoring survival. A dose-response curve was defined and ranged from 14 to 25 days. Based on this study, we elected to inoculate between 1 × 104 and 1 × 105 cells to produce a median survival of 3 weeks from tumor induction to facilitate a rapid and stringent evaluation of the survival effects of the therapeutic viruses.
Neurovirulence.
Previous studies with G207, a genetically engineered HSV-1 currently in human trials for the treatment of malignant glioma, have shown no neurovirulence in this assay at doses of 107 pfu. Thus, the maximum tolerated dose (MTD) was determined (pfu/LD50) for both clones of our IL-12-expressing virus, M002.29 and M002.211. Despite apparently identical clones based on our Southern blot analysis, MTD between the two clones differed somewhat (MTD for clone 211 = 2 × 107 pfu, whereas MTD for clone 29 = 5 × 106 pfu; data not shown). We presume this difference is due to subtle genetic alterations not yet defined. Because clone 211 seemed to be the safer of the two, we tested this virus for experimental therapy of Neuro-2a tumors and refer to it as M002.
Survival of A/J Strain Mice with Intracerebral Neuro-2a Neuroblastomas Treated with M002.
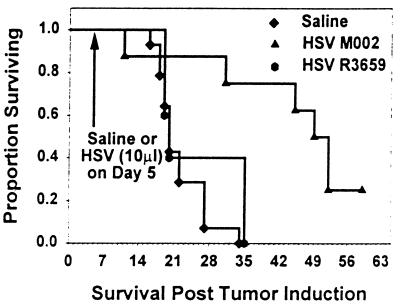
To evaluate the sensitivity of Neuro-2a tumors to HSV infection in A/J strain mice, 1 × 104 tumor cells were injected intracranially into A/J female mice, followed 5 days later by intratumoral injection of 1 × 107 pfu (in 5 μl) of either R3659 or M002. Control mice were injected with 5 μl of the diluent. Data shown in Fig. 4 represent a composite of three experiments; the median survival after tumor induction in mice injected with diluent only was 19.8 days, and all animals were dead by day 34. In contrast, mice with M002-injected tumors had a median survival of 50.5 days (P = 0.00023). Mice that received an intratumoral injection of R3659 had a median survival (19.5 days) that was not significantly different (P = 0.556) from vehicle-treated mice. Survivors were killed at 59 days, and brains were examined histologically. There was no evidence of residual tumor.
Figure 4.
Survival of A/J strain mice with intracerebral Neuro-2a neuroblastomas treated with M002. Neuro-2a cells (1 × 104/5 μl) were injected intracerebrally in A/J mice. After 5 days, intracerebral tumors were injected with 10 μl of saline or 2 × 107 pfu of HSV R3659 or HSV M002, and mouse survival was monitored. Median survival for saline-treated mice was 19.8 days versus 50.5 days for M002-treated mice (P = 0.0002) and 19.5 days for HSV R3659-treated mice. Histologic examination of the brains of survivors killed at 59 days identified no persistent tumor.
Immunohistologic Identification of Inflammatory Cell Infiltrates.
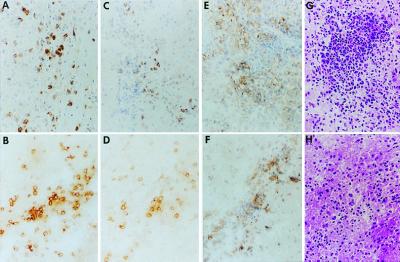
Hematoxylin and eosin staining of mouse brain sections at 5 days after injection of virus identified foci of small cells with a high nuclear to cytoplasmic ratio observable in multiple cerebrospinal fluid compartments. We examined mouse brains intratumorally injected with either the parent γ134.5− HSV (R3659) or M002 HSV at various time points after virus inoculation (see Materials and Methods). R3659 induced a mild but discernible immune-related inflammatory response characterized principally by CD4+ T cells and macrophages with a few CD8+ T cells. These inflammatory cells were scattered throughout the tumor mass with occasional foci predominated by macrophages or CD4+ T cells. In contrast, injection of M002 elicited a pronounced influx of CD4+ T cells and macrophages, with a significant increase in CD8+ T cells as well. Inflammatory responses were first evaluated at 3 days after injection of virus or saline, reached maximum levels around days 5–6, and had begun to regress by day 7 after viral injection (Fig. 5).
Figure 5.
Immunohistologic identification of inflammatory cell infiltrates. A/J female mice were injected intracerebrally with Neuro-2a cells (1 × 105/5 μl) and 5 days later were injected intratumorally with 1 × 107 pfu of HSV R3659 (A, C, E, and G) or HSV M002 (B, D, F, and H). After 6 days, the mice were killed, and their brains were removed intact and embedded in OCT for preparation of frozen sections. Serial 10-μm-thick sections were reacted with rat monoclonal antibodies to CD4+ (A and B) or CD8+ T (C and D) cells or macrophages (E and F); antibody binding was detected by using horseradish peroxidase-labeled anti-rat antibody, and sections were counterstained with Mayer's hematoxylin. Hematoxylin-eosin stained adjacent sections are also shown (G and H).
Discussion
Conditionally replicating, engineered HSVs have been shown to be effective antineoplastic agents in glioma and other animal tumor models. Both growth inhibition of subcutaneous tumors and improvement in survival of mice implanted with intracranial tumors have been shown repeatedly. However, only a fraction of animals seem to be cured by viral therapy with current γ134.5− vectors. To improve the efficacy of this strategy, we have evaluated oncolytic γ134.5− HSVs as gene therapy vectors. M002 is one such vector that expresses mIL-12 via bicistronic expression of the p35 and p40 subunits separated by an IRES sequence. Our study produced a self-assembling, heterodimeric, functionally active molecule in HSV-1.
Additionally, we have shown that p70 heterodimer expression occurs in multiple tumor cell types, including murine neuroblastoma cells (Neuro-2a) and human malignant glioma cells (D54MG and U251MG). Replication of HSV-1 in tumor cells is not affected in vitro by expression of IL-12, and tumoricidal effects in vitro are not diminished and may actually be increased. By using a stringent model of neurovirulence, intracerebral inoculation (up to 2 × 107 pfu of IL-12-expressing virus) could be injected into the hypersensitive A/J strain mouse without producing clinical evidence of toxicity.
Lack of an appropriate immunocompetent animal glioma model required us to develop a different system for studying the effects of our virus in vivo. In comparison to human GBM cells, GL-261 and other C57BL/6 tumor cells are relatively resistant to HSV-1 infection (G.Y.G., unpublished data); rat cells also are resistant to γ134.5− HSV mutants and are thus inappropriate models for determining the neurotoxicity of this treatment (3). The Neuro-2a murine neuroblastoma, another nervous system tumor, was selected for study because of its infectability comparable to human gliomas and its lack of immunogenicity (J.N.P. and J.M.M., unpublished results). In contrast to implanted GL-261 glioma cells, which grow as a solid tumor mass principally at the site of implantation, Neuro-2a cells mimic the tendency of human neuroblastomas to disseminate along cerebrospinal fluid pathways (43) and tend to kill the mice by growing at distant sites. A reproducible survival model was developed by using intracranial inoculation of 104 Neuro-2a cells, which resulted in 100% death of untreated animals within 28 days with a median survival of approximately 23 days. Treatment with R3659 did not appreciably increase survival over control animals. We presume that the tendency of this tumor to metastasize along cerebrospinal fluid pathways might explain the discrepancy between the in vitro and in vivo R3659. However, treatment with M002 resulted in a dramatic increase in median survival with some apparent cures. We hypothesize that, although the γ134.5− HSVs alone have a direct cytolytic effect, M002 had a greater global effect because of production of IL-12 and its resultant antitumor effects, regardless of tumor cell location.
Histopathology and immunohistochemistry showed a significant increase in CD4+ and CD8+ lymphocytes as well as macrophages in the animals treated with M002, whereas animals treated with R3659 had a minimal inflammatory response. This inflammatory response was far greater in regions infiltrated by tumor, even when distant from the site of inoculation. The inflammatory response peaked 5–6 days after inoculation. We have not yet determined whether virus is present at these distal sites.
Enhancement of the antitumor immune response has been studied in a variety of animal models with some success. Such an approach has also been successful with conditionally replicating HSV. An engineered γ134.5− HSV constructed to express mIL-4 showed increased antiglioma activity when compared with its parent virus, R3616, which expressed no foreign genes, or when compared with a similar vector expressing IL-10 (13), which is inhibitory for macrophage-dependent T cell activation. Immunohistochemistry showed the influx of inflammatory cells in murine gliomas treated with IL-4 HSV. This infiltrate, which consisted chiefly of CD4+ lymphocytes and macrophages as well as CD8+ lymphocytes, seemed to account for the increase in survival seen with HSV expressing IL-4.
IL-4 induces a TH-2 response and down-regulates the TH-1 response (44). Although it has been shown to have antitumor efficacy in some models, establishment of a TH-1-type response might be expected to improve antitumor efficacy. Certain central nervous system tumors, such as gliomas, have been shown to down-regulate the induction of a systemic TH-1 response by interfering with the expression of the α-subunit of the IL-2 receptor. However, other cytokines are capable of inducing a TH-1 response without reliance on this receptor. We chose to study one such cytokine, IL-12, for expression in a conditionally replicating γ134.5− HSV. Although IL-12 has been shown to have anti-HSV activity when given in large doses (45), its local expression by M002 did not prevent the vector from producing greater antitumor effects than the parent R3659 virus. Future investigations will be facilitated by the development of an immunocompetent murine model for glioma with GL-261 cells genetically modified such that infectability by HSV-1 mutants resembles that of human gliomas. Comparison of IL-4 and IL-12-expressing viruses in such a system would be informative.
In summary, we have demonstrated that a conditionally replicating γ134.5− HSV expresses IL-12 in a variety of tumors and can provide safe therapy with increased efficacy and survival in a model of nervous system tumors. A major benefit of this type of therapy is that M002-infected tumors experience increased infiltration by a variety of immune effector cells, thus enhancing the antitumor activity mediated by our virus. This capability suggests that M002 may have the potential for use as an antitumor vaccine. As a drawback, increased infiltration of immune effector cells not only contributes to clearance of the virus but could be detrimental to surrounding tissue. Future studies will need to optimize viral dose for therapy as well as evaluate therapies combining M002 with γ134.5− mutant HSV expressing other immunomodulatory molecules. We conclude that a γ134.5− HSV expressing IL-12 increases survival via oncolytic effects combined with immunologic effects. Further research is necessary to explore the use of M002 in other tumor systems as well as to investigate the immunologic and antiangiogenic basis for its antitumor effects.
Acknowledgments
We thank Dr. Bernard Roizman for helpful discussion and comments throughout the course of this work. We also thank Sabrina Jenkins for technical assistance. Studies performed by the authors were initiated and supported by Antiviral Research Branch Contract NO1-AI-62554 of the National Institute of Allergy and Infectious Diseases (to R.J.W.); Program Project Grants PO1 AI 24009 (to R.J.W.) and P01 CA 71933 (to R.J.W.); Unrestricted Grants in Infectious Diseases from Bristol-Myers Squibb (to R.J.W.); General Clinical Research Center Program Grant RR-032; the State of Alabama and the National Institute for Neurologic Disorders and Stroke Mentored Clinical Scientist Development Award 1K08NSO1942 (to J.M.M.); the American Association for Neurological Surgeons Young Investigator Award (to J.M.M.); the American Cancer Society Institutional Award (to J.M.M.); and the American Brain Tumor Association (J.M.M.). J.N.P. was supported in part by a fellowship from the Pediatric Brain Tumor Foundation of the United States.
Abbreviations
- GBM
glioblastoma
- HSV
herpes simplex virus
- tk
thymidine kinase
- pfu
plaque-forming unit
- TH
helper T cell subset
- IRES
internal ribosome entry site
- kb
kilobase
- TD
tolerated dose
- mIL
murine IL
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040557897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040557897
References
- 1.Markert, J. M., Gillespie, G. Y., Weichselbaum, R. R., Roizman, B. & Whitley, R. J. (1999) Rev. Med. Virol., in press. [DOI] [PubMed]
- 2.Cobbs C, Markert J. Persp Neurolog Surg. 1999;10:1–20. [Google Scholar]
- 3.Andreansky S S, He B, Gillespie G Y, Soroceanu L, Markert J, Chou J, Roizman B, Whitley R J. Proc Natl Acad Sci USA. 1996;93:11313–11318. doi: 10.1073/pnas.93.21.11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martuza R L, Malick A, Markert J M, Ruffner K L, Coen D M. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 5.Mineta T, Rabkin S D, Yazaki T, Hunter W D, Martuza R L. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 6.Kramm C M, Chase M, Herrlinger U, Jacobs A, Pechan P A, Rainov N G, Sena-Esteves M, Aghi M, Barnett F H, Chiocca E A, et al. Hum Gene Ther. 1997;8:2057–2068. doi: 10.1089/hum.1997.8.17-2057. [DOI] [PubMed] [Google Scholar]
- 7.Pyles R B, Warnick R E, Chalk C L, Szanti B E, Parysek L M. Hum Gene Ther. 1997;8:533–544. doi: 10.1089/hum.1997.8.5-533. [DOI] [PubMed] [Google Scholar]
- 8.Markert J M, Malick A, Coen D M, Martuza R L. Neurosurgery. 1993;32:597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Chambers R, Gillespie G Y, Soroceanu L, Andreansky S, Chatterjee S, Chou J, Roizman B, Whitley R J. Proc Natl Acad Sci USA. 1995;92:1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou J, Kern E R, Whitley R J, Roizman B. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 11.Mineta T, Markert J M, Takamiya Y, Coen D M, Rabkin S D, Martuza R L. Gene Ther. 1994;1, Suppl. 1:S78. [PubMed] [Google Scholar]
- 12.Andreansky S, Soroceanu L, Flotte E R, Chou J, Markert J M, Gillespie G Y, Roizman B, Whitley R J. Cancer Res. 1997;57:1502–1509. [PubMed] [Google Scholar]
- 13.Andreansky S, He B, van Cott J, McGhee J, Markert J M, Gillespie G Y, Roizman B, Whitley R J. Gene Ther. 1998;5:121–130. doi: 10.1038/sj.gt.3300550. [DOI] [PubMed] [Google Scholar]
- 14.Kaplitt M G, Tjuvajev J G, Leib D A, Berk J, Pettigrew K D, Posner J B, Pfaff D W, Rabkin S D, Blasberg R G. J Neurooncol. 1994;19:137–147. doi: 10.1007/BF01306455. [DOI] [PubMed] [Google Scholar]
- 15.Yazaki T, Manz H J, Rabkin S D, Martuza R L. Cancer Res. 1995;55:4752–4756. [PubMed] [Google Scholar]
- 16.Okada H, Giezeman-Smits K M, Tahara H, Attanucci J, Fellows W K, Lotze M T, Chambers W H, Bozik M E. Gene Ther. 1999;6:219–226. doi: 10.1038/sj.gt.3300798. [DOI] [PubMed] [Google Scholar]
- 17.Wei M X, Li F, Ono Y, Gauldie J, Chiocca E A. J Neurovirol. 1998;4:237–241. doi: 10.3109/13550289809114523. [DOI] [PubMed] [Google Scholar]
- 18.Benedetti S, Dimeco F, Pollo B, Cirenei N, Colombo B M, Bruzzone M G, Cattaneo E, Vescovi A, Didonato S, Colombo M P, et al. Hum Gene Ther. 1997;8:1345–1353. doi: 10.1089/hum.1997.8.11-1345. [DOI] [PubMed] [Google Scholar]
- 19.Tseng S H, Hwang L H, Lin S M. J Immunother. 1997;20:334–342. doi: 10.1097/00002371-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Caruso M, Pham-Nguyen K, Kwong Y L, Xu B, Kosai K I, Finegold M, Woo S L, Chen S H. Proc Natl Acad Sci USA. 1996;93:11302–11306. doi: 10.1073/pnas.93.21.11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bramson J L, Hitt M, Addison C L, Muller W J, Gauldie J, Graham F L. Hum Gene Ther. 1996;7:1995–2002. doi: 10.1089/hum.1996.7.16-1995. [DOI] [PubMed] [Google Scholar]
- 22.Kishima H, Shimizu K, Miyao Y, Mabuchi E, Tamura K, Tamura M, Sasaki M, Hayakawa T. Br J Cancer. 1998;78:446–453. doi: 10.1038/bjc.1998.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meko J B, Tsung K, Norton J A. Surgery. 1996;120:274–283. doi: 10.1016/s0039-6060(96)80298-4. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura T, Watanabe K, Yahata T, Uede T, Saiki I, Herrmann S H, Kobayashi M, Habu S. Annu NY Acad Sci. 1996;795:375–378. doi: 10.1111/j.1749-6632.1996.tb52697.x. [DOI] [PubMed] [Google Scholar]
- 25.Tahara H, Lotze M T, Robbins P D, Storkus W J, Zitvogel L. Hum Gene Ther. 1995;6:1607–1624. doi: 10.1089/hum.1995.6.12-1607. [DOI] [PubMed] [Google Scholar]
- 26.Tahara H, Lotze M T. Gene Ther. 1995;2:96–106. [PubMed] [Google Scholar]
- 27.Majewski S, Marczak M, Szmurlo A, Jablonska S, Bollag W. J Invest Dermatol. 1996;106:1114–1118. doi: 10.1111/1523-1747.ep12340161. [DOI] [PubMed] [Google Scholar]
- 28.Kerbel R S, Hawley R G. J Natl Cancer Inst. 1995;87:557–586. doi: 10.1093/jnci/87.8.557. [DOI] [PubMed] [Google Scholar]
- 29.Toda M, Martuza R L, Kojima H, Rabkin S D. J Immunol. 1998;160:4457–4464. [PubMed] [Google Scholar]
- 30.Kikuchi T, Joki T, Akasaki Y, Abe T, Ohno T. Cancer Lett. 1999;135:47–51. doi: 10.1016/s0304-3835(98)00268-7. [DOI] [PubMed] [Google Scholar]
- 31.Rakhmilevich A L, Janssen K, Turner J, Culp J, Yang N S. Hum Gene Ther. 1997;8:1303–1311. doi: 10.1089/hum.1997.8.11-1303. [DOI] [PubMed] [Google Scholar]
- 32.Bramson J, Hitt M, Gallichan W S, Rosenthal K L, Gauldie J, Graham F L. Hum Gene Ther. 1996;7:333–342. doi: 10.1089/hum.1996.7.3-333. [DOI] [PubMed] [Google Scholar]
- 33.Tahara H, Zitvogel L, Storkus W J, Zeh H J, McKinney T G, Schreiber R D, Gubler U, Robbins P D, Lotze M T. J Immunol. 1995;154:6466–6474. [PubMed] [Google Scholar]
- 34.Myers J N, Mank-Seymour A, Zitvogel L, Storkus W, Clarke M, Johnson C S, Tahara H, Lotze M T. Laryngoscope. 1998;108:261–268. doi: 10.1097/00005537-199802000-00019. [DOI] [PubMed] [Google Scholar]
- 35.Marshall E E. Science. 1995;268:1555. [Google Scholar]
- 36.Post L E, Roizman B. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins F, Roizman B. J Virol. 1986;59:494–499. doi: 10.1128/jvi.59.2.494-499.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagunoff M, Roizman B. J Virol. 1995;69:3615–3623. doi: 10.1128/jvi.69.6.3615-3623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoenhaut D S, Chua A O, Wolitzky A G, Quinn P M, Dwyer C M, McComas W, Familletti P C, Gately M K, Gubler U. J Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
- 40.Zitvogel L, Tahara H, Cai Q, Storkus W J, Muller G, Wolf S F, Gately M, Robbins P D, Lotze M T. Hum Gene Ther. 1994;5:1493–1506. doi: 10.1089/hum.1994.5.12-1493. [DOI] [PubMed] [Google Scholar]
- 41.Lopez C. Nature (London) 1975;258:152–153. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- 42.Macklis J D, Madison R D. J Neurosci. 1991;11:2055–2062. doi: 10.1523/JNEUROSCI.11-07-02055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becker L E, Hinton D. Hum Pathol. 1983;14:538–550. doi: 10.1016/s0046-8177(83)80006-9. [DOI] [PubMed] [Google Scholar]
- 44.Banchereau J, Briere F, Galizzi J P, Miossec P, Rousset F. J Lipid Mediat Cell Signal. 1994;9:43–53. [PubMed] [Google Scholar]
- 45.Carr J A, Rogerson J, Mulqueen M J, Roberts N A, Booth R F G. J Virol. 1997;71:7799–7803. doi: 10.1128/jvi.71.10.7799-7803.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]