Abstract
The mechanosensitive channel of small conductance (MscS) is a bacterial mechanosensitive channel that opens in response to rapid hypoosmotic stress. Since MscS can be opened solely by membrane stretch without help from any accessory protein, the lipid-protein interface must play a crucial role in sensing membrane tension. In this study, the hydrophobic residues in the lipid-protein interface were substituted one by one with a hydrophilic amino acid, asparagine, to modify the interaction between the protein and the lipid. Function of the mutant MscSs was examined by patch-clamp and hypoosmotic shock experiments. An increase in the gating threshold and a decrease in the viability on hypoosmotic shock were observed when the hydrophobic residues near either end of the first or the second transmembrane helix (TM1 or TM2) were replaced with asparagine. This observation indicates that the lipid-protein interaction at the ends of both helices (TM1 and TM2) is essential to MscS function.
INTRODUCTION
Eukaryotic and prokaryotic cells detect mechanical stimuli such as touch, vibration, or swelling through the activation of mechanosensitive (MS) channels (1–3). MS channels have been found in virtually all cell types, probably because monitoring the mechanical condition around and within the cell is essential to the survival and the function of cells. MS channels have also been referred as stretch-activated channels (or stretch-inactivated channels) since the probability of opening changes with the stretch of the cell membrane. How the activities of MS channels are modulated by membrane stretch is one of the fundamental questions in the study of cell mechanosensation.
The bacterial MS channels, MscS and MscL (mechanosensitive channel of small and large conductance, respectively), cooperatively protect cells against cell lysis on hypoosmotic shock (4–9). Escherichia coli MscS is a mechanosensitive channel that has a conductance of ∼1 nS and a slight preference to anions as the permeable ions (10). MscL is a nonselective MS channel that has a conductance as large as 2.5 nS (11). The threshold for MscS gating is 0.5–0.7 times that for MscL when a negative pressure is applied to the excised membrane in patch-clamp experiments. MscS shows sensitivity to voltage in addition to membrane stretch (10,12).
MscS, which has been also known as YggB, is constituted of a 31 kDa, 286 amino acid subunit (6). A crystal structure of MscS resolved at 3.9 Å resolution shows that MscS is a homoheptamer of the subunit and that each subunit has three transmembrane domains (13). The three transmembrane domains have been designated TM1 (residues 29–57), TM2 (residues 68–91), and TM3 (residues 96–127) (13). A channel pore is located at the center of the transmembrane domain and is lined by TM3.
The findings that MscS and MscL retain mechanosensitivity when they are purified and reconstituted in liposomes (11,14) indicate that the stretch of the membrane is directly transmitted from the lipid bilayer to the channel proteins. Further support for this conclusion is provided by the observation that reducing the thickness of the hydrophobic core of the membrane dramatically increases the mechanosensitivity of MscL (15). The finding that incorporation of conical lipids to the lipid bilayer opens MscS and MscL (15,16) also indicates that a change in the membrane tension or the lateral pressure on the channel from the lipids is sufficient to open MscS and MscL. Since MscL and MscS interact with membrane only in the transmembrane domain, MscL and MscS are likely to perceive the membrane tension through the lipid-protein interface.
The key residues in the lipid-protein interface of MscL were first identified by isolating loss-of-function mutants generated by random mutagenesis (17). Several loss-of-function mutants were found at the level of lipid headgroups on both the cytoplasmic and periplasmic side. Some of the second site suppressors of leaky mutants also occurred close to the surface of the bilayer (18). Since those mutants isolated in both studies contained hydrophilic substitutions for hydrophobic residues, whole interface was surveyed by scanning mutagenesis, in which asparagine was used to substitute in turn for each residue on the surface of the transmembrane domain and to modify the interaction between the protein and the lipid (18). Single hydrophilic substitution of the residues near the periplasmic surface of the membrane results in loss of function, whereas substitution in the rest of the transmembrane domain did not significantly affect the MscL function. This finding suggests that MscL senses the membrane tension through the lipid-protein interaction close to the periplasmic ends of the transmembrane domain (18). This idea is consistent with the profile of the lateral pressure that lipids exert on membrane protein. In general, the surface of the bilayer exerts contracting force to prevent exposure of the lipid tails to solvent, whereas the positive pressure by the lipid tails balances this negative pressure (19–23). Therefore, the force that expands MscL is expected to be present only near the surface of the membrane. Decompression of the mid-bilayer may also give rise to the conformational change. Given the general feature of the lateral pressure profile, it is an interesting problem whether the mechanosensitive channels that are directly activated by membrane tension are in common activated through the lipid-protein interaction near the surface of the lipid bilayer.
In this study, we examined whether a disturbance of the lipid-protein interface affects the tension dependence of MscS, which has no amino acid sequence homology to MscL. To identify the residues that impair the channel's function on hydrophilic substitution, each residue in the lipid-protein interface was replaced one by one with asparagine. The asparagine substitution in the protein-lipid interface is expected to allow the mutated residue to interact with lipid headgroups or water and thus to redistribute the lipids and the lateral pressure profile. The substituted asparagine may also form an interaction with hydrophilic residues and protein backbones nearby. Asparagine was used here because: asparagine replacement is effective to assess the interaction between lipid and protein (18); asparagine substitution in the lipid-protein interface does not affect the proper assembly of MscL (18); asparagine is electrically neutral and does not have strong electrostatic interaction with the charged head group of lipid; and asparagine substitution of the residues in the lipid-protein interface has been shown not to affect the voltage-dependent gating of Shaker K+ channel, a stretch-sensitive voltage-gated channel (24,25). The pressure needed to open MscS in patch-clamp experiments and the survival rate on hypoosmotic shock were used as measures of how readily MscS opens. This result indicates that the interaction between the membrane lipids and both ends of the transmembrane helices is important for the tension dependence of MscS.
MATERIALS AND METHODS
Strains and plasmids
E. coli strains PB111 (ΔyggB, ΔrecA) and MJF455 (ΔmscL, ΔyggB∷Cm) were used to host MscS expression in patch-clamp experiments and in hypoosmotic shock experiments, respectively (6,9). E. coli strain DH5α was used for site-directed mutagenesis. Polyhistidine-tagged MscS was described previously (9).
Site-directed mutagenesis
Asparagine mutations were made on the background of pB10b (26), which has an ampicillin resistance gene and the lacUV5 promoter. AAC or AAT was used to code asparagine. Site-directed mutagenesis was performed by the megaprimer method (27) or the DpnI-based method (QuickChange Site-Directed Mutagenesis Kit, Stratagene, La Jolla, CA). Successful mutagenesis was confirmed by DNA sequencing with the CEQ 2000XL DNA Analysis System (Beckman Coulter, Fullerton, CA). We failed to generate I78N MscS by these and other methods although introduction of the I78N mutation into the MscS gene cloned in an antisense direction was successful. We suspect that I78N is a lethal mutation.
Spheroplast preparation
E. coli spheroplasts were prepared essentially as described previously (28). Chephalexin (final concentration 0.06 mg/ml) was added to log-phase cells growing in a modified LB (Luria Bertani) medium that contained 0.5% NaCl instead of 1% NaCl (10). After incubation for 1.5 h, IPTG (isopropyl-β-D-thiogalactoside) was added (final concentration: 1 mM) to induce MscS expression. The induction time was 10 min. The cells were then harvested, lysed with lysozyme (0.02 mg/ml), and collected by centrifugation.
Electrophysiological recording
The channel activities of MscS were examined by the inside-out patch-clamp method as described previously (27). The pipette solution contained 200 mM KCl, 90 mM MgCl2, 10 mM CaCl2, and 5 mM HEPES (pH 6.0), whereas the bath solution contained additional 0.3 M sucrose to stabilize the spheroplasts. Currents were amplified with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) and filtered at 2 kHz. Current recordings were digitized at 5 kHz with a Digidata 1322A interface using pCLAMP 9 software (Axon). A negative pressure was applied by syringe-generated suction through the patch-clamp pipette and measured with a pressure gauge (PM 015R, World Precision Instruments, Sarasota, FL).
Hypoosmotic shock assay
Survival rate after hypoosmotic shock was examined by the method described previously (6,18). Expression was induced by adding IPTG (1 mM) to cells grown to OD600 = 0.14 in minimal medium added with 500 mM NaCl and 200 μg/ml ampicillin. After incubation for 1 h, the cells were diluted 1:20 in the prewarmed minimal medium with or without 500 mM NaCl. A downshock of 500 mM NaCl was applied to the cells for 5 min. The ratio of the number of colony forming units of the cells that experienced osmotic downshock (ndown) to those that did not (ncontrol) was used to calculate the survival rate (ndown/ncontol). The osmotic shock experiment was carried out three times on each mutant.
Membrane protein preparation and Western blots
Western blots were conducted on membrane preparations from cells induced by IPTG (1 mM, 2 h) as described previously (18). Samples were separated on a 12% sodium dodecyl sulfate polyacrylamide gel (Bis-Tris Gel, Invitrogen, Carlsbad, CA). An antibody that recognizes the His6-tag was used to detect histidine-tagged MscS.
RESULTS
Asparagine substitutions in the lipid-protein interface impede MscS function
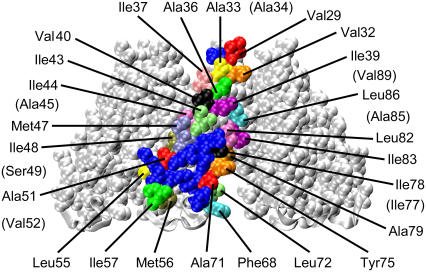
Candidates for the hydrophobic residues that interact with membrane lipid (Fig. 1) were evaluated using the crystal structure of E. coli MscS (13). The residues that are partially buried in the protein were also included because MscS is estimated to show strong fluctuation and flexibility (29). Each of these residues was substituted, one by one, with asparagine. The channel activity of the MscS mutants was examined by applying a negative pressure through a patch pipette to the inside-out membrane patch from the giant spheroplasts expressing MscS. When an increasingly negative pressure was applied to the spheroplast membrane of the cell expressing wild-type MscS, current with steps of ∼15 pA appeared first (arrowhead in Fig. 2 A; at +15 mV pipette potential). This current was judged to be due to MscS by the amplitude of the unit current and by the threshold relative to MscL (see below). MscL, which has a unit amplitude of ∼45 pA, opened at pressure almost twice that needed to open MscS (arrow in Fig. 2 A). Here, we use the threshold of MscL to normalize the threshold of MscS: then the threshold of MscS was consistently ∼0.5 (0.52 ± 0.06, n = 8).
FIGURE 1.
The residues used for asparagine substitution displayed on the crystal structure of MscS. The residues in TM1 and TM2 are shown in space fill model and the loop connecting TM1 and TM2 is shown in ribbon model. The residues in a single subunit are colored arbitrarily except for hydrophilic residues, which are displayed in blue. The residues that are not visible from the outside are in brackets.
FIGURE 2.
Characterization of the wild-type and mutant MscS. (A–C) Channel current through the wild-type (A), I48N (B), and L55N MscS (C). In each panel, the membrane current (top) and the negative pressure applied through patch pipette (bottom) are shown. The first opening of MscS and MscL upon continuously increasing suction is indicated by arrowheads and arrows, respectively. Pipette potential was +15 mV. (D) Threshold for MscS gating as determined by the patch-clamp (mean ± SE). The asterisks in D and E indicate that the threshold is significantly different from wild-type (p < 0.05 by t-test). ND, not determined. (E) Effects of hypoosmotic shock on cells expressing mutant MscS (mean ± SE).
Some mutant MscSs opened only when higher pressure was applied. I48N MscS, for instance, had a threshold of 0.72 ± 0.04 (Fig. 2 B). Furthermore, L55N MscS began to open almost simultaneously with MscL (threshold = 0.92 ± 0.03, Fig. 2 C). Eleven out of 31 mutants were found to have thresholds significantly higher than that of the wild-type MscS (Fig. 2 D). Five of them (A34N, L55N, F68N, A85N, L86N) had thresholds close to MscL ranging from 0.92 to 1.09 (shaded bars in Fig. 2 D). On the other hand, two mutants, I39N (threshold = 0.37 ± 0.03) and I43N (threshold = 0.36 ± 0.01), had significantly smaller thresholds than that of the wild-type (Fig. 2 D). Therefore, MscS changes the sensitivity to membrane stretch upon asparagine substitution depending on the site of the substitution.
When E. coli cells are subjected to hypoosmotic shock, they avoid cell lysis by opening MscS and MscL. In fact, most of the ΔmscL ΔmscS cells harboring an empty vector (pB10b) did not survive upon hypoosmotic shock from 500 mM NaCl down to 0 mM NaCl (Fig. 2 E). To evaluate how easily MscS opens in vivo, the cells expressing MscS mutant were challenged with hypoosmotic shock. Cells expressing MscS with high threshold (A34N, L55N, F68N, A85N, L86N) showed significantly lower survival rate than that of wild-type MscS (shaded bars in Fig. 2 E).
Most of the mutations that did not affect the gating threshold or the ones that decreased the threshold had no significant effect on survival (Fig. 3 A). Exceptions were V89N and I77N, which had a reduced survival rate with little change in the threshold. The five mutations that raised the threshold close to the threshold for MscL significantly declined the survival rate (Fig. 3 A, red symbols; colors represent the gating threshold relative to the wild-type). Those mutants were due to substitutions for alanine, phenylalanine, and leucine (Fig. 3 B).
FIGURE 3.
Summary of the impact (severity of function loss) of asparagine substitution. (A) Survival rate plotted against the gating thresholds. The density of blue and red increases with the decrease and increase in the gating threshold from the wild-type MscS, respectively. (B) Changes in the side chain of the “tight” mutation sites. (C, D) The impact of asparagine substitution shown on net diagram (C) and structural model (D) of TM1 and TM2. (C) Gain-of-function (GOF) mutants are shown in black (V40D is from Okada et al. (9) and I78N is from this experiment). The residues that were not subjected to mutagenesis are in gray. Thick circles indicate that the residues are conserved among MscS from various origins. Parts of each helix facing the lipid in the crystal structure are shown in orange.
The residues responsible for the mutants with high threshold and low survival rate (red) were present near the ends of the transmembrane domain but were absent in the middle (Fig. 3 C). In contrast, the residues responsible for low threshold mutants (blue) were mostly located in the middle of the helix. In the crystal structure, the positions of the “tight” mutations (red) were located in close proximity to the ends of TM1 and TM2 (Fig. 3 D).
In this and previous studies, we have assumed that the asparagine substitution affects the function of MscS and MscL through the change in hydrophobicity of the amino acid (18). To verify this assumption, we substituted Leu-86 with a positively charged amino acid (lysine), a negatively charged amino acid (aspartic acid), or a smaller hydrophobic amino acid (valine). Leu-86 was chosen because L86N is one of the most pronounced loss-of-function mutants (Fig. 3 A) and Leu-86 is exposed to lipid at least in part (Fig. 3 D). The survival rate on hypoosmotic shock was significantly decreased in the cells expressing L86K MscS (25 ± 13%) and L86D MscS (59 ± 8%). The cells expressing L86V MscS showed smaller decrease in the survival rate (72 ± 11%). The threshold examined by patch-clamp was significantly increased in L86K MscS (0.89 ± 0.06) as in L86N MscS (1.02 ± 0.05). The threshold of L86V MscS (0.49 ± 0.09), on the other hand, was similar to that of the wild-type MscS (0.52 ± 0.06). This observation supports the idea that the change in the gating threshold is mostly affected by the change in the hydrophobicity of the residue subjected to mutation.
Double mutations on the same side of the membrane result in total loss of function
The result above shows that a single asparagine substitution is not enough to completely abolish the function of MscS (Fig. 2, D and E). This result contrasts with that of MscL, whose single asparagine substitution at the ends of transmembrane domain results in total disruption of the channel activity (18). To assess whether a combination of two asparagine substitutions can further impair the mechanosensitivity of MscS, we constructed double mutants of the asparagines substitution. The residues for the double mutations are chosen on the bases of the severity of the mutation and the exposure to membrane lipid (Fig. 3, C and D).
First, asparagine substitution of Phe-68, which is located at the cytoplasmic end of TM2, was combined with a second mutation in TM1. Introduction of the second mutation at the periplasmic end of TM1 did not result in further decrease in the survival rate (I37N/F68N, Fig. 4 A), whereas that at the cytoplasmic end showed an additional decrease (A51N/F68N). On the other hand, substitution at Leu-86, a residue at the periplasmic end of TM2, showed a significant decrease with a mutation at the periplasmic end of TM1 (I37N/L86N) but not with a mutation at the cytoplasmic end of TM1 (A51N/L86N, Fig. 4 A).
FIGURE 4.
Effect of combining the mutations that alter MscS stiffer than the wild-type MscS. (A) Survival rate of the double mutants (mean ± SE). The asterisks indicate that the survival rate is significantly different from the corresponding single mutants (p < 0.05). (B) Western blot against the histidine tag attached to the carboxy terminus of MscS. (C, D) Patch clamp experiment on the cells expressing A51N/F68N (C) and I37N/L86N MscS (D). The insets show the magnification of the MscL traces indicated by arrows. Pipette potential was +15 mV.
Second, double mutations were generated in the same transmembrane helix. A51N MscS, which has a mutation at the cytoplasmic end of TM1, showed only a small decrease in the survival rate when a second site mutation was generated at the opposite (periplasmic) end (A34N) of the same helix, TM1. However, a large decrease was observed when the second site mutation was placed at the same (cytoplasmic) end (L55N, Fig. 4 A).
All of the observations above indicate that the asparagine substitutions have additive effect only when they are introduced on the same side of the membrane. The decrease in the survival rate was not due to low expression level, since Western blot shows that the expression was not significantly decreased in the mutants (Fig. 4 B).
When the most pronounced loss-of-function double mutants (A51N/F68N and I37N/L86N) were examined by patch clamp, channel openings of MscS were not observed (Fig. 4, C and D). The current observed in Fig. 4, C and D, is solely due to MscL judging by the unit amplitude (∼45 pA) and the fast kinetics. Application of a high pressure up to membrane lysis, which occurred at pressure 1.6–2.3× the threshold of the wild-type MscL, did not open these mutant MscS. This observation confirms that the decrease in the survival rate is due to the loss of the function of MscS.
DISCUSSION
Because MscS is activated directly by membrane tension, the lipid-protein interaction must be involved critically in its function. In this study, we attempted to modify the distribution of the lipid-derived force on the channel boundary by asparagine substitutions of the hydrophobic residues. Since the residues that are not fully exposed to lipid are also subjected to mutagenesis, it is also possible that some substitutions affected intramolecular interactions. Interestingly, introduction of asparagine into the most hydrophobic environment, i.e., the middle of the transmembrane helices, where protein interacts with lipid tails, did not obstruct the mechanosensitivity (Fig. 3, C and D). This finding indicates that the effectiveness of the mutations is not directly related to the absolute hydrophobicity and the strength of the hydrophobic interaction. Instead, mutations that rise the gating threshold and decrease the survival rate on hypoosmotic shock (“tight” mutations) were present only at the ends of the transmembrane helices (Fig. 3, C and D). The position of the transmembrane domain suggested by the crystal structure (13) has been supported by activities of alkaline phosphatase in fusion with MscS (30) and also by simulating the MscS position in lipid bilayer with molecular dynamics (29). Note, however, that the predicted transmembrane domain in the crystal structure may be distorted due to nonnative crystallization conditions and disordered state of the N-terminus. Together with these data this finding indicates that the residues for “tight” mutation are located near the polar-apolar boundary. These results highlight the residues close to the surface of the lipid bilayer as essential parts for MscS function.
Various types of molecular simulation studies indicate that lipids generate contracting force at polar-apolar boundary of the lipid bilayer, whereas lipid tails generate repulsive pressure at the core of the membrane (19–23). Especially, the lateral pressure is highest at the level of glycerols (23). Thus, membrane proteins are subject to a negative lateral pressure from the lipid just beneath the surface of the membrane. Since an increase in the cross-sectional area occurs during MscS gating (14), MscS should receive tension that expands the closed structure. This pressure is most likely to occur close to the surface of the membrane according to the lateral pressure profile. Thus, this result suggests that the function of MscS is impaired most severely when asparagine substitution is placed close to the sites where the negative tension component acts on MscS to open the channel.
Some residues for the “tight” mutation (Ala-34 and Ala-85) do not face the lipids in the crystal structure (Fig. 3 D). These mutations are likely to affect the interhelical interaction rather than the protein-lipid interaction. Since all transmembrane helices are predicted to move in harmony during the gating of MscS, perturbation of the interhelical interaction is possible to affect the function. We infer that proper intramolecular interactions at the level of water-lipid interface are important for the MscS function. We, however, suspect that these residues are still possible to interact with lipids in the closed or the open state since the crystal structure is suggested to represent neither the closed nor the open state (12,29,31).
Shortening of the hydrophobic region of MscS occurs when asparagine replaces hydrophobic residues at the end of the transmembrane helices. In general, when the hydrophobic region of a membrane protein is shorter than the hydrophobic core of lipid bilayers, hydrophobic matching occurs either through deformation of the lipid bilayer or alteration of the protein conformation (32). These rearrangements take place because exposing hydrophilic residues to hydrophobic lipid tails requires high energy cost. Normally, thinning of the lipid bilayer is more likely to occur than changing the protein structure since the former requires less energy than the latter (33). If MscS also cancels the hydrophobic mismatch through the same mechanisms, we can assume that thinning of the bilayer, or shifting of polar-apolar boundary of the lipids, occurs on asparagine substitution. The introduced asparagine is also likely to attract headgroups or water since molecular dynamic simulation shows that charged and polar residues at the cytoplasmic ends of the TM1 and TM2 helices interact with the headgroups of phosphatidylcholine (29). Similarity in the phenotype of L86N, L86K, and L86D MscS suggests that the strength of this interaction does not differ among asparagine, lysine, and aspartic acid. Because transmembrane helices are likely to behave like a lever arm during gating (13,34), it is possible that the application of the negative pressure at the wrong place reduces the tension sensitivity.
Alternatively, the consequence of the asparagine substitution could be a structural change in MscS. This situation is similar to the one in which an intact channel is embedded in a membrane composed of lipid with longer acyl chains. There are data on the action of mechanosensitive channels under these circumstances: when MscL is incorporated into a thicker membrane, the gating threshold is increased (35); when MscL is incorporated into a thinner membrane the gating threshold is decreased because of tilting of transmembrane helices (36). If we assume that MscS adjusts the height of the hydrophobic surface in the same way, TM1 and TM2 move to a direction more perpendicular to the membrane; however, this is not probable because TM1 and TM2 are thought to be already almost perpendicular to the membrane in the closed state (13,34,37). This explanation that shorter hydrophobic domains straighten up the transmembrane helices is also not consistent with the observation that the double mutants placed at both ends of the transmembrane domain did not have more severe defect than the single mutants (Fig. 4). In addition, this account is not in accord with the result on MscL because severe mutants of MscL were found only at one end of transmembrane helices (18) although the mechanism for tension sensing in MscS may be different from that in MscL.
Still another explanation for the outcome of the asparagine substitution should be considered, at least for the F68N mutant: the deletion of phenylalanine may obscure the location of the transmembrane domain relative to the lipid bilayer. In fact, mutation at Phe-85 or Phe-93 of MscL impairs the function in vitro (38). This rationalization, however, is not applicable to the other “tight” mutation sites since phenylalanine is not located nearby.
The double mutants at the ends of TM1 and TM2 indicate that the effects of mutations are additive only when the two mutations are introduced to the same side of the membrane (Fig. 4 A). The mutations of A51N(TM1)/F68N(TM2) and I37N(TM1)/L86N(TM2) MscSs are located at the cytoplasmic and periplasmic ends, respectively; they impair the MscS function almost entirely. Introduction of two mutations at the same end of TM1 yielded a similar result [A51N(TM1)/L55N(TM1)]. This observation indicates that a similar degree of lipid-protein interaction at both ends of TM1 and TM2 is important for proper MscS function (Fig. 5, A and B). Unbalanced interactions at the two ends result in severe loss of function (Fig. 5, C and D). We suspect that, when two mutations are placed at opposite ends, the effect of the introduction of second mutation is cancelled by restoring the balance of protein-lipid interactions at the two ends. If we assume that the ends of TM1 and TM2 are drawn away from the channel axis through the lipid-protein interaction, the channel opening is brought about by equal displacement of TM1 and TM2 from the axis (Fig. 5, A and B). This suggestion contrasts to the hypothesis that the cytoplasmic ends of TM1 and TM2 are pulled apart from the central axis on gating, whereas the periplasmic ends serve as a pivot (13, 37). However, in support of our idea, a recent molecular dynamic simulation on MscS has shown that the opening of the pore does not correlate with the coordinated tilting of all subunits in the same direction (29). In addition, expansion of the periplasmic pore was observed in that simulation. Taken together with our results, we infer that the ends of TM1 and TM2 are displaced away from the central axis in a similar magnitude (Fig. 5, A and B). It is possible that tilting and pivoting occurs mainly in the circumferential direction but not in the radial direction.
FIGURE 5.
Diagram of the proposed movement of transmembrane helices. Two subunits, each consisting of TM1, TM2, and the membrane embedded part of TM3 (boxes), are shown. Semicircles in the boxes represent the residues for the “tight” mutations in TM1 and TM2. The semicircles are shown as open when a residue within the site is substituted with asparagine. Thin lines at the top and the bottom are the periplasmic and cytoplasmic surface of the membrane, respectively. (A) Closed structure of MscS. (B) Channel opening is brought about by parallel movement of TM1 and TM2. (C, D) When two asparagine substitutions were set to cytoplasmic ends of TM1 and TM2 (C; e.g., A51N/F68N) or to the periplasmic ends (D; e.g., I37N/l86N) tilting of TM1 and TM2 occurs by an unbalanced expansion at both ends. In this case, channel opening does not occur.
V89N and I77N were out of the linear relationship between the survival rate and the gating threshold (Fig. 3 A). Thus, the low survival rate of these two mutants is possibly irrelevant to the decreased tension sensitivity. An MscL mutant with similar phenotype (I41W MscL) was found and its low survival rate was attributed to a change in the dwell time in the open or subopen state (39). V89N and I77N MscS, however, did not show obvious change in the gating kinetics (data not shown). Since neither residue is exposed to the lipid in the crystal structure (Fig. 1), it is possible that the change in the internal structure and the loss of function occurred in a large fraction of the mutant channel population. Patch clamp experiments may have detected a small number of functional channels that were present in the membrane. Alternatively, the threshold of these two mutants may increase only in vivo where the membrane potential, the ionic condition, and the lipid metabolism are different from the patch-clamp experiment.
Because we used spheroplasts rather than the liposomes made of synthetic phosphatidylcholine, further fine modeling or simulation of the lipid-protein interaction may be difficult. However, we believe that characterization of the native membrane, abundant in phosphatidylethanolamine, is as important as that in liposomal membrane usually made of phosphatidylcholine, which is not present in E. coli membrane. How the position of the residues sensitive to asparagine substitution changes with the length of the acyl chain remains to be elucidated.
In this study, we have shown that the asparagine substitution of the residues near both ends of the transmembrane helices impaired the function of MscS. MscS shares the same characteristics with MscL in that the interaction with lipid near the polar-apolar boundary is essential to the function. However, both cytoplasmic and periplasmic ends of transmembrane helices were involved in the tension sensitivity of MscS, whereas asparagine substitution at the cytoplasmic ends did not affect the MscL function (18). Stretching the corresponding residues computationally by molecular simulation or actually by nano-manipulation may provide more information on how increasing the negative pressure at these sites causes a conformational change in mechanosensitive channels.
Acknowledgments
We thank Ian R. Booth for the gift of MJF455, Paul Blount for the gift of PB111 and the plasmid harboring MscS, and R. Weisburd for critically reading the manuscript.
This work was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to M.S. and K.Y.), a grant from the Japan Space Forum (to M.S.), and a contact research from the Japan Science and Technology Agency (to K.Y.).
References
- 1.Hamill, O. P., and B. Martinac. 2001. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81:685–740. [DOI] [PubMed] [Google Scholar]
- 2.Anishkin, A., and C. Kung. 2005. Microbial mechanosensation. Curr. Opin. Neurobiol. 15:397–405. [DOI] [PubMed] [Google Scholar]
- 3.Kung, C. 2005. A possible unifying principle for mechanosensation. Nature. 436:647–654. [DOI] [PubMed] [Google Scholar]
- 4.Blount, P., M. J. Schroeder, and C. Kung. 1997. Mutations in a bacterial mechanosensitive channel change the cellular response to osmotic stress. J. Biol. Chem. 272:32150–32157. [DOI] [PubMed] [Google Scholar]
- 5.Ajouz, B., C. Berrier, A. Garrigues, M. Besnard, and A. Ghazi. 1998. Release of thioredoxin via the mechanosensitive channel MscL during osmotic downshock of Escherichia coli cells. J. Biol. Chem. 273:26670–26674. [DOI] [PubMed] [Google Scholar]
- 6.Levina, N., S. Tötemeyer, N. R. Stokes, P. Louis, M. A. Jones, and I. R. Booth. 1999. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 18:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berrier, C., A. Garrigues, G. Richarme, and A. Ghazi. 2000. Elongation factor Tu and DnaK are transferred from the cytoplasm to the periplasm of Escherichia coli during osmotic downshock presumably via the mechanosensitive channel MscL. J. Bacteriol. 182:248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batiza, A. F., M. M.-C. Kuo, K. Yoshimura, and C. Kung. 2002. Gating the bacterial mechanosensitive channel MscL in vivo. Proc. Natl. Acad. Sci. USA. 99:5643–5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada, K., P. C. Moe, and P. Blount. 2002. Functional design of bacterial mechanosensitive channels. J. Biol. Chem. 277:27682–27688. [DOI] [PubMed] [Google Scholar]
- 10.Martinac, B., M. Buechner, A. H. Delcour, J. Adler, and C. Kung. 1987. Pressure-sensitive ion channel in Escherichia coli. Proc. Natl. Acad. Sci. USA. 84:2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sukharev, S. I., B. Martinac, V. Y. Arshavsky, and C. Kung. 1993. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys. J. 65:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akitake, B., A. Anishkin, and S. Sukharev. 2005. The “dashpot” mechanism of stretch-dependent gating in MscS. J. Gen. Physiol. 125:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bass, R. B., P. Strop, M. Barclay, and D. C. Rees. 2002. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 298:1582–1587. [DOI] [PubMed] [Google Scholar]
- 14.Sukharev, S. 2002. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys. J. 83:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perozo, E., A. Kloda, D. M. Cortes, and B. Martinac. 2002. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 9:696–703. [DOI] [PubMed] [Google Scholar]
- 16.Martinac, B., J. Adler, and C. Kung. 1990. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 348:261–263. [DOI] [PubMed] [Google Scholar]
- 17.Maurer, J. A., and D. A. Dougherty. 2003. Generation and evaluation of a large mutational library from the Escherichia coli mechanosensitive channel of large conductance, MscL. J. Biol. Chem. 278:21076–21082. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura, K., T. Nomura, and M. Sokabe. 2004. Loss-of-function mutations at the rim of the funnel of mechanosensitive channel MscL. Biophys. J. 86:2113–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh, D. 1996. Lateral pressure in membranes. Biochim. Biophys. Acta. 1286:183–223. [DOI] [PubMed] [Google Scholar]
- 20.Cantor, R. S. 1997. Lateral pressures in cell membranes: a mechanism for modulation of protein function. J. Phys. Chem. B. 101:1723–1725. [Google Scholar]
- 21.Lindahl, E., and O. Edholm. 2000. Mesoscopic undulations and thickness fluctuations in lipid bilayers from molecular dynamics simulations. Biophys. J. 79:426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gullingsrud, J., and K. Schulten. 2003. Gating of MscL studied by steered molecular dynamics. Biophys. J. 85:2087–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gullingsrud, J., and K. Schulten. 2004. Lipid bilayer pressure profiles and mechanosensitive channel gating. Biophys. J. 86:3496–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monks, S. A., D. J. Needleman, and C. Miller. 1999. Helical structure and packing orientation of the S2 segment in the shaker K+ channel. J. Gen. Physiol. 113:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu, C. X., P. F. Juranka, and C. E. Morris. 2001. Stretch-activation and stretch-inactivation of shaker-IR, a voltage-gated K+ channel. Biophys. J. 80:2678–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ou, X., P. Blount, R. J. Hoffman, and C. Kung. 1998. One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proc. Natl. Acad. Sci. USA. 95:11471–11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshimura, K., A. Batiza, M. Schroeder, P. Blount, and C. Kung. 1999. Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophys. J. 77:1960–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blount, P., S. I. Sukharev, P. C. Moe, B. Martinac, and C. Kung. 1999. Mechanosensitive channels of bacteria. Methods Enzymol. 294:458–482. [DOI] [PubMed] [Google Scholar]
- 29.Sotomayor, M., and K. Schulten. 2004. Molecular dynamics study of gating in the mechanosensitive channel of small conductance MscS. Biophys. J. 87:3050–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, S., W. Bartlett, S. Chandrasekaran, S. Simpson, M. Edwards, and I. R. Booth. 2003. Domain organization of the MscS mechanosensitive channel of Escherichia coli. EMBO J. 22:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anishkin, A., and S. Sukharev. 2004. Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys. J. 86:2883–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Killian, J. A. 1998. Hydrophobic mismatch between proteins and lipids in membranes. Biochim. Biophys. Acta. 1376:401–416. [DOI] [PubMed] [Google Scholar]
- 33.Harroun, T. A., W. T. Heller, T. M. Weiss, L. Yang, and H. W. Huang. 1999. Experimental evidence for hydrophobic matching and membrane-mediated interactions in lipid bilayers containing gramicidin. Biophys. J. 76:937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards, M. D., I. R. Booth, and S. Miller. 2004. Gating the bacterial mechanosensitive channels: MscS a new paradigm? Curr. Opin. Microbiol. 7:163–167. [DOI] [PubMed] [Google Scholar]
- 35.Perozo, E., A. Kloda, D. M. Cortes, and B. Martinac. 2002. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 9:696–703. [DOI] [PubMed] [Google Scholar]
- 36.Perozo, E., D. M. Cortes, P. Sompornplsut, A. Kloda, and B. Martinac. 2002. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 418:942–948. [DOI] [PubMed] [Google Scholar]
- 37.Bezanilla, F., and E. Perozo. 2002. Force and voltage sensors in one structure. Science. 298:1562–1563. [DOI] [PubMed] [Google Scholar]
- 38.Levin, G., and P. Blount. 2004. Cysteine scanning of MscL transmembrane domains reveals residues critical for mechanosensitive channel gating. Biophys. J. 86:2862–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiang, C.-S., L. Shirinian, and S. Sukharev. 2005. Capping transmembrane helices of MscL with aromatic residues changes channel response to membrane stretch. Biochemistry. 44:12589–12597. [DOI] [PubMed] [Google Scholar]