Abstract
Optical reflectors in animals are diverse and ancient. The first image-forming eye appeared around 543 million years ago. This introduced vision as a selection pressure in the evolution of animals, and consequently the evolution of adapted optical devices. The earliest known optical reflectors—diffraction gratings—are 515 Myr old. The subsequent fossil record preserves multilayer reflectors, including liquid crystals and mirrors, ‘white’ and ‘blue’ scattering structures, antireflective surfaces and the very latest addition to optical physics—photonic crystals. The aim of this article is to reveal the diversity of reflecting optics in nature, introducing the first appearance of some reflector types as they appear in the fossil record as it stands (which includes many new records) and backdating others in geological time through evolutionary analyses. This article also reveals the commercial potential for these optical devices, in terms of lessons from their nano-level designs and the possible emulation of their engineering processes—molecular self-assembly.
Keywords: optics, structural colour, animals, fossils, evolution, biomimetics, self-assembly
1. Introduction
The focusing optics (or lenses) of animal eyes, and even plants, that exist currently have been well documented (see Land & Nilsson 2002 for a review). Some lenses have even found commercial placements in the optics industry. There is a theoretical minimum size for an eye, of around 0.2 mm on land (larvae of tiger beetles—see Land & Nilsson 2002) or larger for marine animals (which must possess more powerful lenses), and so these relatively large sensory structures are common also in the fossil record. In fact the most comprehensive studies of fossil eyes have been made on the oldest of all—the compound eyes of trilobites, now extinct arthropods that benefited from vision. My reason for referring to eyes is partly to contrast the scientific attention paid to them with that given to the less-well-studied optical reflectors, or ‘structural colours’, partly to demonstrate that ancient optics, being physical structures, can survive in the fossil record and partly to introduce the beginnings of vision on Earth.
Before the beginning of the Cambrian period, 545 million years ago (Ma), life forms were exclusively soft-bodied. Then over the next five million years (Myr) the process of evolution accelerated. For the first time, animals from all higher classifications, or phyla, evolved hard external parts. Both hunters and prey developed armaments and defences. The primary speculation relating to this extraordinary event, known as the ‘Cambrian Explosion’, is why it occured. Why did this big bang of evolution happen when it did? This is one case where the fossil record of optical devices could provide important data towards an unrelated subject.
Since eyes have not been discovered in pre-Cambrian fossils, including those preserved in fine detail, we must assume that trilobites were the first animals to host image-forming eyes (subsequently referred to as ‘eyes’; organs contributing to vision as opposed to light level/direction perception). The very first eye evolved in a trilobite at the beginning of the Cambrian Explosion, leading to a hypothesis that it was the development of vision in primitive animals that caused the explosion (Parker 1998a,b, 1999c, 2003). Essentially, the evolution of the first eye enabled the evolution of active predation lifestyles (highly mobile, marine predators generally require eyes). Pre-Cambrian animals were unable to see, making it impossible to find friend or foe (although other senses, such as chemoreception, would have existed). Hence any incidental iridescence appearing before the Cambrian period would have been neutrally selective. With the evolution of the eye, the size, shape, colour and behaviour of animals was suddenly revealed for the first time. Once the lights were ‘turned on’, there was enormous pressure to evolve hard external parts as defences, and swimming and clasping limbs in order to escape predators and to grab prey. The animal kingdom divided rapidly into hunters and hunted, and the types of food webs that exist currently were formed.
The evolution of the first eye, a singular event in the history of life on Earth, probably triggered the Cambrian Explosion. Certainly, it signalled the beginning of the evolution of optical devices in animals. Soon after the first eyes (at least 515 Ma), animals began to evolve sophisticated optical reflectors in response to the new selection pressures set by the presence of eyes, which also became common and diverse (e.g. figure 1). Today we find an array of optical reflectors in animals that have resulted from millions of years of evolutionary ‘fine-tuning’. Perhaps, then, we can benefit from nature's optical designs ourselves and marry the subjects of optics in animals and biomimetics.
Figure 1.

Eyes in the Cambrian period. The head of Waptia fieldensis, a shrimp-like arthropod a few centimetres long, showing well-developed, stalked compound eyes.
Optical reflectors cause the so-called ‘structural colours’ in nature, because they involve the selective reflectance of incident light by the physical nature of their structure. Although the colour effects often appear considerably brighter than those of the more widespread pigments, structural colours usually result from completely transparent materials. To assist biologists not conversant with optical physics, reflectors can be divided into broad categories based on their physical characteristics, regardless that some categories may overlap.
This article will provide a sense of the diversity of optical reflectors in animals, along with the first examples of each reflector type as they have been found in the fossil record (including many new records) or the furthest extrapolations in geological time (for a full review of animal structural colours today, including their biological functions; see Parker 2000). This in turn, will serve as an introduction to the threshold of the exciting new direction for the subject on which we now stand, where optics in animals may help advance optical engineering for several different reasons.
2. Diffraction gratings
According to physics textbooks, the first diffraction grating appeared in 1786 when the American astronomer, David Rittenhouse, made up a square of parallel hairs laid across two fine screws made by a watchmaker (although Joseph von Fraunhofer invented the ruled diffraction grating in 1821). The fossil record, on the other hand, reveals older diffraction gratings.
The earliest known examples of diffraction gratings, or indeed any form of structural colour, are from the Cambrian period and belong to the famous Burgess Shale animals of the Canadian Rockies (515 Ma; Parker 1998a,b). These linear, two-dimensional diffraction gratings have not survived in their entirety, rather as mosaics (e.g. figure 2), but always run in the same direction relative to a spine, even where it curves (suggesting that the gratings are not artefacts of geological processes). Therefore, to observe the original colours, the surface must be reconstructed in photoresist. Most colours in sunlight would have existed in the original environments of the Burgess animals, and their colours probably functioned as warnings to predators with eyes—to emphasize the strong, protective spines on which they are accommodated.
Figure 2.

Micrographs of the Burgess stem-group polychaete Canadia spinosa at increasing magnification—from ×10 to ×4000. The top picture shows the anterior half of the animal, the middle pictures show details of paleae (spines). The bottom picture shows the surface of a palea as removed from the rock matrix, revealing the remnants of a diffraction grating with a ridge spacing of 900 nm.
Diffraction gratings with periods around 650 nm are responsible for the nacreous lustre of pholidostrophiid brachiopods, such as those from the Devonian, around 360 Myr old (Towe & Harper 1966). Here, tabular aragonite platelets, each comprising a linear diffraction grating, form layers (Towe & Harper 1966) although at 600 nm thickness they are too thick to form a multilayer reflector (see below) and it is the exposed surface gratings that interact with light waves.
2.1 How diffraction gratings cause colour1
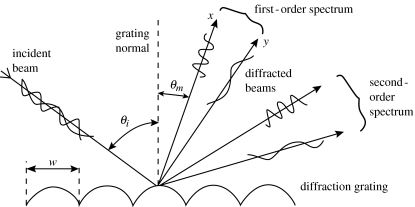
When light interacts with a periodic surface consisting, for example, of a series of parallel grooves, it may be deviated from the direction of simple transmission or reflection. For this to happen, light that is scattered or diffracted from successive grooves should be out of phase by integral values of 2π. This occurs when for a given direction of propagation the optical path difference via successive grooves is mλ, where m is an integer known as the circle number. This may be expressed by the grating equation
| (1) |
where θi and θm are angles of incidence and diffraction, respectively, and w is the period (figure 3).
Figure 3.
Reflection-type diffraction grating dividing white light into spectra.
A diffraction grating gives rise to colouration because different wavelengths are diffracted in different directions. Although the effect changes with angle of incidence, it is less critical than it is with multilayer reflectors (see below) and the visual appearance is different. For a parallel beam of white light incident upon a multilayer, one wavelength will be reflected as determined by the so-called ‘Bragg condition’. The same beam incident upon a grating will be dispersed into spectra. The complete spectrum reflected nearest to the perpendicular (grating normal) is the first order. The first-order spectrum is reflected over a smaller angle than the second-order spectrum, and the colours are more saturated and appear brighter within the former. Diffraction gratings have polarizing properties, but this is strongly dependent on the grating profile.
2.2 The diversity of diffraction gratings today
Diffraction gratings were believed to be extremely rare in nature (Fox & Vevers 1960; Fox 1976; Nassau 1983), but have recently been revealed to be common among extant invertebrates (Parker 2000). They are particularly common on the setae or setules (hairs) of Crustacea. The ostracod (seed-shrimp) Euphilomedes carcharodonta, for example, houses a diffraction grating on the rostrum, a continuous flattened area of the carapace that is corrugated to form periodic ridges. Cylindroleberidid ostracods possess a comb on their maxilla bearing numerous fine setules on each seta, collectively forming a grating with a periodicity of about 500 nm.
Many extant polychaetes possess gratings on their setae. For example, the opheliid Lobochesis longiseta bear gratings with periodicities in the order of 500 nm. The wings of the neurochaetid fly Neurotexis primula bear diffraction gratings only on their dorsal surfaces, and the iridescent effect remains after the insect is gold coated.
3. Liquid crystals
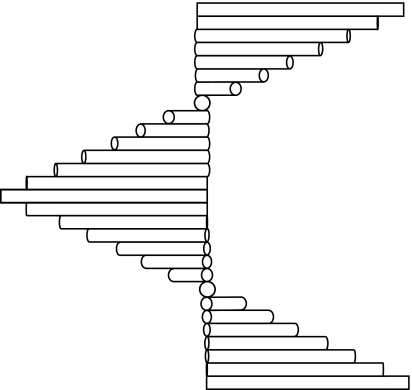
Exceptional, three-dimensionally preserved trilobites (Tapinocalymene nodulosa) have been discovered from the Silurian of the Welsh Borderland (Dalingwater et al. 1993). The outer layer of their cuticle, now phosphatic in composition, reveals a finely layered ultrastructure—stacks of layers in the order of, and separated by, 150 nm thickness. Three-dimensional analysis reveals that these layers are a product of sectioning—the complete structure is composed of layers of individual fibrils where each horizontal layer recesses with regard to the layer above it, in the manner of a liquid crystal. The fibrils stack around a central axis, like stairs spiralling around a central pole (figure 4). Phosphatization has resulted in an opaque fossil, but this was probably not the case in the living animals' exoskeleton. Transparent, chitinous structures of analogous architecture exist in extant arthropods where they cause iridescence—they are essentially liquid crystals.
Figure 4.
Diagrammatic fibrils selected from ‘layers’ within an exoskeleton so that a spiral shape is formed. This acts as a complete ‘pitch’ (2d) of an optical system.
3.1 Liquid crystals in extant animals and how they cause colour
The helicoidal arrangement of the microfibrils comprising the outer 5–20 μm of the cuticle (the ‘exocuticle’) of certain scarabeid beetles, such as Plusiotis resplendens, gives rise to metallic colours (Neville & Caveney 1969). Here, the fibrils are arranged in layers, with the fibril axis in each layer arranged at a small angle to the one above, so that after a number of layers the fibrillar axis comes to lie parallel to the first layer. Thus going vertically down through the cuticle, one corresponding peak and trough of a diffraction grating will be encountered with every 360° rotation of the fibrils—the ‘pitch’ of the system. Polarized light encounters an optically reinforcing plane every half turn of the helix. The system provides a peak reflectance at λ=2nd, where d is the separation of analogous planes, or half the pitch of the helix (figure 4). In fact it approximates a diffraction grating except for the polarization properties; the helical arrangement of fibrils reflects light that is circularly or elliptically polarized (Nassau 1983).
4. Narrow-band (coloured) multilayer reflectors (including single thin films)
The earliest known multilayer reflectors are from the Cretaceous period, where they occur in the shells of some ammonites (Mollusca, relatives of squid with shells), such as in a specimen from South Dakota, 80 Ma (figure 5). Here the original, transparent calcitic material of the shell has preserved, and strong metallic colours are observed. Other ammonites are known from Canada where the original material has changed during fossilization to leave an opal-like structure in this case known as ‘Ammolite’ (a semi-precious gem; figure 6).
Figure 5.
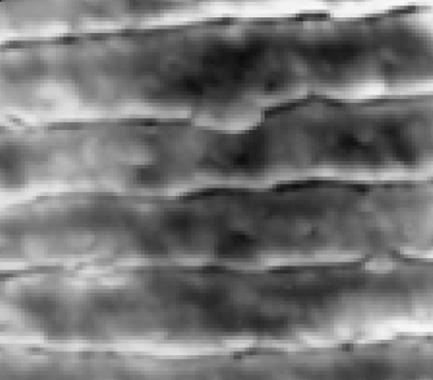
Part of the multilayer reflector in an 80 Ma ammonite ×10 000. The sharp horizontal lines represent the boundary layer pairs (where each pair contains a high and low refractive index layer, represented by dark and light shading, respectively).
Figure 6.

Part of a 70 Ma ammonite that has undergone a change in chemical composition and ultrastructure during the fossilization to form ‘Ammolite’.
I will open the Eocene section with an extraordinary case of a fossil, where not only has the reflector preserved, but also its original visual effect. The Middle Eocene (50 Ma) oil shales of Messel, Germany, are well renowned for their abundant fauna and flora preserved in exceptional detail, including both hard and soft parts (Schaal & Ziegler 1992). Messel rock contains 40% water and has a high content of original organic matter (Schaal & Ziegler 1992). Unusually, beetles have been found at Messel that have retained their original colour (figure 7), although the colour disappears on drying.
Figure 7.
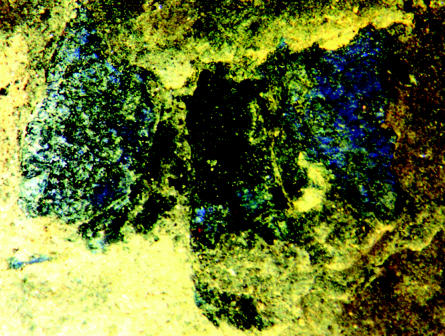
A preserved, flattened beetle with wing covers spread to the sides, 49 Ma, from Messel in Germany, showing original structural colours.
The wing cover (elytron) from one section of a Messel beetle (figure 8a) revealed a reflectance peak at 480–505 nm (figure 8b), corresponding to a blue appearance (to humans), with a maximum reflectivity of 63% (Parker & McKenzie 2003). The exocuticle of the elytron examined was found to contain five layers of electron dense (high refractive index) material, each separated by a layer of low refractive index material (figure 8c; Parker & McKenzie 2003). Each layer was revealed as flat and smooth at the nanoscale (figure 8d).
Figure 8.
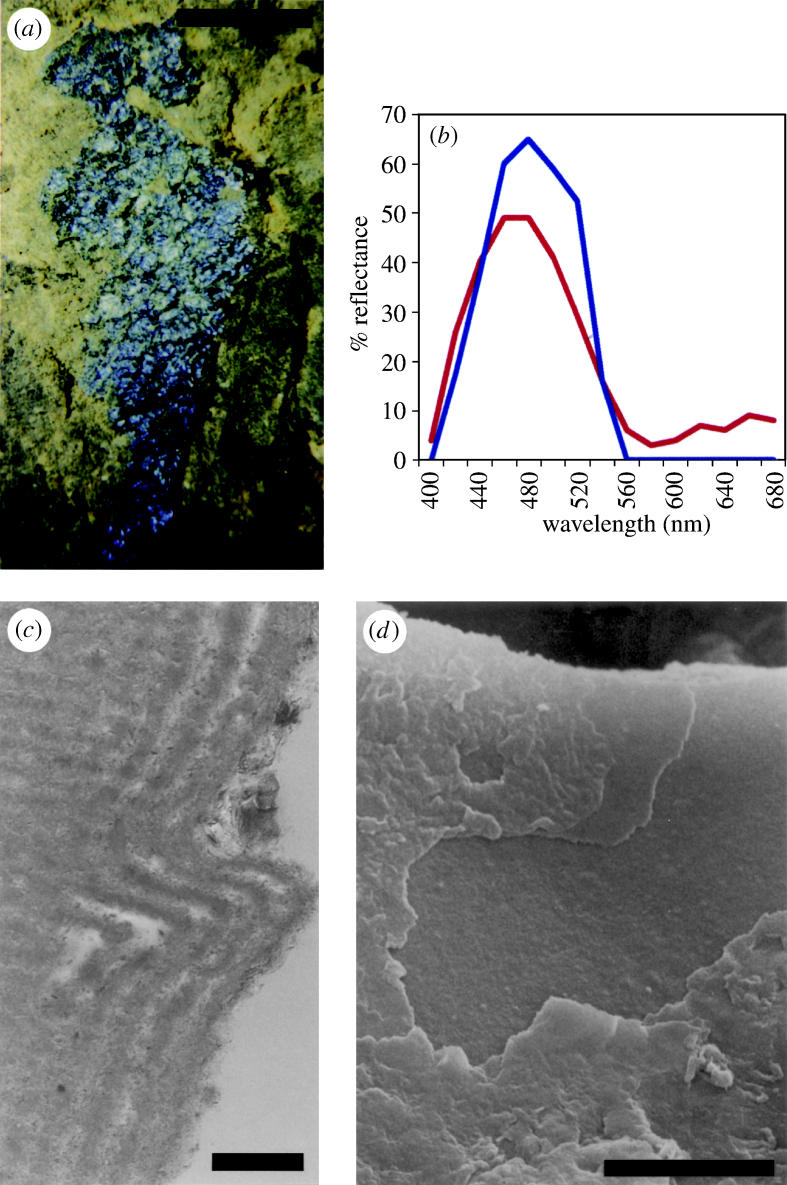
Elytron of an unidentified beetle from Messel, 50 Ma. (a) Light photograph of the specimen examined, embedded in rock. (b) Graphs showing the experimental optical (blue, solid line) and predicted (red, dashed line) reflectance from the elytron. (c) Transmission electron micrograph of the outer surface of a 60‐nm‐wide section of elytron, showing the fine lamination (running vertically in the picture) in a cross-section. The surface of the elytron has become wrinkled; the bend shown here represents a wrinkle. ‘Horizontal’ lines are an artefact of cutting. (d) Scanning electron micrograph of the outer surface of the elytron, perpendicular view, showing internal fine layers where outer layers are broken. Scale bars, a, 1 mm; c, 0.5 μm; d, 5 μm. From Parker & McKenzie (2003).
The close match in carbon:oxygen ratio within the high index layer of the Messel beetle reflector and the extant beetle C. grayanus, compared with that of the rock surrounding the Messel elytron, suggested that the original organic material had been preserved in the Messel specimen examined (Parker & McKenzie 2003). Also, the (principally) water (low index) layers have preserved. Hence, when the Messel beetles are dried, the low index layers of the reflector become altered, which explains the colour disappearance. The same happens in some extant beetles on death.
In fact finely laminate structures within external parts have been preserved and illustrated in, for example, Tertiary arthropods from Riversleigh, Australia (Duncan et al. 1998) and Cretaceous conchostracans from the Crato Formation of Brazil (Martill 1993). No water remains in these fossils and in some cases the original materials have been replaced by opaque materials. Under these conditions, multilayer reflectors would no longer give rise to colour, but the original structure remains and the Messel beetle study has revealed that this can be modelled using computer programs to provide a predicted reflectance spectrum, and consequently original colouration. However, many flies preserved in amber, of various age, appear iridescent from their bodies, owing to multilayer reflectors in their cuticle, and from their wings, which may act as single-layer reflectors.
4.1 How narrow-band multilayer reflectors cause colour
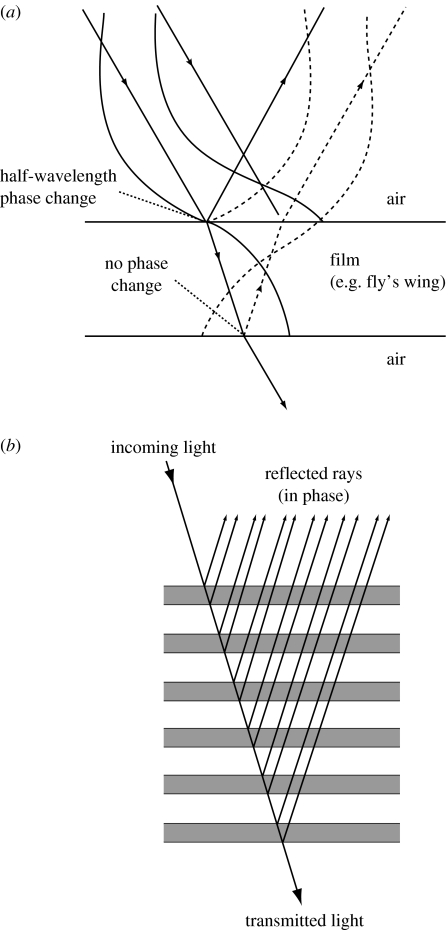
Light may be strongly reflected by constructive interference between reflections from the different interfaces of a stack of thin films (of actual thickness d) of alternately high and low refractive index (n). For this to occur, reflections from successive interfaces must emerge with the same phase, and this is achieved when the ‘Bragg condition’ is fulfilled (figure 9a). The optical path difference between the light reflected from successive interfaces is an integral number of wavelengths and is expressed in the equation
| (2) |
from which it can be seen that the effect varies with angle of incidence (Θ, measured to the surface normal), wavelength (λ) and the optical thickness of the layers (nd). There is a phase change of half a wavelength in waves reflected from every low to high refractive index interface only (figure 9a). The optimal narrow-band reflection condition is therefore achieved where the optical thickness (nd) of every layer in the stack is a quarter of a wavelength. In a multilayer consisting of a large number of layers with a small variation of index the process is more selective than one with a smaller number of layers with a large difference of index. The former therefore gives rise to more saturated colours corresponding to a narrow spectral bandwidth, and these colours, therefore, vary more with a change of angle of incidence. Both conditions can be found in animals today—different coloured effects are appropriate for different functions under different conditions. For an oblique angle of incidence, the wavelength of light that interferes constructively will be shorter than that for light at normal incidence. Therefore, as the angle of the incident light changes, the observed colour also changes. Iridescence caused by such interference disappears after (complete) gold coating because transmission of light through the outer surface is prevented.
Figure 9.
(a) Light rays affected by a single thin layer, such as a fly's wing, in air. The layer is shown in cross-section; the light-ray path and wave profiles are illustrated as solid lines (incoming light) and dashed lines (reflected light). (b) A narrow-band (‘ideal’) multilayer reflector and its effect on light. Reflected rays are in phase when all the layers are approximately a quarter of their wavelength in optical thickness.
If the dimensions of the multilayer system deviate from the quarter-wave condition (i.e. nd is not equal for all layers), then the reflector is known as ‘non-ideal’ (Land 1972) in a theoretical sense (but may be ideal in a behavioural sense). ‘Non-ideal’ reflectors provide a reduced proportional reflectance and narrower bandwidth for a given number of layers. However, a narrow bandwidth, with less conspicuous reflection, is sometimes selected for in animals. Multilayer reflectors polarize light incident at Brewster's angles. This is about 54° for a quarter-wave stack of guanine and cytoplasm. At very oblique angles, all wavelengths are strongly reflected from a multilayer.
4.2 The diversity of narrow-band multilayer reflectors today
Single-layer reflectors are found commonly in nature today, where light is reflected, and interferes, from the upper and lower boundaries (figure 9a). A difference in the thickness of the layer provides a change in the colour observed from unidirectional polychromatic light. The wings of some houseflies act as a single thin film and appear different colours as a result of this phenomenon (Fox & Vevers 1960). A single quarter-wavelength film of guanine in cytoplasm, for example, reflects about 8% of the incident light (Land 1978). However, in a multilayer reflector with 10 or more high index layers, reflection efficiencies can reach 100% (Land 1972; figure 9b). Thus, animals possessing such reflectors may appear highly metallic.
Multilayer reflectors are the most common form of structural colour in animals today (e.g. figure 10). They are usually extracellular, produced by periodic secretion and deposition, but sometimes occur within cells. Guanine (n=1.83) is a common component in invertebrate reflectors because it is one of very few biological materials with a high refractive index and is readily available to most invertebrates as a nitrogenous metabolite (Herring 1994). However, arthropods, including insects, crustaceans and spiders, have largely ignored guanine in favour of pteridines (Herring 1994). Also surprising is that the reflector material of closely related species, for example, the molluscs Pecten (scallop) and Cardium (cockle), may differ (Herring 1994).
Figure 10.

Multilayer reflectors causing colour in the body and corneas of a long-legged fly (Dolichopodidae).
Multilayers produce optical effects in beetle cuticle from highly metallic colours (‘ideal’ system) to rather dull greens (‘non-ideal’ system in combination with scattering; Parker et al. 1998c). They are responsible also for the colours reflected from the wings of many butterflies, where layers of chitin (n=about 1.56) are supported by ribs protruding vertically from the scales. Air (n=1.0) fills in the spaces and provides the alternate layers of the system (Mason 1927; Land 1972). A layer of melanin (a black or brown pigment) often underlies the reflector and intensifies the metallic coloured effect by absorbing the transmitted portion of incident light. For example, in beetles the elytra of Anopognathus parvulus appears metallic gold, green or yellow in reflected light, and diffuse brown in transmitted light (Parker et al. 1998c). Individual butterfly scales have been examined in detail to reveal a number of variations of quarter-wave stacks, sometimes in combination with other optical structures, to provide a range of coloured effects (e.g. Ghiradella 1989; Vukusic et al. 2000; Kinoshita et al. 2002).
The crustaceans Limnadia (Conchostraca), Tanais tennicornis (Tanaidacea), Ovalipes molleri (Decapoda) and the males of Sapphirina (Copepoda) all bear multilayer reflectors in their cuticles, in different forms. In contrast to the usual continuous thin layers, male sapphirinids have 10–14 layers of interconnecting hexagonal platelets within the epidermal cells of the dorsal integument (Chae & Nishida 1994). The reflector of O. molleri comprises layers that are corrugated and also slightly out of phase. The corrugations function to broaden the reflectance band, at the expense of reducing the intensity of reflection (Parker et al. 1998b).
5. Broad-band multilayer reflectors (silver and gold ‘mirrors’)
Structures with the characteristics of iridophores (cells containing broad-band multilayer reflectors) have been found in the fossil record, in placoderm fishes. Fishes did not diversify significantly until the Silurian period, 430 Ma. One group of fishes that appeared in the Silurian dominated waters in the Devonian, 400 Ma ago, until they became extinct 355 Ma. These fishes were the placoderms, or armoured fishes.
A 370 Myr placoderm specimen of Groenlandaspis has been exceptionally well preserved in Aztec Siltstone in Antarctica owing to a lack of weathering. In the trunk shield of this specimen exists packets of numerous, equally spaced, parallel layers, between 70 and 90 nm thick, positioned within a less dense matrix (figure 11). These ‘packets’ may have been iridophores, and were probably originally positioned in the soft tissue (which is not preserved in the fossil) external to the inner bone layer, before the outer bone layer was deposited. However, some fishes today do contain iridophores within their scales and it is possible that the putative iridophores found in Groenlandaspis are in their original location.
Figure 11.
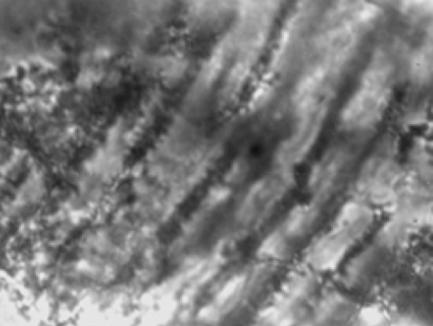
Micrograph of part of a putative iridophore, which causes a silver, mirror-like reflection, from the placoderm Groenlandaspis sp., ×4000 magnification.
The Miocene period reveals another example of multilayer mirrors, in this case as part of photophores (bioluminescent organs) where the mirrors serve to focus bioluminescent light away from the fish's body. Arambourg (1920) described a fossil fish, Myctophum prolaternatum, from the Sahara region near Oran. On the scales of this fossil were reported rows of small hemispheric granules (figure 12) that resemble the photophores of the closely allied but living Myctophum laternatum. M. laternatum is known to possess photophores, arranged in a very similar pattern to that of the extinct species, and so Arambourg's identification of fossil photophores is probably correct, although numerous cases of dubious fossil photophores in fishes also exist (see Harvey 1952).
Figure 12.
Myctophum prolaternatum a Miocene fossil fish with bioluminescent organs (‘dots’), found near Oran.
5.1 How broad-band multilayer reflectors cause silver or gold effects
‘Broad-band’ multilayer reflectors (figure 13), as opposed to the narrow-band types described above, reflect a broad range of wavelengths, such as all of those in white light, thus forming a mirror effect. Simply, they contain layers of different optical thicknesses that each reflect a different wavelength in a given direction. The different wavelengths combine to form an optical effect with a broad range of colours. When all the wavelengths in white light are reflected, the appearance is silver, when all but blue and violet are reflected, the appearance is gold (e.g. figure 14), for instance. The metallic effect (e.g. silver rather than white) is owing to the directional nature of the reflectance; since all rays are reflected into the same direction, the relative intensity is high (the reflection appears bright).
Figure 13.

Three ways of achieving a broad-band wavelength-independent reflector in a multilayer reflector (high refractive index material is shown shaded; Parker et al. 1998c). (a) Three quarter-wave (narrow-band) stacks, each tuned to a different wavelength, such as a ‘red’, ‘green’ and ‘blue’. (b) A ‘chirped’ stack, where layer thickness, and consequently the wavelength reflected in phase, decreases systematically with depth in the stack. (c) A ‘chaotic’ stack, where layers of different thickness are arranged randomly within the stack. Type a can be found in the herring (Denton 1970); type b in a lysianassoid amphipod (Crustacea; Parker 1999b); type c in many other silvery fishes.
Figure 14.
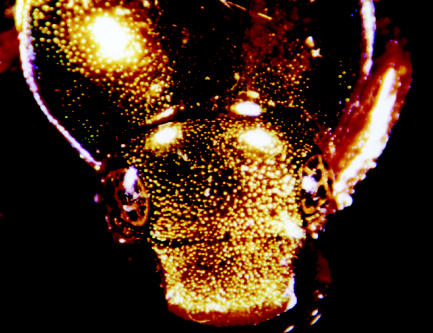
The gold (‘mirrored’) beetle Anopognathus parvulus from Australia. The gold colour results from a (chirped) broad-band multilayer reflector that covers the entire body, but in unidirectional light provides limited colouration over the beetles' ‘hemispherical’ shape.
5.2 The diversity of broad-band multilayer reflectors today
Today broad-band reflectors, or mirrors, are employed in animals such as the chrysalis of the butterfly Euploea core and many silvery fishes to provide camouflage. The surrounding environment is reflected from the mirrored surface so that the animals cannot be seen (Denton 1970; Land 1972; Parker 1999a). However, this means of camouflage can only be achieved in an environment with diffuse light to prevent a strong, direct reflection from the sun. Euploea core indeed lives in forests with diffuse light conditions. Many fishes take advantage of such conditions, to achieve the same effect, in the sea (Denton 1990). Similarly, iridophores camouflage the parts of squids and cuttlefishes that cannot, by their nature, be made transparent, such as eyes and ink sacs (Land 1972).
We are aware of the ultraviolet (wavelengths around 360 nm) vision of many animals, particularly insects, birds and fishes, and consequently examine ultraviolet reflectances from animal optical devices, particularly broad-band reflectors (e.g. figure 15). Many ultraviolet structurally coloured butterflies, with multilayer reflectors, have been revealed, for example (e.g. Ghiradella et al. 1972; Brunton & Majerus 1995).
Figure 15.

An ultraviolet photograph of a silvery fish, demonstrating that its ‘chaotic’ broad-band reflector extends into the ultraviolet (note the strong reflection where angles are accommodating).
6. Zero-order gratings
In contrast to gratings with freely propagating orders, zero-order structures can generate saturated colours even in diffuse illumination (Gale 1989). They may even suppress the reflection of visible light (almost) completely, where they are known as ‘antireflective’ structures.
The first known antireflective optical devices are from the Eocene period. In 1976 in Poland, a 45 Myr old fly's eye preserved in Baltic amber was photographed in an electron microscope to demonstrate the detail of preservation (figure 16). An unusual feature of the cornea was a series of fine parallel ridges. A model of the corneal surface was made in photoresist by lithographic methods and its reflectivity was compared with the same photoresist material with a smooth surface. The fly-eye model was found to have excellent antireflective properties over a wide angular range, at least 60° either side of the surface normal (figure 17; Parker et al. 1998a). This would have aided the fly's vision by permitting more light to pass through the surface of the eye and therefore reach the retina. However, it was found that this new antireflector design provides a 10% increase in energy capture when applied to the surfaces of solar panels (Parker 1999a). Hence this fly-eye antireflector is now manufactured, moulded on large plastic sheets with refractive index matching glue.
Figure 16.
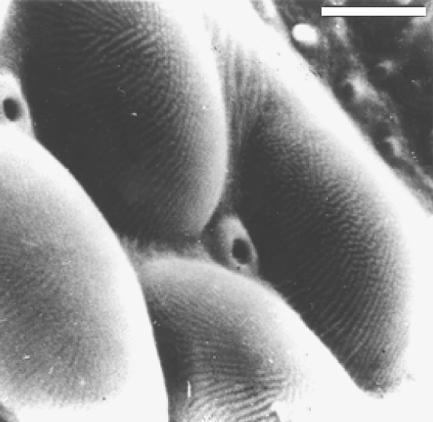
Scanning electron micrograph of the corneal surfaces of four ommatidia from the compound eye of a 45 Ma dolichopodid fly preserved in Baltic amber, showing antireflective gratings. Micrograph courtesy of P. Mierzejewski. Scale bar, 3 μm.
Figure 17.
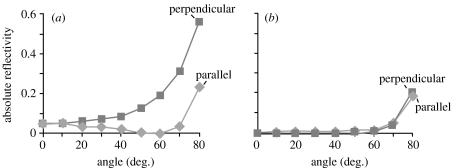
Reflection measurements from photoresist material with (a) a smooth surface and (b) the fly-eye grating surface, showing both polarizations, over a range of angles of incidence with respect to the surface normal.
6.1 How zero-order gratings cause colour or antireflection
In an article in the Encyclopaedia Britannica of 1888, Lord Rayleigh suggested that it was at least theoretically possible to shift energy out of the useless zeroth order into one of the higher-order spectra of a diffraction grating. At least one animal appears to have achieved this, maybe dating back some 160 Myr.
When the periodicity of a grating reduces much below the wavelength of light, it becomes a zero-order grating and its effect on light waves changes (see Hutley 1982). This difference in optical effect occurs because when the periodicity of the grating is below the wavelength of light, the freely propagating diffracted orders are suppressed and only the zero-order is reflected when the illumination is normal to the plane of the grating. To describe accurately the optical properties of a zero-order grating, rigorous electromagnetic theory is required. In an optical system that only accepts the zero-order, what is seen is white light minus that diffracted into the ±1 orders. This may result in no reflection at all (i.e. total transmission).
6.2 The diversity of zero-order gratings today
Such structures occur on the setae of the first antennae of the crustacean Bathynomus (figure 18) that reflect only blue light. Here, there are diffracted orders and the spectral content of the light within the grating is controlled by the groove profile.
Figure 18.
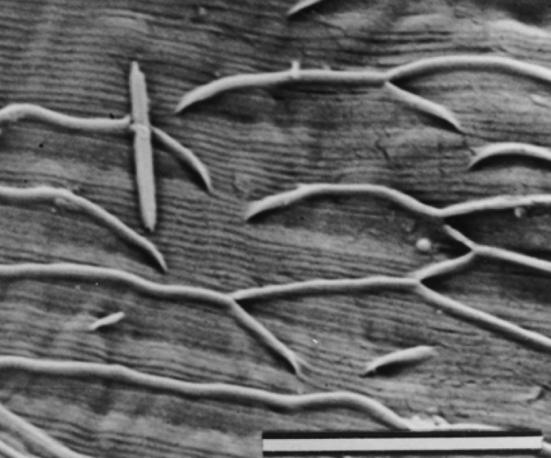
The zero-order grating (fine, parallel lines) on the surface of an antennule hair of Bathynomus immanis. Scale bar, 5 μm.
Bathynomus is a genus of giant isopod crustaceans, resembling a woodlouse/slater although up to half a metre in length. Typically they inhabit seas below 100 m to at least 1000 m depths. Water absorbs light, but selectively for different colours. For example, red is the first colour to disappear with increasing oceanic depth; blue the last (Denton 1990). Beyond about 200 m sunlight is exclusively blue. Some deep-sea animals can detect blue light down to about 1000 m (Denton 1990), and Bathynomus may be one of them. All species of Bathynomus possess blue-reflecting zero-order gratings, and also appear morphologically similar.
Zero-order diffraction gratings that cause total transmission (i.e. there is no reflection) are found also on the corneal surface of Zalea minor (Diptera) today. The periodicity of the corneal gratings of this fly, which have the same profile as that of the Eocene fly above, is 242 nm (Parker et al. 1998a).
Another form of antireflection grating is found on the transparent wings of the hawkmoth Cephonodes hylas (Yoshida et al. 1996) and on the corneal surface of each ommatidium of the compound eyes of moths (Miller et al. 1966) and butterflies (e.g. figure 19). Here, optical-impedance matching is achieved by means of a hexagonal array of tapered cylindrical protuberances, each of about 250 nm diameter (Miller et al. 1966), thus forming a ‘tri-grating’ with grooves transecting at 120°. The protuberances provide a graded transition of refractive index between the air and the cornea/wing. Hence the refractive index at any depth is the average of that of air and the corneal/wing material.
Figure 19.
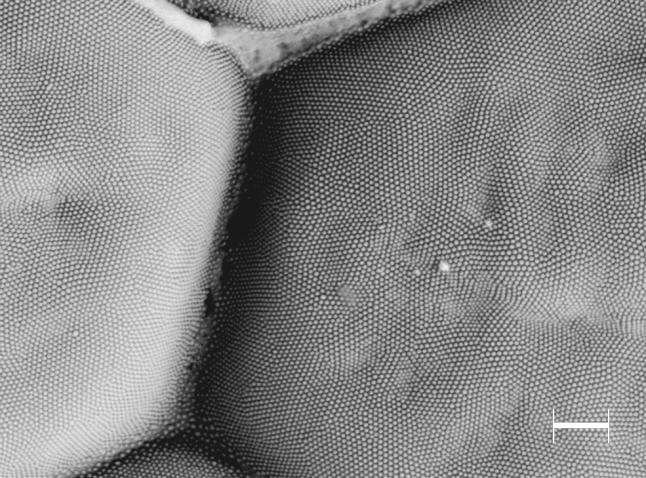
The corneal surface of the compound eye of the butterfly Vanessa kershawi, showing the antireflective surface of protuberances arranged in a hexagonal array. Scale bar, 1 μm.
7. Photonic crystals
Opal is an example of a photonic crystal that occurs naturally—the structure of opal was first revealed in 1964 (Sanders 1964). Many Australian fossils have become opalized, particularly those from the Late Cretaceous period, around 110 Ma (e.g. figure 20). Strictly speaking, however, this opal never belonged to the living animal and so should not belong in this article.
Figure 20.

A 110 Myr opalized bivalve (Mollusca) shell from Australia.
7.1 How photonic crystals cause colour
Photonic crystals are three-dimensional, ordered, subwavelength lattices that can control the propagation of light in the manner that atomic crystals control electrons (see Yablonovitch 1999). Like zero-order gratings, which can be considered a form of photonic crystal, to describe accurately the optical properties of a photonic crystal, rigorous electromagnetic theory is required. In industrial photonic crystals, light rays are trapped via a ‘photonic band gap’ effect within cavities or ‘defects’ in the structure, and hence can be channelled around the crystal in the desired way. However, in the photonic crystals found in nature thus far, no internal cavities have been found and it is the wavelengths reflected that provide a function.
7.2 The diversity of photonic crystals today
To demonstrate the optical effect of opal, it is worthwhile making the contrast with the effect of a multilayer reflector in many species of beetle. In tropical forests many beetles employ structural colours for display purposes (Schultz 1986). Where multilayer reflectors are involved, only part of the beetle is visible (as a ‘spot’ of light) from any direction in direct sunlight because such structures cause mirror-like reflections (e.g. figure 14). However, a beetle (weevil) does exist with metallic colouration visible from every direction, owing to a ‘photonic crystal’ with a structure analogous to that of opal (Parker et al. 2003).
The weevil Metapocyrtus (formerly identified as ‘Pachyrhynchus sp.’) possesses scales, around 0.1 mm in diameter, occurring in patches on the top and sides of its ‘hemispherical’ body (figure 21). Individually, the scales are flat, lying parallel with the body, and consist of two parts—an outer shell and an inner structure. The inner structure of the scales is a solid array of transparent spheres, each 250 nm in diameter (Parker et al. 2003; figure 22). These spheres are arranged in flat layers and have a precise, hexagonal-close-packing order. Since the lattice constant of the crystal approaches half the wavelength of light, it acts like a liquid crystal, although forming several optical domains, oriented in different planes, within a single scale (Parker et al. 2003). This is the most common nano-structure found in opal (occasionally the microspheres show face-centred-cubic packing in opal). It allows for a reflection of a narrow range of wavelengths over a wide range of angles of incidence.
Figure 21.

The weevil Metapocyrtus sp. can achieve a ‘metallic’ coloured appearance, of relatively unvarying colour, from scales positioned anywhere on its body, regardless that the light in its environment is strongly directional.
Figure 22.
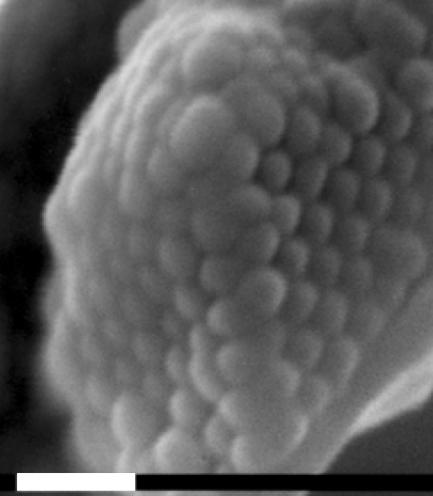
An opal analogue positioned within the flattened, coloured scales on the weevil Metapocyrtus sp. White scale bar, 1 μm.
The first photonic crystal identified as such in animals, however, was a ‘photonic crystal fibre’ (a two-dimensional photonic crystal), which was revealed in a ‘sea mouse’—a marine polychaete worm with the appearance of an iridescent mouse (Parker et al. 2001). The sea mouse is covered in hairs and spines that approximate photonic crystal fibres, causing the coloured effect to change dramatically with orientation of the hairs/spines (figure 23). This was followed by the finding of a three-dimensional ‘photonic crystal’, in the weevil Metapocyrtus sp. (Parker et al. 2003; see ‘Cretaceous’ section above), although three-dimensional photonic crystals in the form of ‘inverse opal’ have been observed in electron micrographs for some time but without photonic identification (e.g. Ghiradella 1989). Currently, many physicists employ rigorous electromagnetic scattering theory on animal reflectors and are uncovering more and more cases of photonic crystals. Further, two-dimensional ‘photonic crystal’ were revealed as the cause of peacock iridescence (Zi et al. 2003) and diatom colours (Fuhrmann et al. 2004), for instance. For a more comprehensive review of this subject see Parker (in press).
Figure 23.
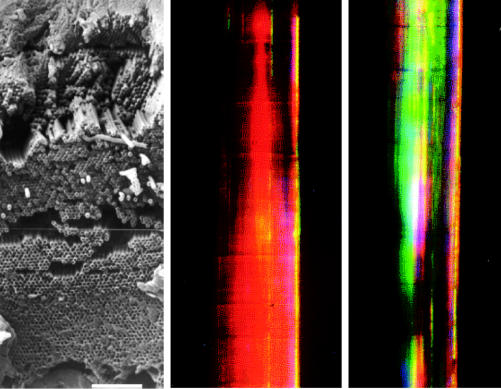
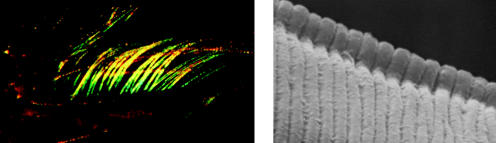
Left: scanning electron micrograph of a ‘photonic crystal’ in the sea mouse Aphrodita sp. (Polychaeta). This shows a cross-section through the wall of a tube, or spine, constructed of nano-tubes, close-packed hexagonally. Internal diameters of the individual nano-tubes increase systematically with depth in the stack. Scale bar, 8 μm. Centre and right: light micrographs of a section of the spine showing the different colours obtained when the orientation in the horizontal plane varies (by 90°) with respect to the direction of the light source.
8. White scattering structures
Many white bird feathers are so coloured owing to non-periodic arrangements of reflecting elements, causing the random scattering of all wavelengths into all directions and thus a diffuse white reflection. This is the case of the white feathers of the Australian sulphur crested cockatoo (e.g. figure 24), but similar feathers may have existed in the Miocene.
Figure 24.

A wing feather of the sulphur crested cockatoo Cacatua galerita where randomly arranged air spaces within a sponge-like lattice cause scattering.
A fossil bill from an extinct cockatoo, Cacatua sp., from the Early to Middle Miocene of Riversleigh, Queensland, Australia has an extremely similar morphology to that of the extant white cockatoos (figure 25), inferring a close evolutionary relationship (Boles 1993). It is suggested that the extinct cockatoo species also possessed white feathers, in line with a trend in this group of parrots (Boles 1993). However, we should consider here that colour may respond to local selective pressures rather than follow evolutionary relatedness. Nonetheless, Nemésio (2001) suggests that the rule in colour evolution in parrots is to lose existing characters and not to acquire new ones.
Figure 25.
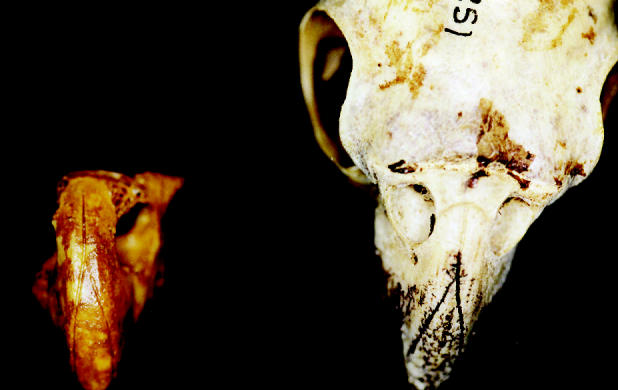
The fossil bill of Cacatua sp. from the Early to Middle Miocene of Riversleigh, Australia (left), and the skull of the extant Cacatua roseicapilla (right), showing similarities in bill morphology.
From some scales of extant butterfly wings, light is scattered uniformly and completely in all directions, owing to the chaotic disposition of the surfaces. Matt or pearly whites may be observed depending on the complexity or the arrangement of the structures, which affects the relative degree of scattering (Mason 1927). If a dark pigment such as melanin is interspersed with the scattering elements, the reflection will appear a shade of grey or brown, and this is the cause of the pale brown coloured effect of Moa feathers (figure 26) and mammoth hair (figure 27). Transmission electron micrographs of both examples reveal a spongy structure containing air spaces, surrounded by melanin granules.
Figure 26.

Feather of the Moa Megalapteryx (Cromwell, South Island, New Zealand). This giant, flightless bird inhabited an Alpine environment well over 1000 m above sea level.
Figure 27.

Mammoth hair.
8.1 How scattering results in white effects
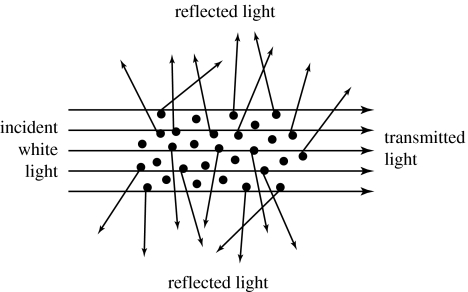
Simple, equal scattering of all spectral wavelengths results in the observation of a diffuse white effect. This commonly arises from the effects of a non-periodic arrangement of colloidally dispersed matter where the different materials involved have different refractive indices (e.g. figure 28), or from solid colourless materials in relatively concentrated, thick layers (Fox 1976). In the colloidal system, the particles are larger than the wavelength of light and can be thought of as mirrors oriented in all directions. The reflection is polarized unless the incident light is at normal incidence on the system and, in the colloidal system, spherical or randomly arranged particles are involved.
Figure 28.
Diagrammatic representation of a scattering system.
8.2 The diversity of white scattering today
The colloidal system involves either a gas-in-solid, gas-in-liquid, liquid-in-liquid (emulsions) or solid-in-liquid (Fox 1976). For example, the gas-in-liquid system is partly responsible for the white body and/or tentacles of certain anemones today (Fox 1976). Light is reflected and refracted at the surfaces of the particles of matter or spaces (with dimensions greater than 1 μm), regardless of the colour of the materials involved (except opaque brown and black compounds, such as melanin; Mason 1927). In insects, the materials involved typically have very low transparencies (Mason 1927).
Variations on white scattering structures can be found in extant animals. Reflection and refraction that occurs at the interfaces of strata with different refractive indices may result in the display of white light. The degree of whiteness depends upon the difference in refractive indices (Fox 1976). This mechanism is evident in the shells of many lamellibranch molluscs (Verne 1930). Between the outer, often pigmented layer, and the mantle is a thick middle layer of crystalline calcium carbonate. The inner surface of this (nacreous) layer is lined with multiple laminations of the same salt. In most species these laminations are sufficiently thick (greater than 1 μm) to render the inner lining white, although in some species they become thin so as to form a multilayer reflector. Calcium carbonate similarly produces whiteness in Foraminifera and in calcareous sponges, corals, echinoderms and crustacean cuticles. Also in the class of white solids is silica in diatom tests and skeletons of hexactinellid sponges (Fox 1976).
An unordered (as opposed to periodic) group of closely spaced setae, such as those in patches on the extant fly Amenia sp., may form a white reflection via random scattering or reflection. However, if the arrangement becomes periodic to some degree, a diffraction grating may be formed, such as the grating of the cylindroleberidid ostracod Tetraleberis brevis.
9. Blue scattering structures
Many ‘subfossils’ are known from the Recent period, where the original, organic parts of extinct animals have been preserved under exceptional environmental conditions, such as permafrost or ice caves. Blue scattering structures can be revealed in some of these, often in combination with pigments.
The Oxford dodo, the last remaining dodo specimen with some soft parts preserved (although only the head and legs survive), shows a blue tint to parts of its skin covering the head and bill (figure 29). It is interesting that some, although not all, seventeenth century colour depictions of the dodo, colour the proximal part of the bill blue. Sections of the blue-coloured skin reveal a randomly arranged, fine-particulate structure in the transmission electron microscope, where the particles are around the size of the wavelength of blue light (the skin is extremely fragile and the particles quickly separate from the structure, forming a powder). Tyndall scattering probably causes the blue colour.
Figure 29.
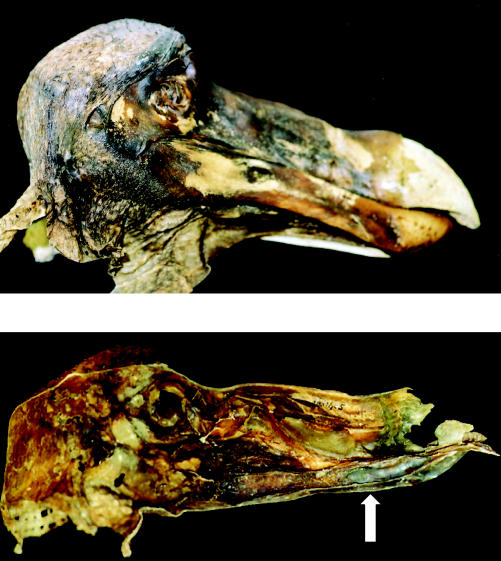
The head of the Oxford dodo specimen. Above: right, lateral (external) view of the skin attached to the skull (note that the end of the bill is bare). Below: medial (internal) view of the skin from the left side of the head, without the skull (note the blue colouration near the end of the lower bill region, arrowed).
10. How scattering results in blue effects
Forms of scattering can result in a blue-coloured effect (red when the system is viewed in transmission). Tyndall or Mie scattering occurs in a colloidal system where the particle size approximates the wavelength of light. Here, diffraction is important. Light is diffracted from the scattering elements but the reflection is inversely proportional to the fourth power of the wavelength. This means that shorter wavelength blues are diffracted more than the longer wavelength reds, and the reflection appears blue (the transmitted portion appears red). Rayleigh scattering may also occur in molecules in a two-photon process by which a photon is absorbed and raises the molecule to an exited electronic state from which it re-radiates a photon when it returns to the ground state. Diffraction is not involved here.
Tyndall scattered light is polarized under obliquely incident light. The relative sizes of particles determine the shade of blue. If the particles responsible for the scattering coalesce to form particles with a diameter greater than about 1 μm, then white light is observed (see ‘white scattering’ above). A gradation from blue to white scattering (‘small’ to ‘large’ particles) occurs on the wings of the extant dragonfly Libellula pulchella (Mason 1927).
10.1 The diversity of blue scattering today
Scattered blues can also be found in other extant dragonflies. In the aeschnids and agrionids, the epidermal cells contain ‘minute’ colourless granules and a dark base. The males of libellulids and agrionids produce a waxy secretion that scatters light similarly over their dark cuticle (e.g. figure 30). The green of the female Aeschna cyanea is the combined result of Tyndall scattering and a yellow pigment, both within the epidermal cells (degradation of the yellow pigment turns the dead dragonfly blue; Fox & Vevers 1960).
Figure 30.

The dragonfly Orthetrum caledonicum (Libellulidae), male. The blue colouration results from Tyndall scattering.
Scattered blues are also observed from the skin of the extant cephalopod (Mollusca) Octopus bimaculatus (Fox 1976), where a broad blue ring surrounds ocelli. Blue light is scattered from this region as a result of fine granules of purine material within cells positioned above melanophore cells (Fox 1976). The colour and conspicuousness of the ring are controlled by the regulation of the melanophores, by varying the distribution of melanin and consequently the density of the absorbing screen. The squid Onychia caribbaea can produce rapidly changing blue colours similarly (Herring 1994). The bright blue patterns produced by some extant nudibranch molluscs (‘sea slugs’) result from reflecting cells containing small vesicular bodies, each composed of particles about 10 nm in diameter and therefore appropriate for Rayleigh scattering (Kawaguti & Kamishima 1964). Importantly, there is no constructive interference between the reflected rays from both white and blue scattering systems—they are known as incoherent reflectors.
Not all assumptions of blue scattering are correct, however. As far back as 1934, C.V. Raman doubted that scattering was the cause of all blue bird feathers (Raman 1934a). In 1971, Jan Dyck suggested that the reflecting elements within blue bird feathers (such as ‘small’ air spaces within a spongy matrix) may actually provide a coherent reflection, whereby they act as ‘layers’ within a multilayer reflector (Dyck 1971). Rick Prum and his colleagues substantiated this idea to great effect using a two-dimensional Fourier analysis to demonstrate that the blues of some extant bird feathers previously thought to result from scattering structures were in fact the result of coherent reflectors (Prum et al. 1998, 1999).
11. Full circle
The subject of bird feathers is where the academic subject of optics in nature began, when Newton (1730) made a tentative link between peacock iridescence (figure 31) and a structural reflector (Hooke (1665) made an even more tentative link between silverfish insects and structural colour). Goureau (1842) discovered that the colours produced from the shells of certain molluscs and the thin, membranous wings of many insects, resulted from physical structures. Nevertheless, until the end of the nineteenth century pigments were generally regarded as the cause of animal colours. Then the Indian scientist C.V. Raman produced a number of papers (e.g. Raman 1934a,b,c) where classical optics was integrated with biological iridescence, such as that from shells and bird feathers. Higher magnification studies of structural colours commenced with Anderson & Richards (1942) following the introduction of the electron microscope to biology, although in this case mistakes were made because efficient sectioning techniques had not been developed.
Figure 31.

The peacock; the subject of the first study of animal reflectors.
With the variety of optical reflectors in nature described, it is worthwhile to consider fields of biology that may benefit from such an understanding, along with the future for the subject.
12. A case of light driving the evolution of a taxon
Optical reflectors can make suitable phenotypes for the study of evolution, since, unlike many other morphologies, they can be quantified. This has led to the conclusion that some invertebrate taxa may have evolved with light as the major stimulus. In this situation, the evolution of structural colours may correlate with the evolution of species.
Many species of cypridinid ostracods (seed-shrimps) possess diffraction gratings on the halophores (the setules) of their first antennae, which cause iridescence (e.g. figure 32). The least derived living cypridinid appears to be Azygocypridina (about 350 Myr old; Parker 1995). The subsequent evolution of Cypridinidae reveals a consistent improvement in the physics of the diffraction gratings. One group of cypridinids continued this trend to the point where the most derived species have very dense ‘iridescent-fans’ (collection of iridescent hairs) with theoretically near-perfect reflectors in males. The females of these derived species, such as Skogsbergia spp., possess very sparse iridescent fans, appearing similar to those of less derived male and female species of Cypridinidae. The males' iridescence is known to be functional. In at least one species of Skogsbergia, when a male ostracod approaches a female its iridescent fan is displayed, which is otherwise held within the carapace that encloses the body (figure 33). The female then becomes sexually receptive to the light displayed and mating follows (Parker 1995, 1997). Divergence in sexual light displays may have generated sufficient sexual isolation among populations to lead to speciation (see Verrell 1991). In fact the whole of the Cypridinidae appear to have evolved with light as the major stimulus (Parker 1995).
Figure 32.
The iridescent effect from the first-antenna of the seed-shrimp Azygocypridina lowryi (left). Scanning electron micrograph of the diffraction grating on a single halophore (right); ridge spacing=600 nm.
Figure 33.
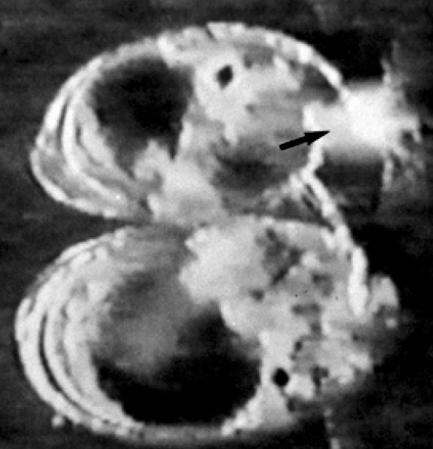
Frame from a video recording of a mating pair of the cypridinid ostracod Skogsbergia sp. The male is above and has released its iridescent hairs (arrowed) from within its shell, and consequently iridescence is displayed to the female (below).
It is useful to know that a structural colour in an animal has a biological function if commercial employment is to be considered—it would be less productive to emulate an incidental reflector than a reflector that has evolved specifically to provide the optical effect observed. And for the same purpose, the more efficient cypridinid gratings are revealed by employing cladistic methods of evolutionary analyses (Parker 1995). Currently, the molecular processes underlying the generation of structural colour patterns are under investigation, beginning with the eyespots of butterfly wings (Carroll et al. 1994).
13. The future for the study of animal reflectors
Photonic crystals carry perhaps the most biomimetic potential for animal reflectors, since these are currently commercially valuable optical devices. Their industrial implications include performance-improvement in lasers, and optical signal processing in the communications industry, making these very recent discoveries within quantum physics at the centre of optical research efforts. Perhaps we can find designs of these and other animal reflectors that could enhance commercial counterparts, particularly since they have been refined over millions of years of evolution. Already, one success story in this field exists—the fly-eye antireflector. Often, however, physicists are ahead of the biologists. For example, it is interesting to note the design of a dielectric reflection grating that has been proposed recently for use in high power lasers. To improve reflected diffraction efficiency Shore et al. (1997) suggest the combination of a multilayer stack with a phase grating on top to diffract a particular wavelength at high efficiency. One of the designs proposed is very similar to the reflective structure discovered more recently in the spider Cosmophasis thalassina (figure 34; Parker & Hegedus 2003).
Figure 34.

The jumping spider Cosmophasis thalassina possesses a combination of a first-order diffraction grating and an underlying broad-band multilayer reflector. The diffraction grating causes one spectral component to be selectively directed away from the direction of incident illumination. The laminar structure beneath produces a broad-band (‘white’) reflectance, and with a significant proportion of the green light being removed from the incident white light by the grating, the reflectance observed at most angles is magenta (white minus green). From Parker & Hegedus (2003).
When considering biomimetics, however, one must be aware that optical reflectors are physical structures and so may have additional functions to that of colour display, hence they may be optically suboptimal. For example, a stack of thin layers (cf. a multilayer reflector) is stronger and more resistant to crack propagation than a single layer of homogeneous material of the same overall thickness (e.g. figure 35), and a corrugated surface (cf. a diffraction grating) is stronger than a flat surface. Indeed, optical reflectors may have their evolutionary origins in structures with a primary mechanical function.
Figure 35.
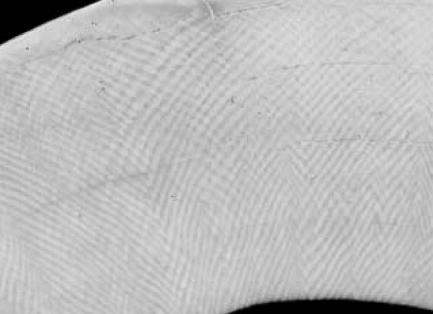
A cross-section of a mammoth tusk, showing layered construction to enhance mechanical strength. The layers here, however, are too thick to have an optical effect, but on a smaller scale could form a multilayer reflector.
An obstacle in the way of the apparently straightforward academic procedure of biomimetics, however, is manufacture. Sometimes animal reflectors are just too intricate and too small. Simply, we cannot make them. A new aim in the study of animal optical structures is to decipher and emulate the animal's manufacturing process. Animals contain the ultimate factories—they engineer via molecular self-assembly. They ‘magically’ mix together chemicals while passing through a suite of specific conditions and perfect reflectors result.
Self-assembly is not an easy process to unravel. There may be intermediate stages involved in the manufacture of nature's complex optics—stages that are not evident from the finished article. Intra-cellular structures may also play their part in the manufacturing process, particularly the smooth endoplasmic reticulum (see Ghiradella 1989) and molecular motors, rulers and templates. However, research on the self-assembly of the sea mouse (Aphrodita sp.) and weevil (Metapocyrtus sp.) ‘photonic crystals’ is underway, beginning with live-cell imaging (e.g. figure 36). Then there are plans to probe the genome of the sea mouse and find the genes that code for its photonic crystals. It may be possible to vary the genome to cause production of crystals with desired properties—we may breed transgenic worms, made to measure.
Figure 36.
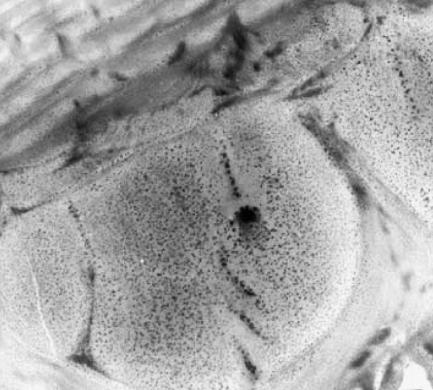
The epidermal (exoskeletal) cell of a beetle in the midst of secreting an unusual addition to a multilayer reflector, giving the structure a unique, three-dimensional quality. It is clues to the manufacturing process that are becoming the most important, at least novel, aspect of the study of animal structural colours. Magnification: ×13 000.
Acknowledgments
This work was funded by The Royal Society (University Research Fellowship) and The Australian Research Council (Large Research Grant).
Footnotes
Note that text following italicized headings will deal with the optics and physics of the system.
References
- Anderson T.F, Richards A.G. An electron microscope study of some structural colours of insects. J. Appl. Phys. 1942;13:748–758. [Google Scholar]
- Arambourg C. Sur un Scopelide fossile a organes luminex: Myctophum ternatum n.sp. du Sahelien oranais. Bull. Soc. Geol. Fr. (Ser. 4) 1920;20:167–168. [Google Scholar]
- Boles W.E. A new cockatoo (Psittaciformes: Cacatuidae) from the Tertiary of Riversleigh, northwestern Queensland, and an evaluation of rostral characters in the systematics of parrots. Ibis. 1993;135:8–18. [Google Scholar]
- Brunton C.F.A, Majerus M.E.N. Ultraviolet colours in butterflies: intra- or inter-specific communication? Proc. R. Soc. Lond. Biol. Sci. 1995;260:199–204. [Google Scholar]
- Carroll S.B, Gates J, Keys D.N, Paddock S.W, Panganiban G.E.F, Selegue J.E, Williams J.A. Pattern formation and eyespot determination in butterfly wings. Science. 1994;265:109–114. doi: 10.1126/science.7912449. [DOI] [PubMed] [Google Scholar]
- Chae J, Nishida S. Integumental ultrastructure and color patterns in the iridescent copepods of the family Sapphirinidae (Copepoda: Poecilostomatoida) Mar. Biol. 1994;119:205–210. [Google Scholar]
- Dalingwater J.E, Hutchinson S.J, Mutveih H, Siveter D.J. Cuticular ultrastructure of some Silurian calymenid trilobites from the Welsh Borderland and Gotland. Palaeontographica A. 1993;229:37–49. [Google Scholar]
- Denton E.J. On the organization of reflecting surfaces in some marine animals. Phil. Trans. R. Soc. Lond. Biol. Sci. 1970;258:285–313. doi: 10.1098/rstb.1970.0037. [DOI] [PubMed] [Google Scholar]
- Denton E.J. Light and vision at depths greater than 200 metres. In: Herring P.J, Cambell A.K, Whitfield M, Maddock L, editors. Light and life in the sea. Cambridge University Press; Cambridge: 1990. pp. 127–148. [Google Scholar]
- Duncan I.J, Briggs D.E.G, Archer M. Palaeontology. 1998;41:835–851. [Google Scholar]
- Dyck J. Structure and spectral reflectance of green and blue feathers of the rosefaced lovebird (Agapornis roseicollis) Biol. Skr. 1971;18:1–67. [Google Scholar]
- Fox D.L. University of California Press; Berkeley: 1976. Animal biochromes and structural colours. [Google Scholar]
- Fox H.M, Vevers G. Sidgwick and Jackson Ltd; London: 1960. The nature of animal colours. [Google Scholar]
- Fuhrmann T, Landwehr S, El Rharbi-Kucki M, Sumper M. Diatoms as living photonic crystals. Appl. Phys. B. 2004;78:257–260. [Google Scholar]
- Gale M. Diffraction, beauty and commerce. Phys. World. 1989;2:24–28. [Google Scholar]
- Ghiradella H. Structure and development of iridescent butterfly scales: lattices and laminae. J. Morphol. 1989;202:69–88. doi: 10.1002/jmor.1052020106. [DOI] [PubMed] [Google Scholar]
- Ghiradella H, Aneshansley D, Eisner T, Silverglied R.E, Hinton H.E. Ultraviolet reflection of a male butterfly: interference colour caused by thin-layer elaboration of wing scales. Science. 1972;178:1214–1217. doi: 10.1126/science.178.4066.1214. [DOI] [PubMed] [Google Scholar]
- Goureau M. Sur l'irisation des ailes des insectes. Annu. Soc. Entomol. France. 1842;12:201–215. [Google Scholar]
- Harvey E.N. Academic Press; New York: 1952. Bioluminescence. [Google Scholar]
- Herring P.J. Reflective systems in aquatic animals. Comput. Biochem. Physiol. 1994;109A:513–546. [Google Scholar]
- Hooke R. Martyn and Allestry; London: 1665. Micrographia. [Google Scholar]
- Hutley M.C. Academic Press; London: 1982. Diffraction gratings. [Google Scholar]
- Kawaguti S, Kamishima Y. Electron microscopic study on the iridophores of opisthobranchiate mollusks. Biol. J. Okayama Univ. 1964;10:83–91. [Google Scholar]
- Kinoshita S, Yoshioka S, Fujii Y, Okamoto N. Photophysics of structural colour in the Morpho butterflies. Forma. 2002;17:103–121. [Google Scholar]
- Land M.F. The physics and biology of animal reflectors. Prog. Biophys. Mol. Biol. 1972;24:75–106. doi: 10.1016/0079-6107(72)90004-1. [DOI] [PubMed] [Google Scholar]
- Land M.F. Animal eyes with mirror optics. Sci. Am. 1978;239:126–134. [Google Scholar]
- Land M.F, Nilsson D.-E. Oxford University Press; Oxford: 2002. Animal eyes. [Google Scholar]
- Martill D.M. The Palaeontological Association; London: 1993. Fossils of the Sanatana and Crato Formations, Brazil. [Google Scholar]
- Mason C.W. Structural colours in insects, II and III. J. Phys. Chem. 1927;31:321–354. (see also pp. 1856–1872) [Google Scholar]
- Miller W.H, Moller A.R, Bernhard C.G. The corneal nipple array. In: Bernhard C.G, editor. The functional organisation of the compound eye. Pergamon Press; Oxford: 1966. pp. 21–33. [Google Scholar]
- Nassau K. Wiley; New York: 1983. The physics and chemistry of colour. [Google Scholar]
- Nemésio A. Colour production and evolution in parrots. Int. J. Ornithol. 2001;4:75–102. [Google Scholar]
- Neville A.C, Caveney S. Scarabeid beetle exocuticle as an optical analogue of cholesteric liquid crystals. Biol. Rev. 1969;44:531–562. doi: 10.1111/j.1469-185x.1969.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Newton, I. 1730 Opticks, reprinted from the 4th edn. by Dover Publications, Inc., New York.
- Parker A.R. Discovery of functional iridescence and its coevolution with eyes in the phylogeny of Ostracoda (Crustacea) Proc. R. Soc. B. 1995;262:349–355. [Google Scholar]
- Parker A.R. Mating behaviour in Myodocopina ostracods (Crustacea): results from video recordings of a highly iridescent cypridinid. J. Mar. Biol. Assoc. UK. 1997;77:1223–1226. [Google Scholar]
- Parker A.R. Colour in Burgess Shale animals and the effect of light on evolution in the Cambrian. Proc. R. Soc. B. 1998;265:967–972. [Google Scholar]
- Parker A.R. Exoskeleton, distribution and movement of the flexible setules on the myodocopine (Ostracoda: Myodocopa) first antenna. J. Crustacean Biol. 1998;18:95–110. [Google Scholar]
- Parker A.R. Light-reflection strategies. Am. Sci. 1999;87:248–255. [Google Scholar]
- Parker A.R. An unusually isolated reflector for host bioluminescence on the second antenna of a lysianassoid (Amphipoda: Gammaridea) In: Schram F.R, von Vaupel Klein J.C, editors. Crustaceans and the biodiversity crisis (Crustaceana) Brill; Leiden: 1999. pp. 879–887. [Google Scholar]
- Parker A.R. The Cambrian light switch. Biologist. 1999;46:26–30. [Google Scholar]
- Parker A.R. 515 million years of structural colour. J. Opt. A. 2000;2:R15–R28. [Google Scholar]
- Parker A.R. Simon & Schuster/Perseus Press; London/Cambridge, MA: 2003. In the blink of an eye. [Google Scholar]
- Parker A.R. Photonic crystals in nature. In: Parker G, Charleston M, editors. Photonic crystals: nanostructures for controlling light. Institute of Physics Press; London: In press. [Google Scholar]
- Parker A.R, Hegedus Z. Diffractive optics in spiders. J. Opt. A. 2003;5:S111–S116. [Google Scholar]
- Parker A.R, McKenzie D.R. The cause of 50 million-year-old colour. Biol. Lett. 2003;270:S151–S153. doi: 10.1098/rsbl.2003.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A.R, Hegedus Z, Watts R.A. Solar-absorber type antireflector on the eye of an Eocene fly (45 Ma) Proc. R. Soc. B. 1998;265:811–815. [Google Scholar]
- Parker A.R, McKenzie D.R, Ahyong S.T. A unique form of light reflector and the evolution of signalling in Ovalipes (Crustacea: Decapoda: Portunidae) Proc. R. Soc. B. 1998;265:861–867. [Google Scholar]
- Parker A.R, McKenzie D.R, Large C.J. Multilayer reflectors in animals using green and gold beetles as contrasting examples. J. Exp. Biol. 1998;201:1307–1313. doi: 10.1242/jeb.201.9.1307. [DOI] [PubMed] [Google Scholar]
- Parker A.R, McPhedran R.C, McKenzie D.R, Botten L.C, Nicorovici N.-A.P. Aphrodite's iridescence. Nature. 2001;409:36–37. doi: 10.1038/35051168. [DOI] [PubMed] [Google Scholar]
- Parker A.R, Welch V.L, Driver D, Martini N. An opal analogue discovered in a weevil. Nature. 2003;426:786–787. doi: 10.1038/426786a. [DOI] [PubMed] [Google Scholar]
- Prum R.O, Torres R.H, Williamson S, Dyck J. Coherent light scattering by blue feather barbs. Nature. 1998;396:28–29. [Google Scholar]
- Prum R.O, Torres R.H, Williamson S, Dyck J. Two-dimensional Fourier analysis of the spongy medullary keratin of structurally coloured feather barbs. Proc. R. Soc. B. 1999;266:13–22. [Google Scholar]
- Raman C.V. The origin of the colours in the plumage of birds. Proc. Indian Acad. Sci. 1934;A1:1–7. [Google Scholar]
- Raman C.V. On iridescent shells—Part I. Introductory. Proc. Indian Acad. Sci. 1934;A1:567–573. [Google Scholar]
- Raman C.V. On iridescent shells—Part II. Colours of laminar diffraction. Proc. Indian Acad. Sci. 1934;A1:574–589. [Google Scholar]
- Sanders J.V. Nature. 1964;204:1151. [Google Scholar]
- Schaal S, Ziegler W. Clarendon Press; Oxford: 1992. Messel. [Google Scholar]
- Schultz T.D. Role of structural colours in predator avoidance by tiger beetles of the genus Cicindela (Coleoptera: Cicindelidae) Bull. Entomol. Soc. Am. 1986;32:142–146. [Google Scholar]
- Shore B.W, Perry M.D, Britten J.A, Boyd R.D, Feit M.D, Nguyen H.T, Chow R, Loomis G.E, Li L. Design of high-efficiency dielectric gratings. J. Opt. Soc. Am. A. 1997;14:1124–1136. [Google Scholar]
- Towe K.M, Harper C.W., Jr Pholidostrophiid brachiopods: origin of the nacreous luster. Science. 1966;154:153–155. doi: 10.1126/science.154.3745.153. [DOI] [PubMed] [Google Scholar]
- Verne J. Armand Colin; Paris: 1930. Couleurs et pigments des êtres vivants. [Google Scholar]
- Verrell P.A. Illegitimate exploitation of sexual signalling systems and the origin of species. Ethol. Ecol. Evol. 1991;3:273–283. [Google Scholar]
- Vukusic P, Sambles J.R, Lawrence C.R. Colour mixing in wing scales of a butterfly. Nature. 2000;404:457. doi: 10.1038/35006561. [DOI] [PubMed] [Google Scholar]
- Yablonovitch E. Liquid versus photonic crystals. Nature. 1999;401:539–541. [Google Scholar]
- Yoshida A, Motoyama M, Kosaku A, Miyamoto K. Nanoprotuberance array in the transparent wing of a hawkmoth, Cephonodes hylas. Zool. Sci. 1996;13:525–526. [Google Scholar]
- Zi J, Yu X, Li Y, Hu X, Xu C, Wang X, Lui X, Fu R. Colouration strategies in peacock feathers. Proc. Natl Acad. Sci. USA. 2003;100:125 76–125 78. doi: 10.1073/pnas.2133313100. [DOI] [PMC free article] [PubMed] [Google Scholar]