Abstract
To understand better the modulation of ryanodine receptors (RyRs) during oxidative stress, the effect of 4,4′-dithiodipyridine (DTDP), a cell-permeant and thiol-reactive oxidant, on global Ca2+ signal and spontaneous Ca2+ sparks of rat ventricular myocytes was investigated. It was shown that a brief Ca2+ transient was elicited by DTDP, when its concentration was raised to 100 μM DTDP. In addition a dose-dependent increase of cytoplasmic free Zn2+ concentration was induced by DTDP. An increase of the frequency of spontaneous Ca2+ sparks appeared at 3 μM DTDP, whereas higher concentration of DTDP caused a biphasic change of the frequency in both intact and permeabilized myocytes. Consistent with the biphasic effect, caffeine-induced Ca2+ transients were similarly affected. Because DTDP did not reduce the free Ca2+ concentration in the sarcoplasmic reticulum lumen, it is likely that the effects of DTDP on the frequency and caffeine-induced Ca2+ transients are due mainly to sulfhydryl oxidation-induced activation and subsequent inactivation of RyRs. Unlike the frequency, the spatio-temporal properties of Ca2+ sparks were not influenced by DTDP. The finding that DTDP does not affect the duration of Ca2+ sparks is inconsistent with that the DTDP-induced increase of the open time of reconstituted RyR channels. The mechanism underlying this discrepancy, especially the possible role of the interaction between arrayed RyRs in myocytes, is discussed. This study suggests that, even if oxidative stress is mild enough not to cause intracellular Ca2+ accumulation, it may affect signaling pathways through directly modulating the RyR or its complex and in turn changing the frequency of spontaneous Ca2+ sparks. Thus, the functional importance of moderate oxidative stress should not be overlooked.
INTRODUCTION
Normal calcium signaling is essential for regular function of cardiomyocytes. In response to oxidative stress, the intracellular Ca2+ accumulation induced by increased formation of reactive oxygen species (ROS) would cause injury and dysfunction of cardiac myocytes (1–3).
In cardiac myocytes the sarcoplasmic reticulum (SR) is a main intracellular Ca2+ store. Ryanodine receptors (RyRs) on the membrane of the SR play a central role in modulating Ca2+ signaling. RyRs contain many cysteine residues in their regulatory domain and putative Ca2+ pore region. These residues are susceptible to modification by oxidants, such as ROS and reactive disulfides. In fact, the effect of ROS on the activity of reconstituted single RyR channel has clearly been shown (4,5). In addition, ROS-induced Ca2+ release from isolated SR vesicles was also observed (3,6). Because the effects of ROS can be prevented or reversed by thiol reducing agents, such as dithiothreitol (DTT), thiol oxidation of RyRs is suggested (4,5). 2,2′- or 4,4′- dithiodipyridine (DTDP) is a cell-permeant and thiol specific reagent, and oxidizes proteins through a thiol-disulfide exchange (7). It has been previously reported that the activation of the cardiac RyR by 2,2′- or 4,4′- DTDP in lipid bilayers, which occurs within minutes, was followed by an irreversible loss of RyR activity (8). All of these results illustrate the important role of sulfhydryl oxidation in regulating RyR gating.
It is well established that RyRs in intact muscle cells are assembled into two-dimensional arrays in the SR membrane (9,10). The interaction between isolated RyRs is modulated by various factors, including monovalent cation and functional states of RyRs (11,12). This interaction and its modulation may play an essential role in the activation and termination of RyRs gating. Recently, by analyzing the quantum nature of Ca2+ sparks, elementary Ca2+ release events mediated by RyRs in the array, Cheng et al. have demonstrated that the gating kinetics of multiple RyRs is reshaped by the array-based interaction between these channels (13). Moreover, it is known that the endogenous antioxidant defense system is present in various cells, including cardiomyocytes. In view of this it is likely that the effect of oxidants on in situ arrayed RyRs is different from that seen with reconstituted single RyR channel.
To understand better the modulation of RyRs during oxidative stress, it is necessary to investigate the effect of oxidants on RyRs in situ. Oxidants usually have complicated effects, including peroxidation of membrane phospholipids, oxidation of protein thiol groups, and mutagenesis of DNA (14). Comparatively, the effect of 4,4′-DTDP is more specific because its oxidizing effect is restricted to thiol groups. Therefore, the effect of 4,4′- DTDP on global and local (spontaneous Ca2+ sparks) Ca2+ signal in isolated rat ventricular myocytes was investigated. The frequency and spatio-temporal parameters of spontaneous Ca2+ sparks were measured. In addition, this study was also performed on saponin permeabilized myocytes. The permeabilized cells probably bridge the gap between intact cell and isolated systems.
METHODS
Cell isolation
Rat ventricular myocytes were enzymatically isolated as described previously (15). Briefly, adult Sprague-Dawley rats (220–300 g) were anaesthetized by sodium pentobarbital (100 mg/kg, intraperitoneal injection). The heart was quickly excised and perfused with O2 saturated Ca2+-free Tyrode's solution at 37°C for 5 min. This solution consisted of (in mM): NaCl 135, KCl 5.4, MgCl2 1, NaH2PO4 0.33, HEPES 5, glucose 10, and was titrated to pH 7.4 with NaOH. Then, the perfusion solution was switched to Tyrode's solution containing 1 mg/ml collagenase (type I), 0.1 mg/ml trypsin (type I), and 0.1 mM CaCl2, and the heart was perfused for another 20 min. Afterwards, the ventricle was dissected, minced, and filtered through nylon mesh. Isolated cells were resuspended in standard physiological solution containing (in mM): NaCl 120, KCl 5.4, MgCl2 1.2, CaCl2 1, HEPES 20, glucose 15, pH 7.4 (NaOH) at room temperature (22-24°C) for 1 h before use.
Cell permeabilization
Isolated myocytes were permeabilized with 0.01% (w/v) saponin according to a modified method described previously (16). The permeabilizing solution contained (in mM): potassium aspartate 100, KCl 20, EGTA 0.1, ATP 3, MgCl2 3.81, HEPES 10, and 8% (w/v) dextran (40,000, to prevent osmotic swelling of the cells); pH 7.2 (KOH). After 1 min permeabilization, the myocytes were perfused with saponin-free internal solution composed of (in mM): potassium aspartate 100, KCl 20, ATP 3, MgCl2 3.81, EGTA 0.5, CaCl2 0.1, phosphocreatine 10, creatine phosphokinase 5 U/ml, HEPES 10, fluo-4 potassium salt 0.04, and 8% (w/v) dextran; pH 7.2 (KOH). As calculated by the computer program WinMAXC 2.5 (Stanford University, Stanford, CA), free [Ca2+] and [Mg2+] in this solution were 43 nM and 1 mM, respectively. The total Ca2+ and Mg2+ necessary for obtaining altered free [Ca2+] and [ATP] were also estimated using this program.
Detection of global Ca2+ transients and spontaneous Ca2+sparks with fluo-4
Intact myocytes were loaded with 20 μM fluo-4 AM for 30 min at room temperature. The cells were attached to a glass coverslip that formed the bottom of a chamber. The chamber was placed on the stage of an inverted microscope (Nicon Diaphot 300). Before taking records, the cells were perfused with standard physiological solution for 30 min for deesterification of fluo-4 AM. For permeabilized cells, the dye fluo-4 potassium salt was added directly into the bathing solution. Both global Ca2+ transients and Ca2+ sparks were observed by a laser scanning confocal microscope (MRC 1024, Bio-Rad, CA) equipped with a ×60 oil-immersion objectives (N.A = 1.4). Fluo-4 was excited at a wavelength of 488 nm, and the fluorescence measured at >522 nm.
To monitor the global Ca2+ transients, a number of regions were chosen. The data of fluorescence intensity were collected at 1 Hz. By averaging the pixel intensities within defined regions, the time course of the fluorescence changes in these regions was obtained. Although the fluorescence signals were not calibrated in terms of Ca2+ concentration, the term of Ca2+ transients was still adopted for the dye signals.
To record spontaneous Ca2+ sparks, the fluorescence was recorded in either two-dimensional (X-Y) or line scan (X-T) mode. In the latter case, each scan line had 512 pixels, and the length of the line was 84 μm (512 pixels × 0.164 μm/ pixel). The scan line was oriented along the long axis of the myocyte, and the cell nuclei were avoided. An X-T image was obtained by stacking 512 scan lines. It took ∼1 s (2 ms/line) to acquire a full image.
The data of line scan mode were processed by IDL software (Research Systems, Boulder, CO) and a modified spark detection algorithm (17). Ca2+ sparks were identified as local peak elevations of fluorescent intensity, which were >3 mean ± SD of the surrounding background levels. The parameters measured in this study included amplitude (F/F0), fullwidth at half-maximum intensity (FWHM), full duration at half-maximum intensity (FDHM). The frequency of Ca2+ sparks was represented by the number of Ca2+ sparks in one image obtained by line scan mode, event s−1 84 μm−1. Because the frequency was low, especially after DTDP exposure, the total Ca2+ sparks in successively taken 10 X-T images were measured, and an averaged frequency was estimated.
Detection of intracellular Zn2+ with mag-fura-5
Mag-fura-5, a fluorescent dye sensitive to many divalent cations, was used to measure intracellular free Zn2+ concentration ([Zn2+]i), because it has high affinity for Zn2+ (KdZn = 28 nM) (18,19). Intact myocytes were loaded with 5 μM mag-fura-5 AM for 30 min at room temperature and then washed with standard physiological solution for 30 min for deesterification of mag-fura-5 AM. By using polychrome system and high-speed cooled charge-coupled device (Imago QE, Till Photonics, Gräfelfing, Germany) image system, the ratio of fluorescence 340 nm/380 nm was analyzed online with Tillvision software (Till Photonics).
Detection of Ca2+ in SR lumen with mag-fluo-4
Mag-fluo-4, a Ca2+ indicator with low affinity (Kd = 22 μM), was used for detecting free [Ca2+] in the SR lumen ([Ca2+]SR). For this purpose, isolated myocytes were loaded with 10 μM mag-fluo-4 AM for 2 h, and then incubated in physiological solution for another 2 h at 37°C to allow deesterification and outward leak of this dye from cytoplasm (20,21). Mag-fluo-4 was excited at 488 nm, and the fluorescence measured at >522 nm. A striation pattern of the fluorescence appeared in the dye-loaded resting myocyte, indicating the localization of the dye in the junctional SR. This localization is supported by the finding that the fluorescence decreased when 10 mM caffeine was briefly applied, which caused translocation of Ca2+ from the SR to the cytoplasm.
Statistical analysis
Results are expressed as mean ± SE for the indicated number of the myocytes isolated from at least three animals. Statistical significance was determined by Student's t-test. P<0.05 was considered to be statistically significant.
RESULTS
Effect of DTDP on [Ca2+]i in intact myocytes
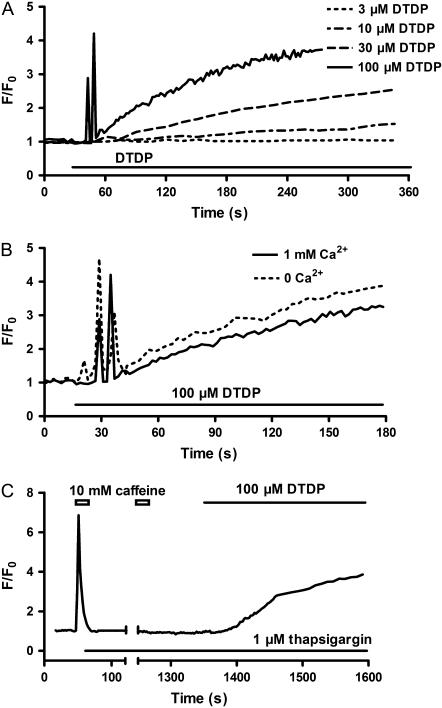
As illustrated in Fig. 1 A, low concentration of DTDP such as 3 μM did not cause any change of the fluorescence within 5 min, whereas a slow rise of fluorescence was seen with 10 μM DTDP. The rate of rise and amplitude of the fluorescence signal increased with increasing DTDP concentration. More interestingly, a complicated change of the fluorescence was consistently observed, when the myocytes were exposed to 100 μM DTDP. A rapid increase of the fluorescence, referred to as phase I, appeared within ∼0.5 min. After the increased levels during phase I returned to the baseline, the fluorescence started to increase again, which is referred to as phase II. The phase I but not the phase II was blocked by 10 μM ryanodine (data not shown).
FIGURE 1.
DTDP-induced change of fluo-4 fluorescence in intact myocytes. (A) The dose dependence. (B) The external Ca2+ dependence. (C) The influence of SR Ca2+ depletion induced by 10 mM caffeine combined with 1 μM thapsigargin in the absence of external Ca2+.
In the absence of external Ca2+, 100 μM DTDP caused a similar change of the fluorescence (Fig. 1 B), indicating that these effects of DTDP are independent of Ca2+ entry. In addition, in the absence of external Ca2+ the phase I but not the phase II was abolished, if Ca2+ in the SR was depleted by caffeine combined with thapsigargin, an inhibitor of Ca2+-ATPase (Fig. 1 C). This illustrates that the increase of Ca2+ during phase I is due mainly to release from the SR, although other sources, e.g., mitochondria etc., cannot be ruled out.
Effect of DTDP on [Zn2+]i in intact myocytes
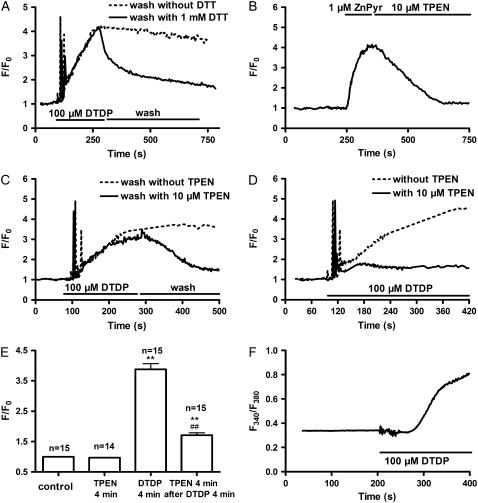
The results described above clearly show that neither extracellular Ca2+ entry nor Ca2+ release from the SR contribute to phase II. In addition, washing out DTDP could not reverse the fluorescence changes that occurred during phase II. However, washing with 1 mM DTT significantly reduced the changes during phase II (Fig. 2 A), indicating that oxidation of some residues might be involved. As a result of the oxidation, an increase of cytoplasmic free Zn2+ concentration ([Zn2+]i) might be induced. In fact, it has previously been shown that oxidants, such as hypochlorous acid and selenite, could increase [Zn2+]i in rabbit ventricular myocytes (22).
FIGURE 2.
DTDP-induced increase of [Zn2+]i in intact myocytes. Myocytes were loaded with fluo-4 (A–E) or mag-fura-5 (F). (A) The myocytes were exposed to 100 μM DTDP followed by washout in the absence or presence of 1 mM DTT, respectively. (B) The antagonizing effect of 10 μM TPEN on the signal induced by 1 μM ZnPyr, a specific Zn2+ ionophore. (C) The myocytes were exposed to 100 μM DTDP followed by washout in the absence or presence of 10 μM TPEN, respectively. (D) The influence of pretreatment with 10 μM TPEN on the effect of DTDP. (E) Summarized results of the effects of 100 μM DTDP and 10 μM TPEN on the fluo-4 fluorescence; n is the number of the examined myocytes. Versus control, **p < 0.01; versus DTDP alone, ##p < 0.01. (F) The effect of DTDP on mag-fura-5 fluorescence.
To explore if the DTDP-induced phase II really is due to an increase of [Zn2+]i, the effect of DTDP in the presence of TPEN, a cell-permeable Zn2+ chelator was studied. In control experiments, exposure of the myocytes to 1 μM Zn2+-pyrithione (ZnPyr), a specific Zn2+ ionophore, caused an increase of the fluo-4 fluorescence (Fig. 2 B). This signal was reversed by 10 μM TPEN. Interestingly, 10 μM TPEN could significantly reverse the DTDP-induced phase II changes (Fig. 2 C). If the myocytes were pretreated with 10 μM TPEN, the DTDP-induced phase I was not affected, but the phase II was clearly depressed (Fig. 2 D).
The effects of DTDP and TPEN on the fluo-4 fluorescence during phase II were summarized in Fig. 2 E. As control, the ratio of F/F0 was 1. TPEN (10 μM) did not change this ratio. Perfusing the myocytes with 100 μM DTDP for 4 min significantly increased the fluorescence signal and most (∼75%) of this increase could be reversed by 10 μM TPEN.
Moreover, mag-fura-5, a Zn2+ indicator, was used to further test if DTDP increases [Zn2+]i. It was apparently observed that only phase II occurred when the myocytes were exposed to 100 μM DTDP (Fig. 2 F). Similar results were observed in the other five myocytes.
Taken together, these observations clearly indicate that oxidation-induced increase of [Zn2+]i contributes largely to phase II.
Effect of DTDP on spontaneous Ca2+ sparks and caffeine-induced Ca2+ transients in intact myocytes
Because the DTDP-induced phase I is due mainly to Ca2+ release from the SR, the effects of DTDP on the local (spontaneous Ca2+ sparks) and global (caffeine-induced Ca2+ transients (CaTs)) Ca2+ release of RyRs in intact myocytes were examined in the following experiments.
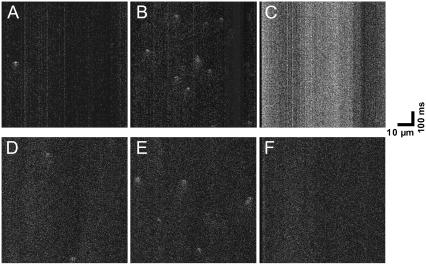
First, the effect of DTDP on the spontaneous Ca2+ sparks in intact myocytes was investigated. Representative confocal images of Ca2+ sparks obtained by line scanning mode are shown in Fig. 3. One spontaneous Ca2+ spark was observed in this myocyte before DTDP exposure (Fig. 3 A). At 0.5 min of 30 μM DTDP perfusion the number of Ca2+ sparks increased to 9 (Fig. 3 B). With prolonged treatment of DTDP, an evident increase of the basal fluorescence appeared and no Ca2+ sparks were detected (Fig. 3 C). According to the results illustrated in Fig. 2, the enhancement of the basal fluorescence is due to an increase of [Zn2+]i. It has been shown that Zn2+ ions have depression effect on the activities of RyRs (23). Moreover, the increase of the basal fluorescence may interfere with the identification of Ca2+ sparks (17). It would be interesting to explore if the DTDP-induced increase of both basal fluorescence and [Zn2+]i is responsible for the vanishing of Ca2+ sparks. Fig. 3, D–F, obtained from another myocyte clearly shows that in the presence of 10 μM TPEN 30 μM DTDP still caused biphasic effect on Ca2+ sparks. In this myocyte 2 spontaneous Ca2+ sparks were found before DTDP exposure (Fig. 3 D). At 0.5 min of 30 μM DTDP perfusion the number of Ca2+ sparks increased to 5 (Fig. 3 E). With prolonged treatment of DTDP the basal fluorescence did not change, but Ca2+ sparks vanished (Fig. 3 F). Therefore, the decrease of Ca2+ sparks induced by DTDP cannot be ascribed to the increase of basal fluorescence and [Zn2+]i. This conclusion is further confirmed by the results of permeabilized myocytes (see the next section).
FIGURE 3.
Representative line scanning images of spontaneous Ca2+ sparks in the intact myocyte. (A–C) The images of one myocyte in the absence of 10 μM TPEN. (D–F) The images of another myocyte in the presence of 10 μM TPEN. (A and D) control; (B and E) 0.5 min after 30 μM DTDP; (C and F) 4 min after 30 μM DTDP.
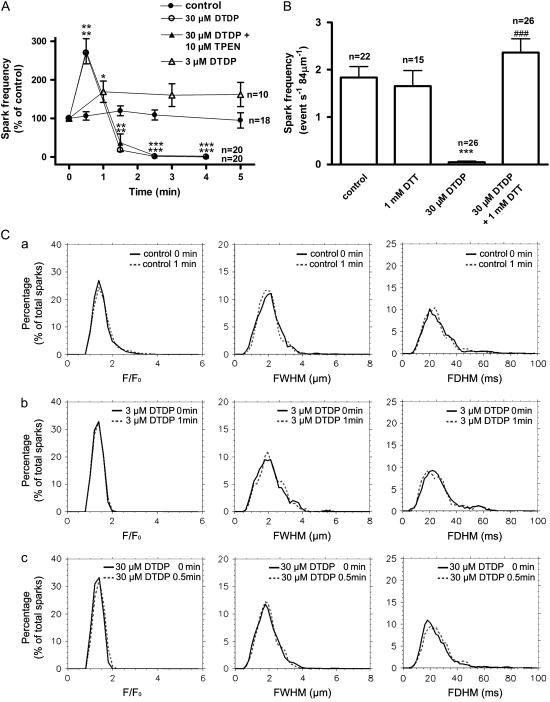
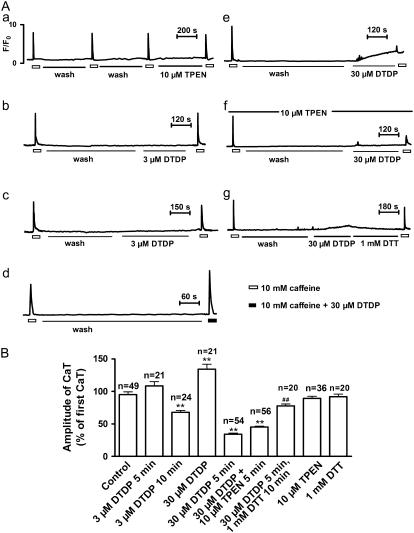
The statistical data are represented in Fig. 4. Fig. 4 A shows that, the frequency of spontaneous Ca2+ sparks in control myocytes was 1.78 ± 0.23 event s−1 84 μm−1 (n = 22), and it was relatively stable within 5 min. Exposure to 3 μM DTDP slightly but significantly increased the frequency, and this increase was sustained within at least 5 min. When DTDP was raised to 30 μM, a biphasic change of the frequency occurred. At 0.5 min, the frequency increased to 270 ± 35% of the control. Then, the frequency gradually decreased. After 4 min, almost no spontaneous Ca2+ sparks could be detected (Fig. 4 A). Fig. 4 A also shows that the DTDP-induced biphasic change of the frequency was not affected by 10 μM TPEN. Although the frequency was affected, all of the spatio-temporal parameters of the spontaneous Ca2+ sparks, including F/F0, FWHM and FDHM, were not changed by 3 μM or 30 μM DTDP (Fig. 4 C). Unlike that in single reconstructed RyRs where DTDP induced an increase of the mean opening time (8), no alteration of temporal parameters, especially FDHM were seen in these experiments.
FIGURE 4.
Effect of DTDP on spontaneous Ca2+ sparks in intact myocyte. (A) The dose dependence and time course of the effect of DTDP on the frequency of spontaneous Ca2+ sparks. (B) The reversal effect of DTT. The columns from the left to the right represent the control, 1 mM DTT for >10 min, 30 μM DTDP for 4 min, and the reversal effect of 1 mM DTT, respectively. The results of the last column were obtained from the myocytes, which were exposed to 30 μM DTDP for 5 min and then to 1 mM DTT for >10 min. (C) The distribution curves of F/F0, FMHM, and FDHM of Ca2+ sparks under various conditions. Each curve was obtained from at least 183 sparks; n is the number of the examined myocytes. Versus control, *p < 0.05, **p < 0.01, ***p < 0.001; versus DTDP alone, ###p < 0.001.
The depression of spontaneous Ca2+ sparks induced by 30 μM DTDP for 5 min could not be restored by washing out DTDP for 10 min (data not shown). To see whether the thiol oxidation of RyRs is involved in this depression, the effect of DTT was examined. One mM DTT alone had no obvious effect on the frequency, indicating that most of the RyRs in resting myocytes probably exist in a reduced form. However, spontaneous Ca2+ sparks depressed by DTDP could be fully recovered by 1 mM DTT (Fig. 4 B).
Second, the effect of DTDP on the CaTs was investigated. In intact myocytes CaTs could be evoked by repetitive exposures to 10 mM caffeine at an interval of 10 min without evident decline in their amplitude, as shown in Fig. 5 A (a). This figure also indicates that the amplitude of CaTs was not affected by 10 μM TPEN. With 3 μM DTDP for 5 min, a slight increase of CaTs was observed (Fig. 5 A (b) and B). Prolonging 3 μM DTDP exposure to 10 min reversed the increase to an evident decrease, 68 ± 3% of the control (Fig. 5 A (c) and B). The biphasic effect of DTDP on CaTs was more clearly seen with 30 μM DTDP. In comparison with the control, a significantly increased CaT was evoked by caffeine combined with 30 μM DTDP (Fig. 5 A (d) and B). However, if the myocytes had been treated with 30 μM DTDP for 5 min before caffeine exposure, the CaT was greatly decreased to 34 ± 2% of the control (Fig. 5 A (e) and B). The DTDP-induced decrease of CaTs could not be prevented by TPEN (Fig. 5 A (f)), indicating that the increase of [Zn2+]i cannot account for the DTDP-induced depression of CaTs. However, as shown in Fig. 5 A (g), 1 mM DTT could antagonize the DTDP-induced depression of CaTs. The summarized results of the effect of DTDP on CaTs are represented in Fig. 5 B.
FIGURE 5.
Effects of DTDP on caffeine-induced Ca2+ transients (CaTs) in intact myocytes. (A) Representative records of CaTs were taken from different myocytes. (a) No obvious decline in the amplitude of CaTs occurred during repetitive exposures to 10 mM caffeine at an interval of 10 min. Besides, CaT was not altered by 10 μM TPEN. (b) An insignificant increase of the CaT at 5 min after 3 μM DTDP treatment. (c) A significant decrease of the CaT at 10 min after 3 μM DTDP treatment. (d) A significant increase of the CaT evoked by 10 mM caffeine combined with 30 μM DTDP. (e) A significant decrease of the CaT at 5 min after 30 μM DTDP treatment. (f) A significant decrease of the CaT at 5 min after 30 μM DTDP treatment in the presence of 10 μM TPEN. (g) Antagonization of the DTDP-induced decrease of the CaT by 1 mM DTT. (B) Summarized results of the effect of DTDP on CaTs; n is the number of the examined myocytes. Versus control, **p < 0.01; versus DTDP alone, ##p < 0.01.
The biphasic effects of DTDP on the spontaneous Ca2+ sparks and global CaTs suggest that thiol oxidation might biphasicly modulate Ca2+ release of RyRs in situ.
Effect of DTDP on spontaneous Ca2+ sparks in permeabilized myocytes
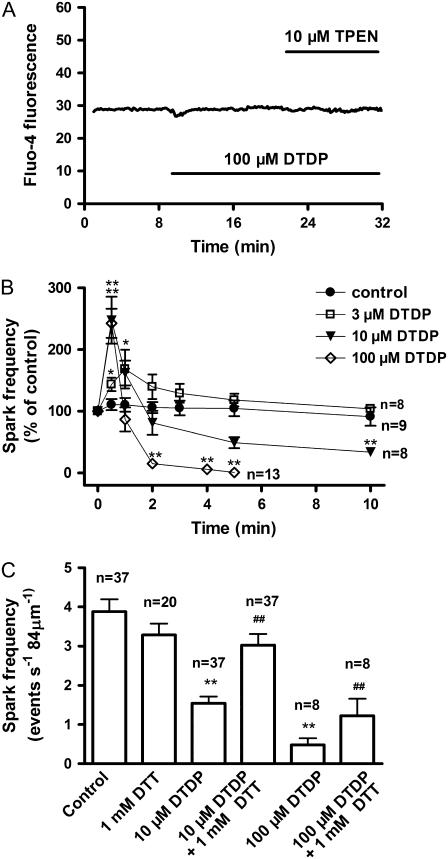
DTDP may change the activity of RyRs through altering [Ca2+]i in intact myocytes. To examine the probable role of DTDP-altered [Ca2+]i in the effects of DTDP, the following experiments were performed on saponin permeabilized myocytes. In these experiments 0.5 mM EGTA was used to buffer [Ca2+]i usually to 43 nM. As shown in Fig. 6 A, under this condition 100 μM DTDP did not change fluo-4 fluorescence in permeabilized myocyte in the presence or absence of 10 μM TPEN, demonstrating proper buffering of [Ca2+]i and [Zn2+]i.
FIGURE 6.
Effect of DTDP on the frequency of spontaneous Ca2+ sparks in permeabilized myocytes. The myocytes were permeabilized with saponin, and 0.5 mM EGTA was present. (A) No evident change of fluo-4 fluorescence was induced by 100 μM DTDP. (B) The dose dependence and time course of the effect of DTDP on the frequency of spontaneous Ca2+ sparks. (C) The reversal effect of DTT. The myocytes were treated with 10 μM DTDP for 10 min or with 100 μM DTDP for 5 min, and then 1 mM DTT was added. Ten minutes later, the reversal effect of DTT was examined; n is the number of the examined myocytes. Versus control, *p < 0.05, **p < 0.01; versus DTDP alone, ##p < 0.01.
The control frequency of spontaneous Ca2+ sparks in permeabilized myocytes was 3.56 ± 0.22 event s−1 84 μm−1 (n = 37). It did not change within 10 min, showing that the activity of RyRs is well maintained after permeabilization (Fig. 6 B). It was observed that 100 μM DTDP caused a biphasic change of the frequency. At 0.5 min, the frequency was raised to 243 ± 24% of the control, and then decreased gradually. Five minutes later, spontaneous Ca2+ sparks were rarely detectable (Fig. 6 B). This time course was basically similar to that seen in intact cells. Biphasic change of the frequency also appeared after 10 μM DTDP exposure, but 10 μM DTDP could not completely abolish Ca2+ sparks even 10 min later (Fig. 6 B). With 3 μM DTDP, only a transient increase of the frequency was observed.
Similar to intact cells, all of the spatio-temporal parameters of spontaneous Ca2+ sparks in permeabilized myocytes were not affected by DTDP (data not shown). One mM DTT alone had no evident effect on the frequency, but DTT could reverse the depression effects of DTDP (Fig. 6 C). The antagonizing effect of DTT further suggests the involvement of the thiol oxidation of RyRs.
Ca2+ accumulation and ATP depletion have been shown in the cells subjected to oxidative stress (1–3,24,25). Both Ca2+ and ATP are known to modulate RyRs. Thus, the effect of DTDP was investigated at different Ca2+ or ATP concentrations. It was found that the biphasic effect of 100 μM DTDP on the frequency was not altered by changing [Ca2+] (from 43 to 115 nM) and [ATP] (from 0.3 to 3 mM) (data not shown). It should be emphasized that, compared with that at 43 nM [Ca2+], an increase of Ca2+ sparks frequency could still be observed at 115 nM [Ca2+], although the basal fluorescence at 115 nM [Ca2+] was increased to an extent similar to that with 30 μM DTDP (Fig. 3 C).
Effect of DTDP on Ca2+ content in the SR lumen
It is well established that the gating of RyRs is modulated by Ca2+ content in the SR lumen (26,27). To observe if the effect of DTDP on the gating of RyRs is mediated by altering Ca2+ content in the SR lumen, mag-fluo-4 was used to directly measure the [Ca2+]SR. Because the Ca2+ affinity of mag-fluo-4 (Kd = 22 μM) is evidently lower than the [Ca2+]SR (∼1 mM), this dye may not be proper for measuring [Ca2+]SR. However, many previous studies have used this dye to successively estimate [Ca2+]SR or free [Ca2+] in the endoplasmic reticulum under various conditions (20,28–30).
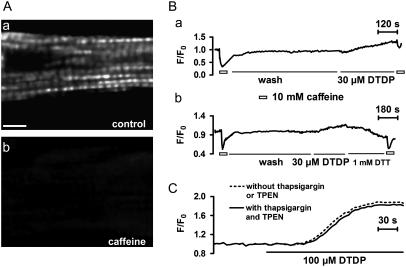
The localization of mag-fluo-4 is illustrated in Fig. 7 A. In the resting myocytes the fluorescence of this dye showed a striation pattern, and this pattern disappeared following caffeine exposure, indicating that this dye was localized in the SR lumen.
FIGURE 7.
Effect of DTDP on the [Ca2+]SR. (A) Confocal X-Y scanning images of a mag-fluo-4 loaded intact myocyte. The fluorescence in resting myocyte showed a striation pattern (a), and this pattern disappeared after 10 mM caffeine application (b). The scale bar, 8 μm. (B) Representative records of mag-fluo-4 signals in intact myocyte. DTDP significantly decreased caffeine-induced reduction of mag-fluo-4 fluorescence (a), and this decrease could be prevented by 1 mM DTT (b). In addition, DTDP caused increase of mag-fluo-4 fluorescence. (C) DTDP-induced increase of mag-fluo-4 fluorescence in permeabilized myocytes.
It was found that caffeine caused an evident reduction of the fluorescence, illustrating a caffeine-induced decrease of the [Ca2+]SR. In the presence of 30 μM DTDP the caffeine-induced response became smaller (Fig. 7 B (a)). Fig. 7 B (b) shows that the DTDP-induced decrease of the caffeine response could be antagonized by 1 mM DTT. More interestingly, it was consistently observed that DTDP increased but not decreased the fluorescence, and this increase could be reversed by DTT (Fig. 7 B (b)). It is clearly indicated that the [Ca2+]SR is not reduced by DTDP. So, the depression and final disappearance of spontaneous Ca2+ sparks caused by DTDP is likely to be due to sulfhydryl oxidation-induced inactivation of RyRs.
To clarify if DTDP-induced rise of the fluorescence in intact myocytes really represents an increase of the [Ca2+]SR, we performed the experiments on permeabilized myocytes using mag-fluo-4. It was seen that DTDP evidently increased mag-fluo-4 fluorescence in permeabilized myocytes (Fig. 7 C). This increase was not affected by the presence of thapsigargin and TPEN, indicating that the change of mag-fluo-4 fluorescence does not result from Ca2+ uptake via Ca2+-ATPase or Zn2+ increase in the SR lumen and DTDP may induce an increase of [Ca2+]SR. Because mag-fluo-4 is not an ideal dye for this purpose, it is desirable to use the dye with lower Ca2+ affinity to repeat these observations in further study.
In addition, it was noted that, DTDP-induced increase of the frequency of Ca2+ sparks appeared evidently earlier than DTDP-induced increase of mag-fluo-4 fluorescence. Thus, the increase of [Ca2+]SR is not the main factor for the activation of RyRs, although it may play some roles.
DISCUSSION
Modulation of RyRs by sulfhydryl oxidation in cardiac myocytes
Consistent with previous results obtained on reconstituted single RyR channels (8), the results of this study show that the activity of RyRs in situ is also affected by DTDP in a dose-dependent manner. An increase of the frequency of spontaneous Ca2+ sparks was seen at 3 μM DTDP, whereas higher concentrations of DTDP caused a biphasic change of the frequency and finally disappearance of spontaneous Ca2+ sparks in both intact and permeabilized myocytes, in which cytoplasmic Ca2+ was buffered with EGTA. Moreover the amplitude of CaTs was similarly altered by DTDP.
In addition, this study showed that [Ca2+]SR was not decreased, but increased by DTDP and this increase was independent of Ca2+ uptake via Ca2+-ATPase in the SR membrane. The exact origin of this increase remains unclear. As one possibility, we suppose that it results from DTDP oxidation-induced dissociation of Ca2+ ions from Ca2+-binding proteins in the SR lumen, e.g., calsequestrin. Calsequestrin is known as the most abundant Ca2+-binding protein in the SR. Its thioredoxin structure suggests that calsequestrin may be sensitive to redox (31,32). Actually, it has been reported that oxidation of calbindin D28, a Ca2+-binding protein, deceases its Ca2+ binding affinity (33). Because DTDP-induced increase of the free [Ca2+] in the SR lumen was later than DTDP-induced increase of Ca2+ sparks frequency, it is unlikely to be the main factor for the activation of RyRs, although it may play some roles.
Taken together these results indicate that the effects of DTDP on the frequency of spontaneous Ca2+ sparks and the amplitude of CaTs are due mainly to sulfhydryl oxidation-induced activation and subsequent inactivation of RyRs. In fact, it has previously been proposed that, there are at least two classes of sulfhydryls in RyR (8). Oxidation of one class of sulfhydryls causes activation, and inactivation would occur due to further oxidation of the other class.
However, compared with previous studies, an apparent disparity is noted. Single channel studies have evidently shown DTDP-induced increase of mean open time of RyR (8). Accordingly, it should be expected that the temporal parameters of spontaneous Ca2+ sparks would be prolonged by DTDP. In contrast, in this study we found no change of the temporal parameters. This discrepancy needs to be explained.
Recent evidence shows that isolated RyRs interact with each other (11,12,34). This interaction can be modulated by several factors, e.g., the concentration of monovalent cations (12), the functional states of RyRs (11). A possible explanation of the inconsistency between the behavior of isolated RyRs and that of RyRs in intact cells could be that the interaction between arrayed RyRs prevents the change of the number of RyRs forming single spontaneous Ca2+ sparks. Because the average release duration of simulated Ca2+ sparks is inversely related to the number of RyRs of which Ca2+ sparks consist (13), the temporal parameters of Ca2+ sparks would not be altered, if the number of RyRs does not change. This study did not find any change of F/F0 in the presence of DTDP, indicating that the mean number of RyRs forming single spontaneous Ca2+ sparks is not affected by DTDP. At the moment, this assumption is speculative. Direct evidence for the role of the interaction between arrayed RyRs for Ca2+ movements in the living cell obviously is needed.
In addition to the interaction between arrayed RyRs, under in situ condition numerous proteins physically associating with RyR are involved in modulating the gating of RyRs (35). Thus, the possibility that the discrepancy between results obtained from intact cells and those obtained from isolated receptors could be due to the absence of some RyR-associated proteins in the isolated system cannot be excluded.
Regardless of the exact reason for this inconsistency, this study clearly indicates that extrapolating in vitro result to in vivo events is not straightforward.
Functional consequence of DTDP-induced change of the frequency of spontaneous Ca2+ sparks
Ca2+ sparks are fundamental SR Ca2+ release events, representing the nearly synchronous activation of a cluster of RyRs at a single junction. In smooth muscle the roles of spontaneous Ca2+ sparks in positive- and negative-feedback regulation have been proposed (36). Although spontaneous Ca2+ sparks are generally thought to play an important role in modulating Ca2+ homeostasis in cardiac myocytes (37), it is unclear what the functional consequence of the change of the frequency of spontaneous Ca2+ sparks is.
The change in the frequency might alter local Ca2+-mediated signaling pathways. Actually, it has recently been shown that Ca2+ sparks are able to elicit miniature mitochondria matrix Ca2+ signal called Ca2+ marks, which are restricted to single mitochondria and last <500 ms (38). As a result, it would affect the metabolism and ATP production in mitochondria. Therefore, the functional consequence of the change of the frequency of spontaneous Ca2+ sparks should not be overlooked, even if the change of the frequency is not accompanied by apparent intracellular Ca2+ accumulation.
Compared with the functional consequences of low concentrations of DTDP those of high concentrations of DTDP are more obvious because they lead to the failure of the RyRs to release Ca2+. Although it would cause dysfunction of excitation-contraction coupling, this failure of RyRs to release Ca2+ may reduce intracellular Ca2+ accumulation and in turn cellular injury. It probably represents a novel mechanism to protect cardiomyocytes from the injury induced by oxidative stress.
DTDP-induced increase of [Zn2+]i and its role in DTDP-induced modulation of RyR
It is known that oxidants, such as hypochlorous acid and selenite, increased [Zn2+]i in rabbit ventricular myocytes (22). More recently, DTDP-induced increase of [Zn2+]i has been reported in neurons (39,40). In this study we provided clear evidence for DTDP-induced increase of [Zn2+]i in rat ventricular myocytes.
We have shown that ryanodine binding to the SR vesicle of calf cardiac muscle was inhibited by Zn2+ (23). Therefore, we were interested in examining if the sulfhydryl oxidation-induced increase of [Zn2+]i influences the effect of DTDP on Ca2+ signaling. It was observed that the effect of DTDP on the frequency of spontaneous Ca2+ sparks was not altered by TPEN (Fig. 4 A), representing that the depression effect of DTDP on spontaneous Ca2+ sparks cannot be ascribed to the increase of [Zn2+]i. In addition, it was found that 3 μM DTDP did not increase [Zn2+]i (Fig. 1 A), but persistently activated RyRs (Fig. 4 A), indicating that the increase of [Zn2+]i also is not the prerequisite for DTDP-induced activation of RyRs.
Although DTDP-induced increase of [Zn2+]i does not influence the effect of DTDP on spontaneous Ca2+ sparks, some of the physiological and pathological processes may be affected by increased [Zn2+]i (41). As an example, it has been shown that Zn2+ regulates KATP channels from both intracellular and extracellular sides (42). This regulation is thought to protect the cardiomyocytes from the injury induced by ischemia and reperfusion. Thus, it is reasonable to propose that fair increase of [Zn2+]i induced by moderate oxidative stress may provide another novel mechanism to protect cardiac myocytes from ischemia-reperfusion insult.
Acknowledgments
The authors thank Dr. W. Z. Zhu for technical help.
This study was supported by the National Basic Research Program of China (G1999054001).
References
- 1.Chakraborti, T., S. K. Ghosh, J. R. Michael, S. K. Batabyal, and S. Chakraborti. 1998. Targets of oxidative stress in cardiovascular system. Mol. Cell. Biochem. 187:1–10. [DOI] [PubMed] [Google Scholar]
- 2.Dhalla, N. S., R. M. Temsah, and T. Netticadan. 2000. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 18:655–673. [DOI] [PubMed] [Google Scholar]
- 3.Ermak, G., and K. J. Davies. 2002. Calcium and oxidative stress: from cell signaling to cell death. Mol. Immunol. 38:713–721. [DOI] [PubMed] [Google Scholar]
- 4.Favero, T. G., A. C. Zable, and J. J. Abramson. 1995. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 270:25557–25563. [DOI] [PubMed] [Google Scholar]
- 5.Boraso, A., and A. J. Williams. 1994. Modification of the gating of the cardiac sarcoplasmic reticulum Ca(2+)-release channel by H2O2 and dithiothreitol. Am. J. Physiol. 267:H1010–H1016. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami, M., and E. Okabe. 1998. Superoxide anion radical-triggered Ca2+ release from cardiac sarcoplasmic reticulum through ryanodine receptor Ca2+ channel. Mol. Pharmacol. 53:497–503. [DOI] [PubMed] [Google Scholar]
- 7.Zaidi, N. F., C. F. Lagenaur, J. J. Abramson, I. Pessah, and G. Salama. 1989. Reactive disulfides trigger Ca2+ release from sarcoplasmic reticulum via an oxidation reaction. J. Biol. Chem. 264:21725–21736. [PubMed] [Google Scholar]
- 8.Eager, K. R., L. D. Roden, and A. F. Dulhunty. 1997. Actions of sulfhydryl reagents on single ryanodine receptor Ca(2+)-release channels from sheep myocardium. Am. J. Physiol. 272:C1908–C1918. [DOI] [PubMed] [Google Scholar]
- 9.Franzini-Armstrong, C., F. Protasi, and V. Ramesh. 1998. Comparative ultrastructure of Ca2+ release units in skeletal and cardiac muscle. Ann. N. Y. Acad. Sci. 853:20–30. [DOI] [PubMed] [Google Scholar]
- 10.Franzini-Armstrong, C., F. Protasi, and V. Ramesh. 1999. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys. J. 77:1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu, X. F., X. Liang, K. Y. Chen, H. Xie, Y. Xu, P. H. Zhu, and J. Hu. 2005. Modulation of the oligomerization of isolated ryanodine receptors by their functional states. Biophys. J. 89:1692–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu, X. F., K. Y. Chen, R. Xia, Y. H. Xu, J. L. Sun, J. Hu, and P. H. Zhu. 2003. Modulation of the interactions of isolated ryanodine receptors of rabbit skeletal muscle by Na+ and K+. Biochemistry. 42:5515–5521. [DOI] [PubMed] [Google Scholar]
- 13.Wang, S. Q., M. D. Stern, E. Rios, and H. Cheng. 2004. The quantal nature of Ca2+ sparks and in situ operation of the ryanodine receptor array in cardiac cells. Proc. Natl. Acad. Sci. USA. 101:3979–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giordano, F. J. 2005. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Invest. 115:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu, H. F., J. W. Dong, W. Z. Zhu, H. L. Ding, and Z. N. Zhou. 2003. ATP-dependent potassium channels involved in the cardiac protection induced by intermittent hypoxia against ischemia/reperfusion injury. Life Sci. 73:1275–1287. [DOI] [PubMed] [Google Scholar]
- 16.Lukyanenko, V., and S. Gyorke. 1999. Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes. J. Physiol. 521 Pt 3:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng, H., L. S. Song, N. Shirokova, A. Gonzalez, E. G. Lakatta, E. Rios, and M. D. Stern. 1999. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys. J. 76:606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sensi, S. L., L. M. Canzoniero, S. P. Yu, H. S. Ying, J. Y. Koh, G. A. Kerchner, and D. W. Choi. 1997. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J. Neurosci. 17:9554–9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sensi, S. L., H. Z. Yin, S. G. Carriedo, S. S. Rao, and J. H. Weiss. 1999. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc. Natl. Acad. Sci. USA. 96:2414–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shmigol, A. V., D. A. Eisner, and S. Wray. 2001. Simultaneous measurements of changes in sarcoplasmic reticulum and cytosolic. J. Physiol. 531:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shannon, T. R., T. Guo, and D. M. Bers. 2003. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ. Res. 93:40–45. [DOI] [PubMed] [Google Scholar]
- 22.Turan, B., H. Fliss, and M. Desilets. 1997. Oxidants increase intracellular free Zn2+ concentration in rabbit ventricular myocytes. Am. J. Physiol. 272:H2095–H2106. [DOI] [PubMed] [Google Scholar]
- 23.Wang, H., Q. Q. Wei, X. Y. Cheng, K. Y. Chen, and P. H. Zhu. 2001. Inhibition of ryanodine binding to sarcoplasmic reticulum vesicles of cardiac muscle by Zn(2+) ions. Cell. Physiol. Biochem. 11:83–92. [DOI] [PubMed] [Google Scholar]
- 24.Nanavaty, U. B., R. Pawliczak, J. Doniger, M. T. Gladwin, M. J. Cowan, C. Logun, and J. H. Shelhamer. 2002. Oxidant-induced cell death in respiratory epithelial cells is due to DNA damage and loss of ATP. Exp. Lung Res. 28:591–607. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, R., A. Pinson, and A. Samuni. 1998. Both hydroxylamine and nitroxide protect cardiomyocytes from oxidative stress. Free Radic. Biol. Med. 24:66–75. [DOI] [PubMed] [Google Scholar]
- 26.Satoh, H., L. A. Blatter, and D. M. Bers. 1997. Effects of [Ca2+]i, SR Ca2+ load, and rest on Ca2+ spark frequency in ventricular myocytes. Am. J. Physiol. 272:H657–H668. [DOI] [PubMed] [Google Scholar]
- 27.Lukyanenko, V., I. Gyorke, and S. Gyorke. 1996. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflugers Arch. 432:1047–1054. [DOI] [PubMed] [Google Scholar]
- 28.Li, N., J. Y. Sul, and P. G. Haydon. 2003. A calcium-induced calcium influx factor, nitric oxide, modulates the refilling of calcium stores in astrocytes. J. Neurosci. 23:10302–10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park, M. K., K. K. Lee, and D. Y. Uhm. 2002. Slow depletion of endoplasmic reticulum Ca(2+) stores and block of store-operated Ca(2+) channels by 2-aminoethoxydiphenyl borate in mouse pancreatic acinar cells. Naunyn Schmiedebergs Arch. Pharmacol. 365:399–405. [DOI] [PubMed] [Google Scholar]
- 30.Shmygol, A., and S. Wray. 2005. Modulation of agonist-induced Ca2+ release by SR Ca2+ load: direct SR and cytosolic Ca2+ measurements in rat uterine myocytes. Cell Calcium. 37:215–223. [DOI] [PubMed] [Google Scholar]
- 31.Beard, N. A., D. R. Laver, and A. F. Dulhunty. 2004. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog. Biophys. Mol. Biol. 85:33–69. [DOI] [PubMed] [Google Scholar]
- 32.Wang, S., W. R. Trumble, H. Liao, C. R. Wesson, A. K. Dunker, and C. H. Kang. 1998. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat. Struct. Biol. 5:476–483. [DOI] [PubMed] [Google Scholar]
- 33.Cedervall, T., T. Berggard, V. Borek, E. Thulin, S. Linse, and K. S. Akerfeldt. 2005. Redox sensitive cysteine residues in calbindin D28k are structurally and functionally important. Biochemistry. 44:684–693. [DOI] [PubMed] [Google Scholar]
- 34.Yin, C. C., H. Han, R. Wei, and F. A. Lai. 2005. Two-dimensional crystallization of the ryanodine receptor Ca2+ release channel on lipid membranes. J. Struct. Biol. 149:219–224. [DOI] [PubMed] [Google Scholar]
- 35.Bers, D. M. 2004. Macromolecular complexes regulating cardiac ryanodine receptor function. J. Mol. Cell. Cardiol. 37:417–429. [DOI] [PubMed] [Google Scholar]
- 36.Jaggar, J. H., V. A. Porter, W. J. Lederer, and M. T. Nelson. 2000. Calcium sparks in smooth muscle. Am. J. Physiol. Cell Physiol. 278:C235–C256. [DOI] [PubMed] [Google Scholar]
- 37.Bers, D. M. 2001. Excitation-Contraction Coupling and Cardiac Contractile Force. Kluwer Academic Publisher, Netherland.
- 38.Pacher, P., A. P. Thomas, and G. Hajnoczky. 2002. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc. Natl. Acad. Sci. USA. 99:2380–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aizenman, E., A. K. Stout, K. A. Hartnett, K. E. Dineley, B. McLaughlin, and I. J. Reynolds. 2000. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J. Neurochem. 75:1878–1888. [DOI] [PubMed] [Google Scholar]
- 40.Malaiyandi, L. M., K. E. Dineley, and I. J. Reynolds. 2004. Divergent consequences arise from metallothionein overexpression in astrocytes: zinc buffering and oxidant-induced zinc release. Glia. 45:346–353. [DOI] [PubMed] [Google Scholar]
- 41.Vallee, B. L., and K. H. Falchuk. 1993. The biochemical basis of zinc physiology. Physiol. Rev. 73:79–118. [DOI] [PubMed] [Google Scholar]
- 42.Prost, A. L., A. Bloc, N. Hussy, R. Derand, and M. Vivaudou. 2004. Zinc is both an intracellular and extracellular regulator of KATP channel function. J. Physiol. 559:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]