Abstract
Acidification of the extracellular milieu of malignant tumors is reported to increase the invasive behavior of cancer cells. In normal tissues, production of acid is catalyzed by carbonic anhydrases (CAs), some of which are known to be overexpressed in certain cancers. To investigate the functional role of CA activity in such cancer cells, we analyzed the effect of acetazolamide, a potent CA inhibitor, on the invasive capacity of four renal carcinoma cell lines (Caki-1, Caki-2, ACHN, and A-498). We found that 10 μM acetazolamide inhibited the relative invasion rate of these cell lines between 18–74%. The Caki-2 and ACHN cell lines displayed the highest responsiveness, and their responses clearly depended on the acetazolamide concentration in the culture medium. Immunocytochemical and Western blotting results identified the presence of CA isoenzyme II in the cytoplasm of all four cell lines and CA XII on the plasma membrane in three of four cell lines. Because acetazolamide alone reduced invasiveness of these cancer cells in vitro, we conclude that the CAs overexpressed in these renal cancer cells contribute to invasiveness, at least in vitro, and suggest that CA inhibitors may also reduce invasiveness in other tumors that overexpress one or more CAs.
Microelectrode-measured pH values have indicated that the extracellular pH in solid tumors is more acidic than in adjacent normal tissue (1). In contrast, the intracellular pH estimated by 31P-magnetic resonance spectroscopy is identical or slightly more basic in tumor compared with normal tissue (2). To establish the pH gradient between the extracellular and intracellular compartments, tumor cells express ion transport proteins, including vacuolar H+-ATPase, Cl−/HCO3− exchanger, and Na+/H+ exchanger (3, 4). Many tumors also express carbonic anhydrases (CAs), which catalyze the production of H+ and HCO3− ions in the reversible reaction H2O + CO2 ⇄ H+ + HCO3− (5–12).
At present, 11 enzymatically active CA isoenzymes have been identified from mammals, namely, cytosolic CA I, II, III, and VII, mitochondrial CA VA and VB, secretory CA VI, and membrane-associated CA IV, IX, XII, and XIV. Three recently discovered isoenzymes (CA IX, XII, and XIV) are transmembrane proteins whose active sites are located on the cell exterior (11–15). Two of them (CA IX and XII) are highly expressed in some tumors and may be functionally related to oncogenesis (9–12). Previous immunohistochemical studies have also indicated that CA II is highly expressed in several tumors, including malignant brain tumors (6) and gastric and pancreatic carcinomas (5, 16).
Although the exact role of CA activity in carcinogenesis has not been established, Ivanov et al. (12) recently hypothesized that “tumor-associated” transmembrane isoenzymes CA IX and XII may be implicated in acidification of extracellular milieu surrounding the cancer cells and thus create a microenvironment conducive to tumor growth and spread. In this study, we analyzed in vitro the significance of CA activity for invasion capacity in four renal cancer cell lines by using acetazolamide, a potent CA inhibitor. The results obtained from the invasion assays were correlated with the presence of CA isoenzymes II, IV, IX, and XII in the same cell lines. The results indicated that acetazolamide can, indeed, inhibit the invasive capacity of renal cancer cells in vitro, and this effect might be attributable to the inhibition of CA II and/or CA XII in these cells.
Materials and Methods
Cell Culture and the Invasion Assay.
Human renal carcinoma cell lines (Caki-1, Caki-2, ACHN, and A-498) were obtained from the American Type Culture Collection. The cells were grown in DMEM (Life Technologies, Paisley, U.K.) supplemented with GlutaMAX, 4,500 mg/liter d-glucose, and 10% (vol/vol) FBS in a humidified atmosphere of 5% CO2-95% air at 37°C. The cells were grown for 3 days to confluence, trypsinized, centrifuged at 200 × g rpm for 5 min, and resuspended in the culture medium. The invasion assay was performed in a Biocoat Matrigel invasion chamber purchased from Becton Dickinson. Cells (n = 1.8 × 105) were added to the chamber and cultured for 48 h in the presence or absence of acetazolamide, which was administered twice during the 48-h period: the first dose at the beginning of the assay and the second after 24 h. After growing for 48 h, noninvasive cells were removed from the upper surface of the membrane with a cotton swab. The invasive cells on the lower surface of the membrane were fixed with 4% (vol/vol) neutral-buffered formaldehyde, stained with toluidine blue, and counted in 20 separate areas with a Leitz Diaplan microscope (Leica, Heidelberg, Germany). To determine the effect of acetazolamide on cell proliferation, the cells were grown on Nunclon dishes (diameter = 92 mm; Nunc A/S) for 2 days in the presence or absence of 100 μM acetazolamide, which was administered twice during the 48-h period. The cells were fixed with 4% (vol/vol) neutral-buffered formaldehyde, stained with toluidine blue, and counted in 10 separate areas.
Antibodies.
Murine monoclonal antibody M75 recognizing the N-terminal domain of MN/CA IX protein has been described by Pastoreková et al. (17). Polyclonal rabbit antisera to human CA II, IV, and XII have been produced and characterized previously (18–20). The anti-CA IV and anti-CA XII antibodies were raised against the secretory forms of human recombinant isoenzymes. All these antibodies have been tested by Western blotting and have shown high isoenzyme specificity.
Immunocytochemistry.
The Caki-1, Caki-2, ACHN, and A-498 cells grown in chamber slides for microscopy were rinsed with PBS and fixed with 4% (vol/vol) neutral-buffered formaldehyde for 25 min. Then the cells were rinsed with PBS and subjected to immunofluorescence staining that consisted of the following steps: (i) pretreatment with 0.1% BSA in PBS (BSA-PBS) for 40 min and rinsing in PBS, (ii) incubation for 1 h with the first antibody diluted 1:100 (anti-CA II and anti-CA XII), 50 μg/ml (affinity-purified anti-CA IV IgG), or 1:10 (M75 hybridoma medium) in 0.1% BSA-PBS, and (iii) incubation with diluted (1:100) fluorescein-conjugated goat anti-mouse IgG (Sigma) or fluorescein-conjugated swine anti-rabbit IgG (Dakopatts, Copenhagen) in 0.1% BSA-PBS. The cells were washed three times for 5 min after the incubation steps. All incubations and washings were done in the presence of 0.05% saponin. The cells were viewed with a confocal laser-scanning microscope (Leitz CLSM, Leica Laser Technics). The specimens were excited with a laser beam at a wavelength of 488 nm, and the emission light was focused through a pinhole aperture. The full field was scanned in square image formats of 512 × 512 pixels and built-in software was used to reconstruct the images obtained from the confocal sections.
SDS/PAGE and Western Blotting.
Samples containing 25 μg of protein from homogenized Caki-1, Caki-2, ACHN, and A-498 cells were analyzed by SDS/PAGE under reducing conditions according to Laemmli (21). All the reagents for SDS/PAGE were from Bio-Rad or Sigma. The electrophoreses were performed in a Mini-Protean electrophoresis unit (Bio-Rad) with a 10% acrylamide separating gel and a 4% acrylamide stacking gel. The proteins were transferred electrophoretically from the gel to a poly(vinylidene difluoride) membrane (Millipore) in a NOVEX Blot Module (NOVEX, San Diego). After the transblotting, the sample lanes were first incubated with TBST buffer (10 mM Tris⋅HCl, pH 7.5/150 mM NaCl/0.05% Tween-20) containing 10% (vol/vol) cow colostral whey (Biotop Oy, Oulu, Finland) for 30 min. Then, the membranes were incubated with the first antibody diluted 1:2,000 (anti-CA II and anti-CA XII sera), 1 μg/ml (affinity-purified anti-CA IV IgG), or 1:100 (M75 hybridoma medium) in TBST buffer for 1 h. The membranes were washed five times for 5 min with TBST buffer and incubated for 30 min with alkaline phosphatase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (both from Bio-Rad) diluted 1:3,000 in TBST buffer. After washing four times for 5 min in TBST buffer, the polypeptides were visualized by a chemiluminescence substrate (Bio-Rad). All the steps were carried out at room temperature.
Results
Effect of Acetazolamide on Malignant Cell Invasion.
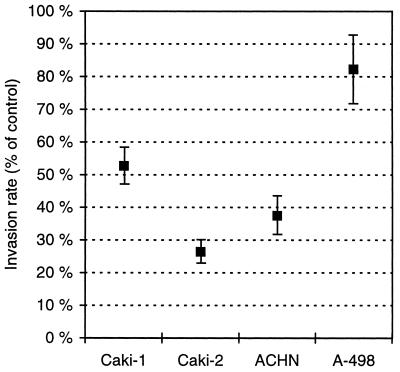
To assess the role of the CA activity in malignant cell invasion, we cultured renal cancer cells in the invasion chamber in the presence or absence of a CA inhibitor, acetazolamide. After culturing for 2 days, the invasive cells were stained and counted. Experiments repeated three to four times provided clear evidence that acetazolamide administered to the culture medium reduces the invasion potential in renal cancer cells in vitro. Fig. 1 shows that, although the degree of response was cell-line specific, all the analyzed renal carcinoma cell lines responded to the acetazolamide administration. In the most responsive cell line, Caki-2, acetazolamide reduced the number of invasive cells by 74%. The least response was observed in the A-498 cells, the reduction being 18%.
Figure 1.
Effect of 10 μM acetazolamide on cell invasion rate. The invasion assay was repeated three (Caki-1 and A-498 cells) or four (Caki-2 and ACHN cells) times. The results are shown as means ± SEM.
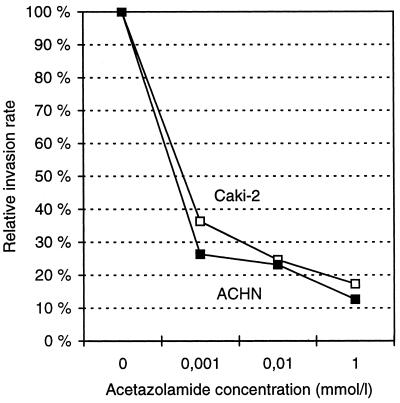
The concentration dependence of the acetazolamide effect on cell invasion was tested in two cell lines, Caki-2 and ACHN, both of which were found to be highly responsive to 10 μM acetazolamide treatment. Fig. 2 shows that 1 μM acetazolamide caused a highly significant inhibition of invasion activity in both cell lines, and this effect was enhanced further when the concentration of acetazolamide was increased to 10 μM or 1 mM.
Figure 2.
Effect of acetazolamide concentration on the invasion rate in Caki-2 and ACHN cells.
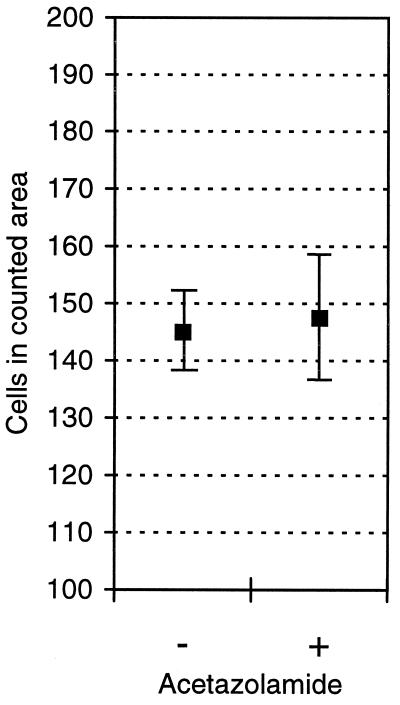
To exclude the possibility that the effect of acetazolamide is attributable to reduced cell proliferation, we cultured ACHN cells in the presence of 100 μM acetazolamide and counted the cell number. Fig. 3 shows that 100 μM acetazolamide did not affect the number of cells grown on the cell culture plates.
Figure 3.
Effect of 100 μM acetazolamide on cell proliferation in ACHN cells. The results are shown as means ± SEM.
Expression of CA Isoenzymes II, IV, IX, and XII in Renal Cancer Cells.
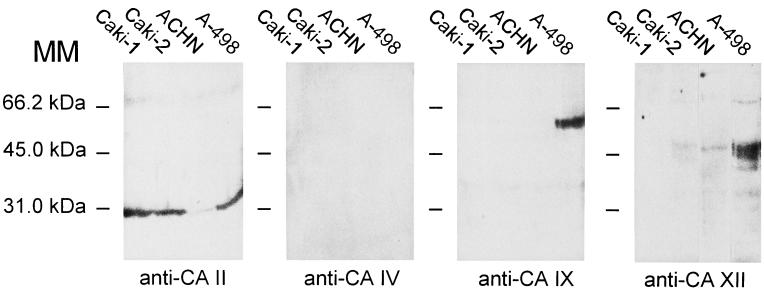
Because normal or neoplastic renal epithelium contains at least four potential CA isoenzymes as targets for acetazolamide (CA II, IV, IX, and XII), we immunostained these isoenzymes in the same renal cancer cell lines whose invasion capacity was tested with the invasion assay. Fig. 4 shows that Caki-1, Caki-2, and A-498 cells showed strong cytosolic signals for CA II, whereas ACHN cells showed a weaker reaction. None of the cell lines expressed CA IV. Only A-498 cells showed a positive signal for CA IX under these experimental conditions. Caki-2, ACHN, and A-498 cells displayed a positive reaction for CA XII.
Figure 4.
Immunocytochemical staining of renal cancer cells for CA II, IV, IX, and XII. Caki-1, Caki-2, and A-498 cells show strong positive staining for CA II, whereas the signal in ACHN cells is less intense. None of the cell lines express CA IV. Only A-498 cells show positive immunostaining for CA IX, whereas Caki-2, ACHN, and A-498 are all positive for CA XII.
Western blotting of the renal cancer cells for CA isoenzymes confirmed the immunocytochemical staining results (Fig. 5): all the cell lines expressed CA II (less so in ACHN cells); none of them expressed CA IV; only A-498 cells expressed CA IX; and all but Caki-1 cells expressed detectable amounts of CA XII.
Figure 5.
Western blotting of Caki-1, Caki-2, ACHN, and A-498 cells with antibodies raised against human CA II, IV, IX, and XII. The results confirm the pattern of expression of different CA isoenzymes in renal cancer cells as indicated by immunocytochemistry (compare with Fig. 4). MM, molecular mass.
Discussion
The present results showing the inhibitory effect of acetazolamide on malignant cell invasion raise two interesting questions. First, how does acetazolamide reduce the invasion rate? Second, what is the target isoenzyme for acetazolamide in the analyzed renal cancer cells? One explanation for the effect of acetazolamide is that it might inhibit cell proliferation during the invasion assay and consequently reduce the number of cells capable of invasion. We found, however, that 100 μM acetazolamide did not affect the number of cells grown on the cell culture plates. This result excludes the possibility that the reduction in the invasion rate observed after acetazolamide administration is attributable to reduced proliferation of the cancer cells. Rather, this result suggests a specific effect of acetazolamide on the invasion process itself.
Acetazolamide inhibits CA activity by binding to the active site of the enzyme molecule, and it can affect several active CA isoenzymes. Among these, CA II and CA IV have been identified in the mammalian kidney and implicated in the acidification of the excreted tubule fluid (22). Two more recently characterized transmembrane isoenzymes, CA IX and CA XII, are overexpressed in renal cell carcinomas and down-regulated by the product of the von Hippel–Lindau tumor suppressor gene (11, 12, 23, 24). Based on immunocytochemistry and Western blotting of renal cancer cells, we can conclude that the effect of acetazolamide on invasion capacity is most likely attributable to inhibition of CA II and/or CA XII. The only CA IX-positive cell line, A-498, showed the least response to the acetazolamide treatment. A role for CA IX in invasion cannot be totally excluded, however, because its level of expression is normally density dependent (9, 10) and may also be influenced by other experimental conditions. As shown in the present data, the effect of acetazolamide was highest in the cell lines expressing high levels of CA II and CA XII. CA II, although cytosolic, is a high-activity isoenzyme that could play a major role in the regulation of pH homeostasis between cancer cells and their microenvironment. (The relative specific activities of human CA II and secretory forms of CAs IX and XII are 2,500, 2,200, and 200–400 enzyme units/mg protein, respectively; A.W. and W.S.S., unpublished work.)
Interestingly, Martinez-Zaguilan et al. (25) have shown that human melanoma cells cultured at acidic pH were more aggressive than control cells when tested at the same medium pH. The authors proposed that a change in intracellular pH caused by extracellular acidification results in activation of gelatinase B, which in turn leads to the higher invasion potential of cancer cells. In tumors, cytosolic CA II may be functionally coupled to both intracellular and extracellular pH regulation, as it is in the kidney where it contributes to bicarbonate reabsorption in the proximal tubules and to luminal acidification in distal parts of the nephron (22). The inhibition of this enzyme by acetazolamide might prevent the activation of matrix-degrading enzymes and reduce the invasive potential of cancer cells.
Acetazolamide is clinically used to reduce intraocular pressure in glaucoma, to correct metabolic alkalosis, and to manage cerebral edema. Interestingly, Teicher et al. (26) have shown that acetazolamide treatment may also be beneficial as an adjunct to cancer chemotherapy, because it can produce additive tumor growth delays with anticancer drugs in vivo. The present results provide evidence that acetazolamide alone can inhibit the invasive potential of cancer cells in vitro. This finding may explain the observations of Teicher et al. (26) and provide further impetus for exploring therapeutic strategies that target intracellular and extracellular CAs in the treatment of some cancers.
Acknowledgments
We acknowledge Ms. Elizabeth Torno for editorial assistance. Dr. Ismo Virtanen (Institute of Biomedicine, University of Helsinki) kindly provided the renal cancer cells. This work was supported by National Institutes of Health Grants DK40163 and GM34182 to W. S. S. as well as by grants from the Sigrid Juselius Foundation to S. P. and from Chiron Diagnostics Corporation and the Slovak Scientific Grant Agency to S. P. and J. P.
Abbreviation
- CA
carbonic anhydrase
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040554897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040554897
References
- 1.Webb S D, Sherratt J A, Fish R G. J Theor Biol. 1999;196:237–250. doi: 10.1006/jtbi.1998.0836. [DOI] [PubMed] [Google Scholar]
- 2.Gerweck L E. Semin Radiat Oncol. 1998;8:176–182. doi: 10.1016/s1053-4296(98)80043-x. [DOI] [PubMed] [Google Scholar]
- 3.Montcourrier P, Silver I, Farnoud R, Bird I, Rochefort H. Clin Exp Metastasis. 1997;15:382–392. doi: 10.1023/a:1018446104071. [DOI] [PubMed] [Google Scholar]
- 4.Lee A H, Tannock I F. Cancer Res. 1998;58:1901–1908. [PubMed] [Google Scholar]
- 5.Parkkila S, Parkkila A-K, Juvonen T, Lehto V-P, Rajaniemi H. Histochem J. 1995;27:133–138. doi: 10.1007/BF00243908. [DOI] [PubMed] [Google Scholar]
- 6.Parkkila A-K, Herva R, Parkkila S, Rajaniemi H. Histochem J. 1995;27:974–982. [PubMed] [Google Scholar]
- 7.Saarnio J, Parkkila S, Parkkila A-K, Haukipuro K, Pastoreková S, Pastorek J, Kairaluoma M I, Karttunen T J. Am J Pathol. 1998;153:279–285. doi: 10.1016/S0002-9440(10)65569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kivelä, A., Parkkila, S., Saarnio, J., Karttunen, T. J., Kivelä, J., Parkkila, A.-K., Waheed, A., Sly, W. S., Grubb, J. H., Shah, G., et al. (1999) Am. J. Pathol., in press. [DOI] [PMC free article] [PubMed]
- 9.Závada J, Závadová Z, Pastoreková S, Ciampor F, Pastorek J, Zelník V. Int J Cancer. 1993;54:268–274. doi: 10.1002/ijc.2910540218. [DOI] [PubMed] [Google Scholar]
- 10.Pastorek J, Pastoreková S, Callebaut I, Mornon J P, Zelník V, Opavský R, Zatovicova M, Liao S, Portetelle D, Stanbridge E J, et al. Oncogene. 1994;9:2877–2888. [PubMed] [Google Scholar]
- 11.Türeci Ö, Sahin U, Vollmar E, Siemer S, Göttert E, Seitz G, Parkkila A-K, Shah G N, Grubb J H, Pfreundschuh M, et al. Proc Natl Acad Sci USA. 1998;95:7608–7613. doi: 10.1073/pnas.95.13.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanov S V, Kuzmin I, Wei M-H, Pack S, Geil L, Johnson B E, Stanbridge E J, Lerman M I. Proc Natl Acad Sci USA. 1998;95:12596–12601. doi: 10.1073/pnas.95.21.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opavský R, Pastoreková S, Zelník V, Gibadulinová A, Stanbridge E J, Závada J, Kettmann R, Pastorek J. Genomics. 1996;33:480–487. doi: 10.1006/geno.1996.0223. [DOI] [PubMed] [Google Scholar]
- 14.Mori K, Ogawa Y, Ebihara K, Tamura N, Tashiro K, Kuwahara T, Mukoyama M, Sugawara A, Ozaki S, Tanaka I, et al. J Biol Chem. 1999;274:15701–15705. doi: 10.1074/jbc.274.22.15701. [DOI] [PubMed] [Google Scholar]
- 15.Fujikawa-Adachi K, Nishimori I, Taguchi T, Onishi S. Genomics. 1999;61:74–81. doi: 10.1006/geno.1999.5938. [DOI] [PubMed] [Google Scholar]
- 16.Pastoreková S, Parkkila S, Parkkila A-K, Opavský R, Zelník V, Saarnio J, Pastorek J. Gastroenterology. 1997;112:398–408. doi: 10.1053/gast.1997.v112.pm9024293. [DOI] [PubMed] [Google Scholar]
- 17.Pastoreková S, Závadová Z, Kost'ál M, Babusiková O, Závada J. Virology. 1992;187:620–626. doi: 10.1016/0042-6822(92)90464-z. [DOI] [PubMed] [Google Scholar]
- 18.Parkkila A-K, Parkkila S, Juvonen T, Rajaniemi H. Histochemistry. 1993;99:37–41. doi: 10.1007/BF00268018. [DOI] [PubMed] [Google Scholar]
- 19.Parkkila S, Parkkila A-K, Juvonen T, Waheed A, Sly W S, Saarnio J, Kaunisto K, Kellokumpu S, Rajaniemi H. Hepatology. 1996;24:1104–1108. doi: 10.1002/hep.510240521. [DOI] [PubMed] [Google Scholar]
- 20.Karhumaa P, Parkkila S, Türeci Ö, Waheed A, Grubb J H, Shah G N, Parkkila A-K, Kaunisto K, Tapanainen J, Sly W S, et al. Mol Hum Reprod. 2000;6:68–74. doi: 10.1093/molehr/6.1.68. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Sly W S, Hu P Y. Annu Rev Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 23.McKiernan J M, Buttyan R, Bander N H, Stifelman M D, Katz A E, Chen M-W, Olsson C A, Sawczuk I S. Cancer Res. 1997;57:2362–2365. [PubMed] [Google Scholar]
- 24.McKiernan J M, Buttyan R, Bander N H, de la Taille A, Stifelman M D, Emanuel E R, Bagiella E, Rubin M A, Katz A E, Olsson C A, et al. Cancer. 1999;86:492–497. doi: 10.1002/(sici)1097-0142(19990801)86:3<492::aid-cncr18>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Zaguilan R, Seftor E A, Seftor R E, Chu Y W, Gillies R J, Hendrix M J. Clin Exp Metastasis. 1996;14:176–186. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 26.Teicher B A, Liu S D, Liu L T, Holden S A, Herman T S. Anticancer Res. 1993;13:1549–1556. [PubMed] [Google Scholar]