Abstract
The prototypic oncogene c-MYC encodes a transcription factor that can drive proliferation by promoting cell-cycle reentry. However, the mechanisms through which c-MYC achieves these effects have been unclear. Using serial analysis of gene expression, we have identified the cyclin-dependent kinase 4 (CDK4) gene as a transcriptional target of c-MYC. c-MYC induced a rapid increase in CDK4 mRNA levels through four highly conserved c-MYC binding sites within the CDK4 promoter. Cell-cycle progression is delayed in c-MYC-deficient RAT1 cells, and this delay was associated with a defect in CDK4 induction. Ectopic expression of CDK4 in these cells partially alleviated the growth defect. Thus, CDK4 provides a direct link between the oncogenic effects of c-MYC and cell-cycle regulation.
The protooncogene c-MYC has been implicated in a variety of human and experimental tumors (for review see refs. 1–4). In some cases, the overexpression of c-MYC can be traced to genetic alterations of the oncogene itself, whereas in others, this dysregulation is caused by genetic defects in upstream regulators of c-MYC expression. In either case, the ability of c-MYC to promote proliferation through cell-cycle reentry seems critical to its oncogenic function. Accordingly, expression of c-MYC is induced by a variety of mitogens and repressed under conditions of growth arrest. Furthermore, ectopic c-MYC expression, in some cases, can promote reentry of resting cells into the cell cycle and facilitate proliferation in the absence of external growth factors (5).
The c-MYC gene encodes a transcription factor of the helix–loop–helix leucine zipper class (for review see refs. 1 and 2). c-MYC binds to E-boxes (CACGTG) in the vicinity of target genes, which are then activated. The DNA binding activity requires dimerization with another helix–loop–helix leucine zipper protein called MAX. MAX also can interact with transcriptional repressors such as MAD and Mxi1, which presumably down-regulate expression of c-MYC target genes. Despite many advances and identification of a number of c-MYC target genes, the direct mediators of c-MYC's effects on cell-cycle reentry have not yet been identified.
Materials and Methods
Cell Culture, Medium, and Reagents.
Human umbilical vein cord (HUVEC) cells and their respective media were obtained from Clonetics (San Diego). The RAT1 fibroblast subclone TGR-1 and the c-Myc −/− derivatives have been described (6). RAT1 fibroblasts and BOSC23 (7) packaging lines were cultured in growth medium (DMEM supplemented with 10% calf serum; Life Technologies, Rockville, MD).
Adenovirus (Ad) Generation.
High-titer adenovirus expressing c-MYC or MADMYC was generated by using the AdEasy system as described (8). In brief, a fragment containing the cytomegalovirus promoter and a human c-MYC cDNA fused to a hemagglutinin (HA)-epitope-tag was excised from the construct HH67 (9), using the restriction enzymes XhoI and HindIII, and inserted into the shuttle vector pAdTrack. To generate an HA-epitope-tagged MADMYC cDNA, the previously described MADMYC-encoding plasmid (10) was used as a template in a PCR using the primers 5′-GTCTCAGGTACCTTCCACCATGGCGGCGGCGGTTCGG-3′ and 5′-GATCATCGATGTTATTGTATGGTAACATGG-3′. The resulting fragment was cut with KpnI and ClaI and ligated into the HH67 vector digested with the same enzymes. A fragment containing the cytomegalovirus promoter and the MADMYC ORF then was transferred to pAdTrack. After recombination with the vector pAdEasy, high-titer virus was generated in 911 and 293 cells. Viruses were purified by a CsCl gradient, and the effective titer was determined by the frequency of green fluorescent protein (GFP)-positive cells after infection. The efficiency of the infection was normalized to the frequency and intensity of GFP-positive cells.
Northern Blot Analysis.
Total RNA was prepared by CsCl gradient ultracentrifugation of guanidine isothiocyanate-lysed cells as described (11). Probes directed against the 3′ untranslated region of the respective mRNAs were generated by PCR using expressed sequence tags as templates and by subsequent gel purification. Hybridizations were performed in QuickHyb, following the manufacturer's instructions (Stratagene).
Serial Analysis of Gene Expression (SAGE).
Total RNA was harvested 12 h after Ad-MYC or Ad-GFP infection of HUVEC cells, which had been arrested by serum starvation for 48 h. SAGE was performed as described (11, 12), and a total of 92,478 tags representing ≈8,500 different transcripts were analyzed to identify candidate c-Myc-induced genes.
Western Blot Analysis.
For Western blot analysis, cells were lysed in 2× Laemmli buffer. Proteins were separated on SDS/polyacrylamide gels (NOVEX, San Diego) and transferred to nitrocellulose membranes (Millipore). Membranes were preblocked in 5% nonfat dry milk/Tris-buffered saline (TBS) for 30 min and then probed with different primary antibodies diluted in 5% milk/TBS/0.05% Tween 20 for 60 min and then for 30 min with a horseradish peroxidase-coupled secondary antibody. After washing the membranes for 30 min in TBS/0.05% Tween 20, enhanced chemiluminescence detection was performed according to the manufacturer's instructions (NEN). Primary antibodies used for detection were AB-1/DCS-35 (Neomarkers, Union City, CA) for Cdk4, A-12 (Santa Cruz Biotechnology) for cyclin D1, rat anti-HA (catalog no. 1867423, Roche Molecular Biochemicals) for tagged proteins, and TU-02 (Santa Cruz Biotechnology) for α-tubulin. For analyses of CDK4 protein, we found that the use of the AB-1/DCS-35 antibody was critical, because other commercially available antibodies detected crossreacting non-CDK4 proteins of similar size to CDK4.
Isolation of the Human and Murine CDK4 Genes.
The primer pair 5′-CAGCATCACCTCTGGTACCC-3′ and 5′-CCCGAATTCCGGGGCGAACGCCGGACG-3′, respectively, was derived from the cosmid sequence (ref. 13 and GenBank accession no. HSU81031) containing the CDK4 promoter region and used to screen a human bacterial artificial chromosome library. A bacterial artificial chromosome (662M22, Research Genetics, Huntsville, AL) containing the CDK4 promoter was digested with KpnI. A 2-kb fragment containing the CDK4 promoter was identified by using PCR and then subcloned into pBR322 (corrected sequence deposited in GenBank, accession no. AF224272). For isolation of the murine Cdk4 gene, the primer pair 5′-CTGCCACTCGATATGAACCCG-3′ and 5′-TAGATCCTTAATGGTCTCAACCG-3′, derived from the mouse Cdk4 cDNA, was used to identify a bacterial artificial chromosome (509, Research Genetics) containing the mouse Cdk4 gene. A 4-kbp KpnI fragment containing the promoter, exons 1 and 2, and the first intron was then subcloned into pBR322 and partially sequenced (sequence deposited in GenBank, accession no. AF223390).
Gel Electrophoretic Mobility Shift Assays.
DNA binding assays were performed in 25 mM Tris⋅HCl, pH 7.5/80 mM NaCl/35 mM KCl/5 mM MgCl2/1 mM DTT/6 μg/ml poly(dI-dC)/10% glycerol/2.4% NP-40. Proteins were generated by a coupled in vitro transcription/translation with the TNT T7 Quick System (Promega) and using MAX and ct-MYC (a truncated version of c-MYC) encoding plasmids described in ref. 14. Per reaction, ≈106 cpm of end-labeled oligonucleotides (40 ng DNA) was used. The respective wild-type and mutant DNA CDK4 promoter fragments were released by a KpnI/BamHI digestion from the reporter constructs described below. DNA and proteins were incubated for 30 min at room temperature. Anti-HA antibody (catalog no. 1867423, Roche Molecular Biochemicals) was added for the last 15 min of this incubation. The complete reactions were then loaded on a nondenaturing 5% acrylamide gel and separated in 0.5× TBE (1× = 0.1 M Tris, pH 8.4/0.09 M boric acid/1 mM EDTA) for 6 h at 4°C at 100 V.
Reporter Assays.
To generate reporter constructs, the following oligonucleotides were used: 5′-CCGGTACCGGGTTGTG- GCAGCCAGTCACGTGCCCGCCGCGTAGCCACACC- TCTGCTCCTCAGAGCAATGTCAAGCGGTCACGTG- TGATAGCAACAGATCACGTGGCTGCCATCGCCCC- TC-3′ [Oligo A, for wild-type c-MYC binding sites (MBS)1–3], 5′-ATGAATTCCGGACGTTCTGGGCACGTGACCGC- CACCCATG CGCTGAGGGGCGGACAGGAGGTGCTTCGACTGGGAGGAGGGCGAAGAGTGTAAGGGGGCG- GAGGGGCGATGGCAGCC-3′ [Oligo B, for wild-type MBS4], 5′-CCGGTACCGGGTTGTGGCAGCCAGTCACCTGC C CGCCGCGTAGCCACACCTCTGCTCCTCAG- AGCAATGTCAAGCGGTCACCTGTGATAGCAACAG- ATCACCTGGCTGCCATCGCCCCTC-3′ [Oligo C , for mutant MBS1–3], and 5′-ATGAATTCCGGACGTTCTGGGCAGGTGACCGCCACCCATGCGCTGAGGGGC- GGACAGGAGGTGCTTCGACTGGGAGGAGGGCG- AAGAGTGTAAGGGGGCGGAGGGGCGATGGCAG- CCAGG-3′ [Oligo D, for mutant MBS4]. Different combinations of oligonucleotide pairs (A+B, A+D, C+B, C+D) were annealed and converted to double-stranded fragments through one PCR cycle. These promoter fragments were subcloned into the KpnI/BamHI sites of pBV-luc, a modified pGL3-basic-derived reporter containing a minimal promoter (15). Further polymerase-derived mutants (mutMBS2 and mutMBS3+4) were identified while sequencing the reporter constructs. For reporter assays in RAT1 cells, transfections were performed by using Lipofectamine (Life Sciences, St. Petersburg, FL), 1 μg of reporter plasmid, and 0.1 μg of a β-galactosidase reporter to control for transfection efficiency. Luciferase and β-galactosidase activities were assessed 24 h after transfection by using reagents from Promega and ICN, respectively. To test the ability of exogenous c-Myc to transactivate reporters, subconfluent NIH 3T3 fibroblasts were transfected by Lipofectin (GIBCO) with 2 μg of reporter plasmid and different amounts of either murine leukemia virus-long terminal repeat (MLV-LTR)-driven plasmids expressing wild-type c-Myc or mutant c-Myc with the helix–loop–helix domain deleted (deletion of amino acids 371–412) (16). Luciferase activity was measured 48 h after transfection, following the manufacturer's protocol (Promega). Total DNA amount was equalized by adding different amounts of empty MLV-LTR vector.
Retrovirus Generation.
The CDK4 ORF was generated by PCR using the expressed sequence tag W77860 as a template and the primers 5′-GCGGATCCGCGGCCGCCTTCCACCATGGCTACCTCTCGATCTGAGC-3′ and 5′-CGGTCGACTCACTCCGGATTACCTTCATC-3′. The resulting product was digested with the enzymes NotI and SalI and inserted into the respective sites of the vector G1BgSVNA (a retroviral vector encoding a hygromycin resistance gene and β-galactosidase), replacing the β-galactosidase gene. The unmodified vector was used as a control. BOSC23 packaging cells (7) were transfected, and the supernatant of resistant, pooled cells was used to infect RAT1 cells.
Results
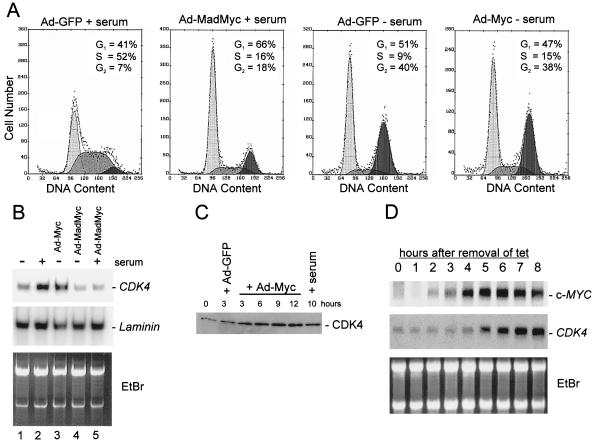
Infection of HUVEC cells with an adenovirus containing a dominant-negative mutant of c-MYC (MADMYC; ref. 10) prevented their serum-induced reentry into the cell cycle (Fig. 1A). Infection with an adenovirus containing a wild-type c-MYC gene did not efficiently induce reentry in the absence of serum (Fig. 1A). In combination, these results suggest that c-MYC expression is necessary but not sufficient for HUVEC cell-cycle reentry. Furthermore, this system provided a way to potentially identify the genes regulated by c-MYC in the absence of incidental changes associated with proliferation.
Figure 1.
Effects of ectopic c-MYC and MADMYC expression on cell-cycle distribution and CDK4 mRNA/protein levels. (A) Flow cytometric analysis of serum-starved HUVEC cells (48 h in 0.5% serum) that were infected with the indicated viruses and maintained in 0.5% serum (− serum) or restimulated by addition of 2% serum (+ serum). Cells were harvested 12 (2 left plots) or 24 h (2 right plots) after viral infection and subjected to flow cytometric analysis as described in ref. 8. (B) Northern blot analysis with RNA (2.5 μg) from HUVEC cells serum-starved (0.5%) for 24 h and then subjected to the serum stimulation (2%) and/or adenoviral infection as indicated. Membranes were hybridized with a probe for CDK4 or a control probe for laminin mRNA. (C) Western blot analysis of lysates from serum-starved HUVEC cells (48 h in 0.5% serum) infected with Ad-MYC or Ad-GFP, or serum-stimulated and harvested at the indicated times. Membranes were probed with a CDK4-specific antibody (see Materials and Methods). (D) Northern blot analysis with RNA from a human B cell line (P493–6) after activation of a conditional c-MYC allele. P493–6 cells harbor a c-MYC gene under control of a tetracycline-responsive element (24). EtBr, ethidium bromide.
SAGE was used to determine which genes are induced by expression of c-MYC in these human cells. SAGE was performed on serum-starved HUVEC cells 12 h after infection with either a c-MYC-expressing virus (Ad-MYC) or a control virus containing the gene for GFP (Ad-GFP). The most intriguing c-MYC induced transcript in terms of cell-cycle regulation was that encoding CDK4 (17). This transcript was of particular interest because ectopic expression of CDK4 had been shown previously to mimic some of the effects of c-MYC overexpression. For example, expression of CDK4 or c-MYC is sufficient to prevent the cell-cycle arrest associated with serum starvation (5, 14), exposure to transforming growth factor-β (18, 19), or ectopic expression of p53 (20, 21). Likewise, c-MYC and CDK4 genes can both immortalize primary cells (22, 23).
Induction of CDK4 mRNA was detectable as early as 6 h after infection with Ad-Myc and increased 3- to 4-fold by 15 h after infection (Fig. 1B and data not shown). This increase in CDK4 mRNA was accompanied by an induction of CDK4 protein (Fig. 1C). CDK4 mRNA was also induced after the addition of serum to serum-starved cells (compare lanes 1 and 2 in Fig. 1B). This induction of CDK4 by serum depended on c-MYC, as adenoviral expression of the dominant-negative mutant MADMYC prevented the induction of CDK4 mRNA after serum stimulation (compare lanes 2 and 5 in Fig. 1B). Expression of MADMYC also led to a reduction in the low level of CDK4 mRNA present in serum-starved cells (compare lanes 1 and 4 in Fig. 1B).
To test whether other cell types displayed c-MYC regulation of CDK4, human primary B cells engineered with a tetracycline-inducible c-MYC gene were used (24). Induction of c-MYC RNA was detectable 4 h after removal of tetracycline. Induction of CDK4 mRNA lagged 1 h behind the c-Myc induction (Fig. 1D). Induction of CDK4 protein lagged 2 h behind the induction of CDK4 mRNA (M.S. and D.E., unpublished data).
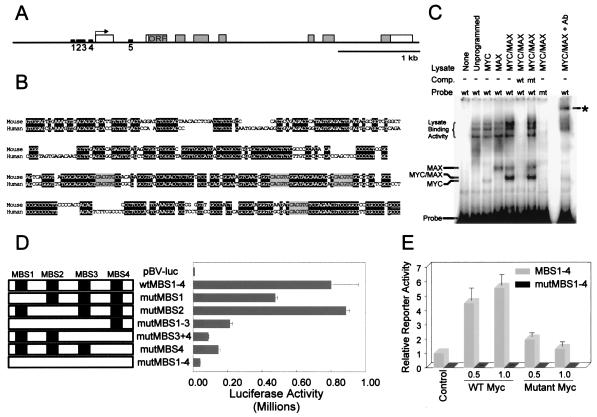
Taken together, these results suggested that c-MYC directly regulates CDK4 mRNA expression. This possibility was further supported by examination of the human CDK4 gene sequence. There were only five potential MBS within the entire 45,976 bp within and surrounding the CDK4 coding sequence, four of which were clustered in a 200-bp region immediately upstream of the transcription start site (Fig. 2 A and B). If CDK4 were a general target of c-MYC, MBS would be expected to be present in the murine CDK4 gene promoter. To evaluate this possibility, we determined the sequence of the murine Cdk4 gene promoter after isolating a mouse bacterial artificial chromosome containing this gene. Remarkably, the murine promoter contained the same four MBS (MBS1–4), identical to those observed in humans in sequence and in position with respect to the Cdk4 transcription start site (Fig. 2B). MBS5 was not found to be conserved.
Figure 2.
MBS in the CDK4 promoter. (A) Map of the human CDK4 gene indicating the position of E boxes (MBS) in the promoter of the human CDK4 gene (black rectangles, MBS1–5). Gray shading represents the CDK4 ORF. The arrow indicates the transcription start site (TSS). (B) Alignment of the human and mouse CDK4 promoter sequence upstream of the TSS (underlined). Identical residues are shaded black, and the identical MBS are shaded gray. (C) Gel electrophoretic mobility shift assay. Oligonucleotides encompassing the first 200 bp upstream of the TSS depicted in B containing either wild-type (wt) or mutant (mt) MBS were end-labeled with [γ-32P]ATP and incubated with combinations of in vitro-translated MYC and MAX proteins (38). DNA–protein complexes were separated by electrophoresis and detected as “shifts” from the position of the free probe. Addition of an antibody directed against an HA-epitope engineered to the C terminus of MAX was able to generate a “supershifted” band, as indicated by the asterisk. Unlabeled oligonucleotides (40× excess) were used as competitors in some reactions. (D) Luciferase activity of CDK4 promoter constructs was measured in RAT1 cells cotransfected with the indicated reporter and a β-galactosidase-expressing vector as control. Luciferase activity is presented as the average of three separate experiments with SD as error bars. (E) Luciferase activity of indicated CDK4 promoter constructs (MBS1–4 or mutMBS1–4) was measured in NIH 3T3 cells cotransfected with empty vector (Control) or the indicated amounts (in μg) of expression vectors for wild-type c-Myc (WT) or mutant c-Myc (16). Luciferase activity was measured 48 h after transfection and presented as relative activity normalized to the control activity of the wild-type promoter (MBS1–4). Values are the average of four determinations with the SD as error bars.
To test whether c-MYC actually binds these putative MBS, gel electrophoretic mobility shift assays were performed with the MBS-containing portion of the CDK4 promoter. c-MYC/MAX complexes specifically bound a CDK4 promoter fragment containing MBS1–MBS4, but not a CDK4 promoter fragment containing mutant MBS1–MBS4 in which each MBS had a single nucleotide substitution (CACGTG → CACCTG) (Fig. 2C). The specificity of the observed complexes was demonstrated by competition with wild-type CDK4 MBS but not mutant CDK4 MBS (Fig. 2C). Addition of an antibody directed against an HA-epitope present in the recombinant MAX protein was able to generate a “supershift” of the putative MYC/MAX and MAX/MAX complexes bound to DNA.
To test whether the four potential MBS sequences were required for transactivation of CDK4 by c-MYC, reporter constructs with specific point mutations in the MBS1–4 sequences (CACGTG → CACCTG) were generated in different combinations (Fig. 2D). A fragment encompassing 200 bp of the region directly upstream of the CDK4 transcription start site conferred strong transcriptional activity to a reporter after introduction into RAT1 cells (Fig. 2D). The activity of this reporter was mediated through MBS1–4 sequences, because mutation of all four sites almost completely abrogated transactivation. Mutation of individual MBS elements suggested that MBS3 and MBS4 were particularly important for mediating the c-MYC responsiveness of the CDK4 promoter (Fig. 2D). To further evaluate the c-MYC responsiveness of the CDK4 promoter, we tested the ability of exogenous c-Myc to activate the CDK4 reporters in NIH 3T3 fibroblasts (Fig. 2E). These studies indicated that wild-type c-Myc, but not a mutant c-Myc lacking the helix–loop–helix domain, transactivated the CDK4 promoter by 4- to 5-fold. Point mutations of the four MBS (mutMBS1–4) resulted in a markedly diminished basal activity of the mutant promoter, whose activity remained about 100-fold less than that of the wild-type promoter, even in the presence of cotransfected wild-type c-Myc. These data suggest that c-Myc directly activates the CDK4 promoter in an E box-dependent manner.
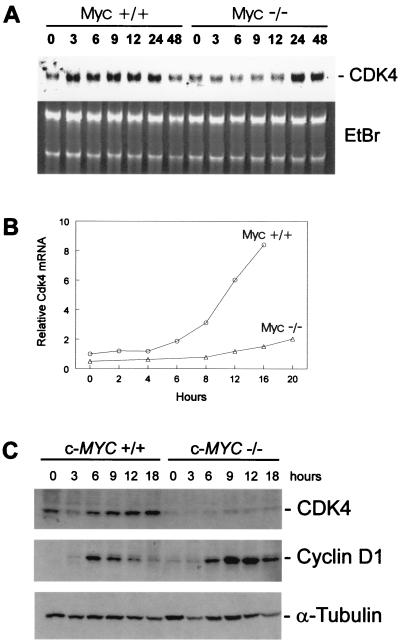
To determine whether c-MYC plays a role in the induction of CDK4 by mitogens, we studied RAT1 fibroblasts in which the c-Myc gene had been inactivated by homologous recombination (6). These cells exhibit an extension of their G1 and G2 phases, leading to an increase in cell-doubling time from 18 h to ≈50 h (25). Serum-stimulated induction of Cdk4 mRNA was attenuated and delayed in c-Myc-deficient cells. This attenuation was evident whether normalized for total cellular RNA (Fig. 3A) or cell count (Fig. 3B) and was ≈2-fold greater than the deficit observed for the induction of glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and other housekeeping genes in the c-Myc-deficient cells. Consistent with this deficit, both serum-starved and exponentially growing c-Myc-deficient cells displayed lower basal levels of Cdk4 mRNA than their wild-type counterparts (Fig. 3B and data not shown). Additionally, Cdk4 expression was restored in c-Myc −/− cells that ectopically expressed c-Myc from a retroviral construct (Fig. 4A and data not shown). The defect in Cdk4 mRNA induction also was reflected by a defect in induction of Cdk4 protein (Fig. 3C). In contrast to Cdk4, cyclin D1 showed higher than normal levels of induction after serum stimulation of c-Myc-deficient cells (Fig. 3C), confirming that c-Myc-deficient cells do not have a general defect in their mitogenic signaling cascades as reported previously (25).
Figure 3.
Requirement of c-Myc for normal induction of Cdk4 after serum stimulation. (A) RAT1 c-Myc +/+(TGR-1) and Rat1 c-Myc −/−(HO15.19) were serum-starved for 48 h in DMEM containing 0.25% calf serum. RAT1 and RAT1 c-Myc −/− were restimulated with 10% calf serum/DMEM, and RNA lysates were prepared at the indicated times. Northern blot analysis was performed with a probe for rat Cdk4 (Upper) and total RNA was stained with ethidium bromide (Lower). (B) RAT1 c-Myc +/+(TGR-1) and Rat1 c-Myc −/−(HO15.19) were serum-starved for 48 h in DMEM containing 0.25% calf serum. RAT1 and RAT1 c-Myc −/− were restimulated with 10% calf serum/DMEM, and RNA lysates were prepared at the indicated times. Northern blot analysis was performed with a probe for rat Cdk4 and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) as an internal control. Relative Cdk4 mRNA levels were determined by quantitating the hybridization signal with a PhosphorImager (Molecular Dynamics), followed by correction for the number of cells loaded by using the internal Gapdh standards. (C) RAT1 c-Myc +/+(TGR-1) and Rat1 c-Myc −/−(HO15.19) were serum-stimulated as described in A, and protein lysates were prepared at the indicated times. Western blot analyses were performed with antibodies against CDK4, cyclin D1, and α-tubulin.
Figure 4.
Growth enhancement of c-Myc-deficient cells by ectopic CDK4 expression. (A) Western blot analysis of CDK4 expression in c-Myc-deficient RAT1 cell infected with a CDK4-encoding retrovirus and a gene conferring hygromycin resistance. CDK4-P1, -P2, and -P3 represent pools of hygromycin-resistant c-Myc −/− cells. CDK4, endogenous CDK4. (B) The pools from A were analyzed for growth rates. Cells were seeded in DMEM containing 10% calf serum and counted at 24-h intervals. Each time point represents the average of two independent experiments. β-Gal, β-galactosidase.
We next hypothesized that the failure to form active Cdk4/cyclin D1 complexes contributed to the previously observed prolongation of the G1 phase in c-Myc-deficient RAT1 cells grown in the presence of serum. To test this conjecture, c-Myc −/− RAT1 cells were infected with retroviruses conferring expression of either CDK4 or β-galactosidase. Analysis of the CDK4 retrovirus-infected cells revealed expression of CDK4 at levels comparable to those seen in wild-type RAT1 cells (Fig. 4A). Ectopic CDK4 expression led to a significant increase in growth rate (Fig. 4B). The doubling time of CDK4-expressing c-Myc −/− RAT1 cells was reduced to 29.75 h (SD = 2.3, n = 8) when compared with parental or β-galactosidase-expressing cells, which doubled every ≈42.8 h (SD = 5.27, n = 4).
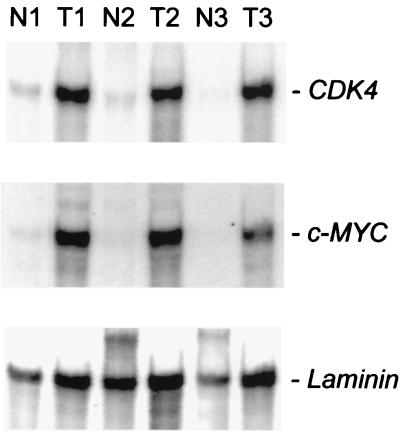
To determine whether the link between c-MYC and CDK4 extends to naturally occurring human tumors, we evaluated colorectal cancers. It previously has been shown that these cancers overexpress c-MYC (see examples in refs. 26 and 27), usually because of genetic defects in adenomatous polyposis coli (APC) or β-catenin, which regulate the activity of the c-MYC promoter (15). Northern blot analysis revealed a concordant increase in c-MYC and CDK4 expression in colorectal cancers when compared with normal colorectal epithelium derived from the same patients (Fig. 5). This observation was consistent with previous reports showing increases in CDK4 levels in early adenomas of mice and humans with APC mutations (28, 29).
Figure 5.
Correlation between c-MYC and CDK4 mRNA in colorectal tumors. Northern blot analysis with RNA isolated from normal colonic epithelial cells and tumor cells derived from three different patients is shown.
Discussion
The above results suggest that the ability of c-MYC to promote cell-cycle reentry is in part due to its ability to induce directly the transcription of CDK4. This mechanism is consistent with several previous observations. First, embryonic fibroblasts derived from Cdk4 −/− mice show a prolonged transition from G1 to S phase after serum stimulation (30, 31), similar to the phenotype of c-Myc-deficient fibroblasts (6). Second, a striking defect in cyclin/Cdk activity was recently demonstrated in c-Myc-deficient fibroblasts, with a 12-fold reduction in the activity of Cdk4/cyclin D1 and Cdk6/cyclin D1 complexes (25). Our results suggest that one factor contributing to the reduction was the reduced amount of Cdk4 protein in c-Myc-deficient cells. Because Cdk4 is regulated at multiple levels, it is likely that other Myc-dependent factors also contribute to the defect in Cdk4 activity in c-Myc-deficient cells. Indeed, the reduction of Cdk4 activity is significantly greater than the reduction in Cdk4 protein (ref. 25 and unpublished data). Third, c-MYC can antagonize the growth inhibition mediated by three different CDK inhibitors (p21, p27, and p16), suggesting that c-Myc induces a protein that can compensate for such inhibition (21, 32, 33). CDK4 is a protein that clearly could function in this manner, because it can serve to sequester p21, p27, and p16 (34, 35). This sequestration may account for the ability of c-Myc overexpression to substitute for p16 deficiency in mouse fibroblast transformation (36). Finally, a target of CDK4 phosphorylation is the retinoblastoma tumor-suppressor gene product pRB (37, 38), and, as noted above, CDK4 can inhibit the activity of p16. The ability of CDK4 to functionally inactivate the products of two tumor suppressor genes, RB and p16, provides a link between c-MYC and the CDK4/cyclin D1/pRB/p16 pathway and may account for the lack of genetic alterations of RB and p16 in some cancers. In such cancers, the elevated c-MYC expression and the consequent elevation of CDK4 expression could obviate the driving force for mutations in RB and p16. Consistent with this model, expression of CDK4 was shown to transform primary rat embryo fibroblasts in cooperation with activated Ha-rasG12V (39). Furthermore, ectopic expression of a fusion gene between CDK4 and Cyclin D1 is able to immortalize primary rat embryo fibroblasts and cooperates with activated Ha-ras to transform rat embryo fibroblasts, conferring anchorage-independent growth in vitro and formation of tumors in vivo (40). Cyclin D1 and Ha-rasG12V coexpression alone did not lead to transformation, suggesting that cdk4 is necessary for transformation and immortalization (40). In these assays, CDK4/Cyclin D1 could be replaced by c-MYC (40).
One puzzling observation made in the course of our studies is that Cdk4 transcription was not induced by Myc estrogen receptor (MycER) chimeras in RAT1 cells (data not shown). We do not know whether this is due to a subtle defect in the MycER protein as compared with native protein, to physiological alterations in the MycER cell lines, or to a more complex regulation of Cdk4 by c-Myc than suggested by our model.
Transcriptional targets of c-MYC have long been sought. CDK4 is especially interesting for several reasons. The induction of CDK4 was observed after c-MYC expression independent of species (human or mouse) and cell type (endothelial, fibroblast, B cell, or epithelium), albeit to varying degrees. The regulation of CDK4 by c-MYC seemed to be direct, as suggested by the conservation of MBS in the CDK4 promoter and by their ability to confer responsiveness to exogenous MYC in reporter assays. Finally, the experiments reported here, as well as those reviewed above, provide plausible mechanisms that explain how this tar- get (CDK4) can mediate some of the effects of c-MYC on the cell cycle. Though any single target is unlikely to explain all of c-MYC's activities, CDK4 provides a direct link between c-MYC's ability to promote tumorigenesis and cell-cycle regulation.
Acknowledgments
We thank Tong-Chuan He for experimental advice, Lin Zhang for RNA samples, Christoph Lengauer for retroviral constructs, Jim Flook for assistance with fluorescence-activated cell sorter analysis, Katrin Berns and Rene Bernards for MADMYC plasmids, and Kornelia Polyak and members of our laboratories for critical reading of this manuscript. This work was supported by National Institutes of Health Grants CA-57345 (to K.W.K.), CA57341 (to C.V.D.), GM-41690 (to J.M.S.), and GM-07601 (to M.K.M.). A.J.O. was supported by a postdoctoral fellowship from the Ministerio Educación y Cultura de España. K.W.K. received research funding from Genzyme Molecular Oncology (Genzyme). Under a licensing agreement between The Johns Hopkins University and Genzyme, the SAGE technology was licensed to Genzyme for commercial purposes, and K.W.K and B.V. are entitled to a share of royalties received by the University from sales of the licensed technology. The SAGE technology is freely available to academia for research purposes. K.W.K. and B.V. are consultants to Genzyme. The Johns Hopkins University, K.W.K., and B.V. own Genzyme stock, which is subject to certain restrictions under University policy. The terms of this arrangement are being managed by the University in accordance with its conflict-of-interest policies.
Abbreviations
- SAGE
serial analysis of gene expression
- MBS
c-MYC binding sites
- HUVEC cells
human umbilical vein cord cells
- HA
hemagglutinin
- GFP
green fluorescent protein
- Ad
adenovirus
Footnotes
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AF224272 and AF223390).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050586197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050586197
References
- 1.Amati B, Alevizopoulos K, Vlach J. Front Biosci. 1998;3:250–268. doi: 10.2741/a239. [DOI] [PubMed] [Google Scholar]
- 2.Dang C V. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eick D, Hermeking H. Trends Genet. 1996;12:4–6. doi: 10.1016/0168-9525(96)81374-6. [DOI] [PubMed] [Google Scholar]
- 4.Garte S. Crit Rev Oncog. 1993;4:435–449. [PubMed] [Google Scholar]
- 5.Eilers M, Schirm S, Bishop J M. EMBO J. 1991;10:133–141. doi: 10.1002/j.1460-2075.1991.tb07929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mateyak K M, Obaya A J, Adachi S, Sedivy J M. Cell Growth Differ. 1997;8:1039–1048. [PubMed] [Google Scholar]
- 7.Pear W S, Nolan G P, Scott M L, Baltimore D. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermeking H, Wolf D A, Kohlhuber F, Dickmanns A, Billaud M, Fanning E, Eick D. Proc Natl Acad Sci USA. 1994;91:10412–10416. doi: 10.1073/pnas.91.22.10412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berns K, Hijmans E M, Bernards R. Oncogene. 1997;15:1347–1356. doi: 10.1038/sj.onc.1201280. [DOI] [PubMed] [Google Scholar]
- 11.Hermeking H, Lengauer C, Polyak K, He T C, Zhang L, Thiagalingam S, Kinzler K W, Vogelstein B. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- 12.Velculescu V E, Zhang L, Vogelstein B, Kinzler K W. Science. 1995;270:484–487. doi: 10.1126/science.270.5235.484. [DOI] [PubMed] [Google Scholar]
- 13.Elkahloun A G, Krizman D B, Wang Z, Hofmann T A, Roe B, Meltzer P S. Genomics. 1997;42:295–301. doi: 10.1006/geno.1997.4727. [DOI] [PubMed] [Google Scholar]
- 14.Kohlhuber F, Hermeking H, Graessmann A, Eick D. J Biol Chem. 1995;270:28797–28805. doi: 10.1074/jbc.270.48.28797. [DOI] [PubMed] [Google Scholar]
- 15.He T C, Sparks A B, Rago C, Hermeking H, Zawel L, da Costa L T, Morin P J, Vogelstein B, Kinzler K W. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 16.Shim H, Dolde C, Lewis B C, Wu C S, Dang G, Jungmann R A, Dalla-Favera R, Dang C V. Proc Natl Acad Sci USA. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushime H, Ewen M E, Strom D K, Kato J Y, Hanks S K, Roussel M F, Sherr C J. Cell. 1992;71:323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- 18.Ewen M E, Sluss H K, Whitehouse L L, Livingston D M. Cell. 1993;74:1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- 19.Alexandrow M G, Kawabata M, Aakre M, Moses H L. Proc Natl Acad Sci USA. 1995;92:3239–3243. doi: 10.1073/pnas.92.8.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latham K M, Eastman S W, Wong A, Hinds P W. Mol Cell Biol. 1996;16:4445–4455. doi: 10.1128/mcb.16.8.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermeking H, Funk J O, Reichert M, Ellwart J W, Eick D. Oncogene. 1995;11:1409–1415. [PubMed] [Google Scholar]
- 22.Wang J, Xie L Y, Allan S, Beach D, Hannon G J. Genes Dev. 1988;12:1769–1774. doi: 10.1101/gad.12.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holland E C, Hively W P, Gallo V, Varmus H E. Genes Dev. 1998;12:3644–3649. doi: 10.1101/gad.12.23.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuhmacher M, Staege M S, Pajic A, Polack A, Weidle U H, Bornkamm G W, Eick D, Kohlhuber F. Curr Biol. 1999;9:1255–1258. doi: 10.1016/s0960-9822(99)80507-7. [DOI] [PubMed] [Google Scholar]
- 25.Mateyak M K, Obaya A J, Sedivy J M. Mol Cell Biol. 1999;19:4672–4683. doi: 10.1128/mcb.19.7.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erisman M D, Rothberg P G, Diehl R E, Morse C C, Spandorfer J M, Astrin S M. Mol Cell Biol. 1985;5:1969–1976. doi: 10.1128/mcb.5.8.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Augenlicht L H, Wadler S, Corner G, Richards C, Ryan L, Multani A S, Pathak S, Benson A, Haller D, Heerdt B G. Cancer Res. 1997;57:1769–1775. [PubMed] [Google Scholar]
- 28.Zhang T, Nanney L B, Luongo C, Lamps L, Heppner K J, DuBois R N, Beauchamp R D. Cancer Res. 1997;57:169–175. [PubMed] [Google Scholar]
- 29.Zhang T, Nanney L B, Peeler M O, Williams C S, Lamps L, Heppner K J, DuBois R N, Beauchamp R D. Cancer Res. 1997;57:1638–1643. [PubMed] [Google Scholar]
- 30.Rane S G, Dubus P, Mettus R V, Galbreath E J, Boden G, Reddy E P, Barbacid M S, Rane G. Nat Genet. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- 31.Tsutsui T, Hesabi B, Moons D S, Pandolfi P P, Hansel K S, Koff A, Kiyokawa H. Mol Cell Biol. 1999;19:7011–7019. doi: 10.1128/mcb.19.10.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiner P, Philipp A, Lukas J, Godden-Kent D, Pagano M, Mittnacht S, Bartek J, Eilers M. EMBO J. 1995;14:4814–4826. doi: 10.1002/j.1460-2075.1995.tb00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlach J, Hennecke S, Alevizopoulos K, Conti D, Amati B. EMBO J. 1996;15:6595–6604. [PMC free article] [PubMed] [Google Scholar]
- 34.Reynisdottir I, Polyak K, Iavarone A, Massague J. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 35.Serrano M, Hannon G J, Beach D. Nature (London) 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 36.Serrano M, Lin A W, McCurrach M E, Beach D, Lowe S W. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 37.Koh J, Enders G H, Dynlacht B D, Harlow E. Nature (London) 1995;375:506–510. doi: 10.1038/375506a0. [DOI] [PubMed] [Google Scholar]
- 38.Lukas J, Parry D, Aagaard L, Mann D J, Bartkova J, Strauss M, Peters G, Bartek J. Nature (London) 1995;375:503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 39.Haas K, Staller P, Geisen C, Bartek J, Eilers M, Moroy T. Oncogene. 1997;15:179–192. doi: 10.1038/sj.onc.1201171. [DOI] [PubMed] [Google Scholar]
- 40.Rao R N, Stamm N B, Otto K, Kovacevic S, Watkins S A, Rutherford P, Lemke S, Cocke K, Beckmann R P, Houck K, et al. Oncogene. 1999;18:6343–6356. doi: 10.1038/sj.onc.1203009. [DOI] [PubMed] [Google Scholar]