Abstract
Menkes disease, a fatal neurodegenerative disorder resulting in seizures, hypotonia, and failure to thrive, is due to inherited loss-of-function mutations in the gene encoding a copper-transporting ATPase (Atp7a) on the X chromosome. Although affected patients exhibit signs and symptoms of copper deficiency, the mechanisms resulting in neurologic disease remain unknown. We recently discovered that Atp7a is required for the production of an NMDA receptor-dependent releasable copper pool within hippocampal neurons, a finding that suggests a role for copper in activity-dependent modulation of synaptic activity. In support of this hypothesis, we now demonstrate that copper chelation exacerbates NMDA-mediated excitotoxic cell death in primary hippocampal neurons, whereas the addition of copper is specifically protective and results in a significant decrease in cytoplasmic Ca2+ levels after NMDA receptor activation. Consistent with the known neuroprotective effect of NMDA receptor nitrosylation, we show here that this protective effect of copper depends on endogenous nitric oxide production in hippocampal neurons, demonstrating in vivo links among neuroprotection, copper metabolism, and nitrosylation. Atp7a is required for these copper-dependent effects: Hippocampal neurons isolated from newborn Mobr mice reveal a marked sensitivity to endogenous glutamate-mediated NMDA receptor-dependent excitotoxicity in vitro, and mild hypoxic/ischemic insult to these mice in vivo results in significantly increased caspase 3 activation and neuronal injury. Taken together, these data reveal a unique connection between copper homeostasis and NMDA receptor activity that is of broad relevance to the processes of synaptic plasticity and excitotoxic cell death.
Keywords: excitotoxicity, Menkes disease, brain, newborn
Copper is an essential transition metal that plays a critical role in the biochemistry of the nervous system, functioning as an enzymatic cofactor in pathways for cellular respiration, iron oxidation, peptide amidation, neurotransmitter synthesis, and antioxidant defense (1). Consistent with this, prolonged copper deficiency in humans results in a neurologic presentation similar to the ataxic myelopathy observed in animals on copper-poor diets (2, 3). Inherited loss of the multicopper oxidase ceruloplasmin is associated with progressive neurodegeneration of the retina and basal ganglia (1), whereas gain-of-function mutations in the cytosolic copper enzyme superoxide dismutase result in the motor neuron degeneration of amyotrophic lateral sclerosis (4). Several studies have now implicated copper in the pathogenesis of neuronal injury in Alzheimer's disease and prion-mediated encephalopathies (5), and recent biochemical work revealing a critical role for copper in molybdenum cofactor biosynthesis suggests a potential role for copper in the pathogenesis of neurologic deterioration in molybdenum cofactor deficiency (6).
Considerable experimental data also reveal that copper is essential for nervous system development, including studies of copper deficiency during pregnancy in a variety of mammals where the outcomes are often neurologic impairment at birth (7). In children with Menkes disease, an impairment in copper acquisition in utero due to loss-of-function mutations in the gene encoding a copper-transporting ATPase, Atp7a, results in fatal neurodegeneration associated with intractable seizures, severe hypotonia, and profound developmental delay (8). Pathologic analysis of brain tissue from affected patients reveals neurodegeneration in the cerebral cortex, cerebellum, and hippocampus, consistent with the known sites of the expression of Atp7a within the developing central nervous system (9–14).
Despite these experimental and clinical observations, the molecular and cellular basis for copper function within the nervous system is not well understood, and the mechanisms of neurologic dysfunction in patients with Menkes disease remain unknown. Recent studies in the murine olfactory epithelium support a role for Atp7a in axon extension, suggesting mechanisms for the neurodegenerative process in affected patients (11). Previous studies have revealed copper release into the synaptic cleft after neuronal depolarization (15, 16). Consistent with this, we recently demonstrated a critical role for Atp7a in the production of an NMDA receptor-dependent, releasable pool of copper in hippocampal neurons, suggesting a role for copper in activity-dependent modulation of synaptic activity (12). We now show that copper is specifically protective toward NMDA-mediated excitotoxic cell death in primary hippocampal neurons. Furthermore, we demonstrate a direct role for Atp7a in this process by using hippocampal neurons from Mobr mice, a murine model of Menkes disease, and an in vivo model of NMDA-mediated excitotoxic neuronal injury in these mice. Our findings provide a biochemical link between copper homeostasis and the mechanisms of synaptic plasticity and excitotoxic cell death that has broad relevance for neuronal development and direct implications for the pathogenesis and treatment of Menkes disease.
Results
To examine the role of copper in NMDA receptor activation, rat hippocampal neurons were exposed for 5 min to excitotoxic levels of glutamate/glycine and then allowed to recover for 24 hr; they then were stained with green fluorescent membrane-permeant calcein AM. All experiments were done in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), an inhibitor of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptors, thus ensuring that the effects of glutamate/glycine were specific to the NMDA receptor. As can be seen in Fig. 1A, glutamate/glycine resulted in cell death in these neurons; this result was markedly increased by prior treatment with the copper chelator bathocuproine disulfonate (BCS) for 24 hr to reduce intracellular copper content. In contrast, the addition of 200 μM CuCl2 only at the time of treatment completely abrogated cell death after NMDA receptor activation (Fig. 1A). Quantitation of findings from multiple such experiments revealed that control neurons exhibited an average cell viability of 56.5 ± 3.3% after glutamate/glycine treatment (Fig. 1B). Consistent with the immunofluorescent data, depletion of intracellular copper increased susceptibility to NMDA receptor-mediated excitotoxicity (34.5 ± 2.5% viability), whereas acute treatment with copper protected against neuronal death (106 ± 2.7% viability). This effect of copper is most likely due to a modulation of NMDA receptor function or direct downstream signaling rather than to changes in receptor trafficking or localization, because immunofluorescent staining for NR1 (17) and synapsin I did not reveal any change in the synaptic localization of NMDA receptors after BCS or copper treatment (data not shown). In support of this hypothesis, hippocampal neurons treated with BCS that received the excitotoxic insult with the addition of CuCl2 were also significantly rescued from excitotoxic death compared with those treated with BCS alone or under control conditions (91.3 ± 6.7% viability) (Fig. 1B). This effect of copper is immediate, because protection is not conferred in neurons that are only preincubated with 200 μM CuCl2 for 5 min and then washed (57.4 ± 6.1% viability) (Fig. 1B, Cu 5′). These findings were not due to an effect of copper on glutamate or glycine, because incubation of these compounds with copper, followed by copper removal with chelex resin, did not alter their excitotoxic potential (data not shown).
Fig. 1.
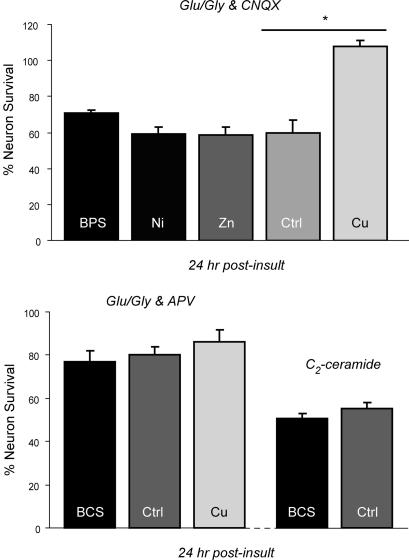
Copper protects hippocampal neurons from NMDA receptor-mediated excitotoxicity. (A) Rat hippocampal neurons were cultured and stimulated (300 μM glutamate/30 μM glycine/10 μM CNQX in ECS) or mock-treated (ECS with 2 mM MgCl2) for 5 min, followed by incubation in conditioned media for 24 hr after insult. Neurons were then incubated with 2 μM calcein AM for 30 min at 37°C, washed in dye-free ECS with 2 mM MgCl2, and assayed for viability via epifluorescent microscopy as described in Materials and Methods. In some experiments, neurons were preexposed to 200 μM BCS for 24 hr (BCS) or treated with 200 μM CuCl2 during the insult (Cu). Mock-treated coverslips were exposed to ECS with 2 mM MgCl2. (B) Neuronal survival was quantitated after an insult of 300 μM glutamate plus 30 μM glycine with an additional 200 μM CuCl2 (Glu/Gly & CNQX) as described above. In some experiments, neurons were preexposed to 200 μM BCS for 24 hr (BCS), treated with 200 μM CuCl2 during the insult (Cu), preexposed to 200 μM BCS for 24 hr and treated with 200 μM CuCl2 during the insult (BCS + Cu), or pretreated with 200 μM CuCl2 for 5 min before the insult (Cu 5′). (C) Rat hippocampal neurons were cultured and stimulated with Glu/Gly plus CNQX alone (upper trace) or with 200 μM CuCl2 (lower trace) for the times indicated, and Ca2+ influx was assayed as described in Materials and Methods. (D) Rat hippocampal neurons were cultured and stimulated with Glu/Gly plus CNQX alone (Control) or with 200 μM CuCl2 (Cu), and the relative Ca2+ influx at 5 min was determined as described. (∗, P < 0.05.)
These experiments suggest that a temporally specific interaction between copper and stimulated neurons underlies the basis of protection against excitotoxicity. To further examine this concept, the proximal event of Ca2+ influx was analyzed in rat hippocampal neurons after NMDA receptor hyperactivation with glutamate/glycine. A rapid and sustained elevation of Ca2+ was observed in rat hippocampal neurons during NMDA receptor hyperactivation, and this was significantly diminished by exposure to 200 μM CuCl2 immediately before and during treatment with glutamate/glycine (Fig. 1 C and D).
This protection by copper against excitotoxicity in isolated hippocampal neurons is specific, because no significant differences in cell death compared with control were observed in either neurons exposed to bathophenanthroline disulfonate (BPS), an iron-specific chelator, before NMDA receptor-dependent excitotoxic insult (70.5 ± 2.0% viability) or neurons after the addition of NiCl2 (58.9 ± 4.2% viability) or ZnCl2 (58.5 ± 4.7% viability) (Fig. 2 Upper). Furthermore, this effect of copper is specific for NMDA receptor-mediated excitotoxicity, because no such copper status-dependent differences in sensitivity to cell death were observed when these same experiments were repeated in hippocampal neurons exposed to glutamate/glycine in the presence of 100 μM 2-amino-5-phosphonovalerate (APV) to examine AMPA/kainate receptor-mediated excitotoxicity (Fig. 2 Lower). Similarly, treatment with BCS had no effect on survival after the exposure of hippocampal neurons to C2 ceramide, a treatment resulting in significant neuronal apoptosis (Fig. 2 Lower).
Fig. 2.
Specificity of copper protection from NMDA receptor-mediated excitotoxicity. (Upper) Rat hippocampal neurons were cultured and stimulated as described in Materials and Methods in the presence of the AMPA receptor antagonist CNQX for 5 min [300 μM glutamate plus 30 μM glycine with an additional 200 μM CuCl2 (Glu/Gly + CNQX)], rinsed in ECS with 2 mM MgCl2, incubated in conditioned media for 24 hr after insult, and then assayed for viability (Ctrl). In some experiments, neurons were preexposed to 200 μM bathophenanthroline disulfonate, an iron-specific chelator, for 24 hr (BPS) or treated during the insult with 200 μM NiSO4 (Ni), 200 μM ZnCl2 (Zn), or 200 μM CuCl2 (Cu). (∗, P < 0.05.) (Lower) Rat hippocampal neurons were cultured and treated as described above in the presence of the NMDA receptor antagonist APV (Glu/Gly & APV) for 5 min (Ctrl). In some experiments, neurons were preexposed to 200 μM BCS for 24 hr (BCS) or treated with 200 μM CuCl2 during the insult (Cu). In other experiments, hippocampal neurons were cultured and treated in identical fashion but also were exposed to 50 μM C2 ceramide for 24 hr (C2-ceramide).
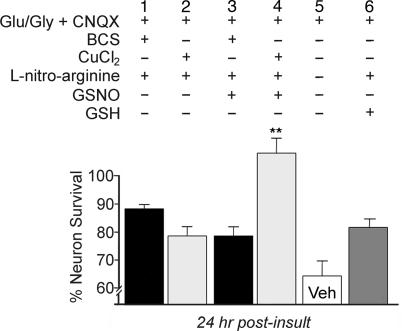
Redox modulation by nitric oxide is a well recognized mechanism of regulation of NMDA receptors involving nitrosylation at specific cysteine residues (18, 19). Given the role of copper as an electron source for thiol chemistry determining nitrosylation specificity (20), we hypothesized a role for nitric oxide in the neuroprotective effect of copper against NMDA receptor-mediated excitotoxicity. Inhibition of nitric oxide production in neurons has a protective effect on survival independent of NMDA activation (21), which was also observed in our studies when control, vehicle-treated neurons were compared with those in which nitric oxide was inhibited and l-glutathione, reduced (GSH) was added (Fig. 3, column 5 vs. 6). Nevertheless, when nitric oxide production was abrogated in hippocampal neurons by using the nitric oxide synthase inhibitor l-nitroarginine, copper did not provide any additional neuroprotection after NMDA receptor-dependent excitotoxic insult (Fig. 3, column 2 vs. 6). In support of a direct role for nitric oxide in these studies, the neuroprotective effect of copper seen in our previous studies (12) was restored after the addition of the exogenous nitric oxide donor S-nitrosoglutathione (GSNO) (Fig. 3, column 4). Interestingly, accentuation of NMDA-mediated cell death after treatment with BCS was not observed in these experiments even when GSNO was added (Fig. 3, column 1 vs. 3), suggesting that, under these experimental conditions, the neuroprotective effect of nitric oxide inhibition is predominant. These experiments support the concept that the observed neuroprotective effect of copper is mediated through the known effect of nitric oxide on NMDA receptor function and suggest that this effect may serve as a rapid modulator of neuronal function.
Fig. 3.
Copper protection depends on nitric oxide production. Rat hippocampal neurons were cultured and stimulated as described in Materials and Methods in the presence of the AMPA receptor antagonist CNQX for 5 min, rinsed in ECS with 2 mM MgCl2, incubated in conditioned media for 24 hr after insult, and then assayed for viability (Veh). In some experiments, neurons were pretreated for 5 min with 1 mM l-nitroarginine to block endogenous nitric oxide production and were additionally treated with l-nitroarginine for the 5-min duration of the excitotoxic stimulus, with or without the addition of 10 μM S-nitrosoglutathione (GSNO), an exogenous nitrosylating agent, or 10 μM l-glutathione, reduced (GSH) as a control. In some experiments neurons were preexposed to 200 μM BCS for 24 hr (BCS) or treated with 200 μM CuCl2 during the insult (Cu) as indicated.
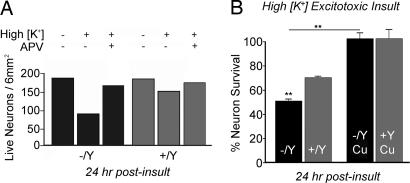
To directly assess the role of Atp7a and endogenous copper release in modulating NMDA-mediated neuronal excitotoxicity, we next examined hippocampal neurons derived from newborn male mice hemizygous for wild-type allele (+/Y) or the brindled mutant functionally null Menkes ATPase, Atp7a (Mobr/Y). Hippocampal neurons from these Mobr/Y mice were shown previously to lack the ability to release copper after NMDA receptor activation (12). In wild-type hippocampal neurons, Atp7a was localized to the late Golgi network as demonstrated by overlap with syntaxin 6, and glutamate/glycine stimulation resulted in trafficking to a cytoplasmic vesicular compartment that extended down the neuronal processes (Fig. 4, +/Y), consistent with our previous observations on rat hippocampal neurons (12). In marked contrast, trafficking of Atp7a was abrogated in Mobr neurons after glutamate receptor activation (Fig. 4, −/Y). Given the essential role of such trafficking in hippocampal copper homeostasis (12), these findings suggest a direct link between impaired copper homeostasis and NMDA receptor-mediated excitotoxic cell death. Consistent with this concept, Mobr neurons exhibited an increased sensitivity to excitotoxic injury after exposure to high K+ for 5 min to induce synaptic release of glutamate (52.1 ± 5.5% cell viability vs. 79.8 ± 6.2% cell viability in wild-type neurons, P < 0.05) (Fig. 5A). These findings were not the result of impaired growth or metabolism in neurons derived from Mobr pups during the experimental period, because, in all cases, neurons cultured from male hemizygous wild-type littermates or Mobr pups did not differ in survival rates before excitotoxic insult (Fig. 5A). Addition of exogenous copper before and during excitotoxic insult abrogated this increased sensitivity to excitotoxic injury in Mobr neurons and resulted in an equivalent, marked increase in survival in both wild-type and Mobr neurons (Fig. 5B). The increased survival of wild-type neurons with copper treatment beyond that already observed under control conditions (Fig. 5B) is consistent with the survival noted above in rat neurons and, given that Atp7a-dependent copper release is the only known route for copper access in vivo, strongly supports a physiologic role for copper under wild-type conditions.
Fig. 4.
NMDA-dependent trafficking of Atp7a is impaired in Mobr neurons. Mouse hippocampal neurons were isolated and cultured as described in Materials and Methods. At 10 days in culture, neurons were treated with 200 μM BCS for 24 hr and then exposed to ECS with 2 mM Mg2+ (Control) or 50 μM glutamate and 5 μM glycine (Glu/Gly), fixed and stained with antisera to Atp7a (Menkes and red in Merged) and syntaxin 6 (green in Merged) as indicated, and analyzed with confocal microscopy. After the analysis, the genotype of the cultures was determined by PCR as either wild type (+/Y) or functionally null (−/Y). Arrowheads indicate Menkes ATPase in processes outside the late Golgi network after glutamate receptor activation in +/Y neurons. (Scale bar: 10 μm.)
Fig. 5.
Mobr hippocampal neurons display increased sensitivity to excitotoxicity. (A) Representative experiment in which neurons were stimulated in high K+ buffer for 5 min, rinsed in ECS with 2 mM MgCl2, and then incubated in conditioned media for 24 hr after insult (High K+). Neurons were then assayed for viability as described in Materials and Methods. In some experiments, neurons were treated as described above in the presence of the NMDA receptor antagonist APV (where indicated). (B) Percent survival across all experiments was calculated from the ratio of live neurons on experimentally treated coverslips to live neurons on mock-treated coverslips from the same dish of plated coverslips. In some experiments, neurons were treated with 200 μM CuCl2 during the insult (Cu), which increased neuronal survival rates in both the +/Y and −/Y genotypes. (∗∗, P < 0.001.)
Taken together, these observations on cultured hippocampal neurons reveal a direct neuroprotective role for copper in NMDA receptor-mediated excitotoxicity that depends on the function of Atp7a. To examine the physiologic relevance of these in vitro observations, excitotoxic neuronal cell death was examined in wild-type and Mobr newborn mice by using an established model of neonatal hypoxia/ischemia (22). In this model of immature stroke, excitotoxic injury leads to cell death by a combination of apoptosis and necrosis. Here we used a mild hypoxic/ischemic injury model consisting of unilateral carotid ligation followed by exposure to 8% oxygen for 37 min. Neuronal injury was reflected by caspase 3 cleavage of Asp-Glu-Val-Asp (DEVD) and was increased in the left (injured) hemisphere of wild-type and Mobr littermates. In response to the mild injury, wild-type mice had a 2-fold increase in caspase 3 activity in the injured hippocampus as compared with the noninjured hippocampus (Fig. 6A, R +/Y) (P < 0.005). Increased caspase 3 activity was also detected as an increase in the p120 cleavage product of spectrin, and, as can be seen in Fig. 6B, this cleavage product was more intense in the injured left hippocampus of Mobr (−/Y) mice than in the injured left hippocampus of wild-type mice. In addition, an increase in the activity of the calcium-activated protease calpain was observed. When lysates were probed for the calpain-dependent cleavage products of spectrin, p145 and p150, an increase in the level of these bands was observed in the hippocampal lysates from the Mobr mice, as compared with wild-type littermates, in the injured hemisphere (Fig. 6B). Calpain activation is independent of caspase 3 activation and most likely reflects necrotic cell death after neonatal hypoxic/ischemic injury (23). Interestingly, this increase in cell death in the Mobr mice was specific to the hemisphere of the brain injured by hypoxia/ischemia, because no change in the apoptotic or necrotic markers was observed in the right (noninjured) hemisphere between genotypes (Fig. 6A). These in vivo experiments demonstrate that neonatal Mobr mice are more severely affected when presented with an excitotoxic insult.
Fig. 6.
Increased caspase 3 and calpain activation in Mobr mice after mild neonatal hypoxic/ischemic insult. (A) Mice undergoing a mild hypoxic/ischemic insult were killed 24 hr after injury, and the activity of caspase 3 was analyzed by examining the cleavage of 7-amino-4-methylcoumarin, N-acetyl-l-aspartyl-l-glutamyl-l-valyl-l-aspartic acid amide (Ac-DEVD-AMC) (see Materials and Methods) in the left injured (L) and right uninjured (R) hippocampal lysates from wild-type (+/Y, n = 14) and Mobr (−/Y, n = 11) littermates. (B) Lysates from injured (L) and uninjured (R) hippocampus of wild-type and Mobr (−/Y) littermates were subjected to SDS/PAGE, transferred to Immobilon P membranes, and stained with primary antibody against spectrin and the caspase 3-dependent p120 cleavage product, as well as the p145 and p150 calpain-dependent cleavage products. The lower band reveals this same membrane reprobed with antibody against tubulin as loading control. KO, knockout.
Discussion
The data in this study reveal that copper modulates the excitotoxic responsiveness of the NMDA receptor in hippocampal neurons, mediating a neuroprotective effect that is rapid and associated with a significant decrease in intracellular calcium elevation (Fig. 1). Loss of function of the copper transporter Atp7a markedly accentuates NMDA receptor excitotoxicity in hippocampal neurons in vitro and in an in vivo model of hypoxic/ischemic injury. Taken together with previous results indicating a critical role for Atp7a in the availability of an NMDA receptor-dependent, releasable pool of copper in hippocampal neurons (12), the current findings reveal a physiologic role for copper in the modulation of NMDA receptor activity. These observations provide a direct link between copper homeostasis and neuronal function that has important implications for our understanding of central nervous system function and disease.
Copper treatment of hippocampal neurons significantly decreased Ca2+ elevation (Fig. 1) without affecting the distribution or localization of NMDA receptors (data not shown), indicating a direct effect on NMDA receptor function or downstream signaling. Consistent with this, exogenously applied copper can inhibit NMDA receptor-mediated current in hippocampal neurons (24–26) and can selectively reduce NMDA-mediated potentials and synaptic plasticity in hippocampal slices (27). Importantly, the concentration of copper here shown to protect hippocampal neurons from excitotoxic death is consistent with physiologic measurements of extracellular copper content within synaptosomes and in the synaptic cleft after brief depolarization (15, 16). Redox activity is a recognized mechanism of NMDA receptor modulation dependent on disulfide bond formation and S-nitrosylation of specific cysteine residues on the extracellular domains of the NR1 and NR2A subunits (19, 28, 29). The release of catalytic amounts of copper would provide a readily available electron acceptor for such redox activity; in direct support of such a mechanism, our data demonstrate an essential role for endogenous nitric oxide production in the neuroprotective effect of copper (Fig. 3). This proposed mechanistic role for copper is also consistent with our finding that FeCl2 also protects hippocampal neurons from NMDA receptor excitotoxicity, albeit significantly less than copper and at supraphysiologic concentrations of iron (data not shown). Furthermore, a similar neuromodulatory role has been proposed for zinc, which is released from excitatory synapses in the hippocampus, where it inhibits NMDA receptors and influences signaling pathways (30). Although extracellularly released zinc has been shown to reenter neurons through activated voltage-gated calcium channels, either Ca2+-permeable AMPA/kainate receptors or NMDA receptors (31), no such reentry of copper was detected in these current studies (data not shown). Thus, although similarities exist in the effects of these two transition elements, the Atp7a-dependent copper pathway demonstrated here is unique and separate from any cellular systems using zinc in these neurons. In support of such differences, no effect of zinc on NMDA receptor function was observed under the conditions used in this work (Fig. 2 Upper).
Menkes disease is a fatal disorder of copper metabolism resulting from loss-of-function mutations in the gene encoding an intracellular copper transporter, Atp7a. Affected patients develop severe, intractable seizures and neuronal degeneration, most prominently in the hippocampus and cerebellum. Consistent with its direct role in this neuronal pathology, Atp7a is abundantly expressed predominantly in neurons in the CA2 region of the hippocampus (13). Despite extensive clinical and experimental studies, the pathogenesis of the neuronal disease remains poorly understood, but it does not result from the absence of function of any of the known cuproenzymes expressed within the developing brain (32). Recent studies in a murine model of Menkes disease suggested a role for Atp7a in axon extension and synaptogenesis based on the expression and localization of Atp7a in early development (11). Our findings now reveal a mechanistic role for copper in neuronal physiology that could account for such a role in synaptogenesis and indicate that, in the absence of Atp7a function, the resulting impairment in copper release markedly accentuates NMDA receptor-mediated excitotoxicity (Figs. 5 and 6). Thus, in Menkes disease, impairment of NMDA receptor-mediated copper efflux in hippocampal neurons could directly impair modulation of receptor function, contributing to both the seizures and the rapid neuronal degeneration that are hallmarks of this disease. Our data provide support for the hypothesis that excitotoxic mechanisms underlie the neurologic disease in affected patients and raise the possibility of therapeutic approaches based on NMDA receptor blockade (33).
These observations on copper and excitotoxic cell injury have implications relevant to additional clinical situations. Indeed, the excessive release of copper due to prolonged or inappropriate NMDA receptor activation would prove harmful to neurons, analogous to the role proposed for zinc during ischemic brain injury (34). In this case, spillover of copper from excitatory synapses could then result in the inhibition of heterosynaptic GABA receptors and the creation of a runaway circuit with excitotoxic consequences, as may occur with zinc (35). Experimental and clinical studies reveal that hypoxic/ischemic encephalopathy in newborn infants results in neuronal excitotoxicity, including seizures and subsequent neurodegeneration triggered by a profound disruption in the function of glutamate synapses (36). In support of these ideas, our data clearly indicate that experimental hypoxic/ischemic injury is markedly exacerbated in a murine model of Menkes disease (Fig. 6). Our data suggest that impairment of NMDA receptor-mediated copper efflux in such newborns could directly alter receptor function, contributing both to seizures and to rapid neuronal degeneration through excitotoxic neuronal death. Because excitotoxicity from hypoxic/ischemic injury results in significant neonatal morbidity and mortality, these findings point toward therapeutic approaches aimed directly at reducing excitotoxic damage in affected patients through the manipulation of copper metabolism. Last, these findings suggest a potential role for Atp7a in modulating memory and learning, processes that are also critically dependent on precise levels of NMDA receptor activity, thus greatly expanding our current concepts regarding copper metabolism within the central nervous system and providing paradigms for experimental analysis.
Materials and Methods
Animals.
Brindled (Mobr) C57BL/6 heterozygous female and C57BL/6 male mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and screened for the brindled mutation as described (37). The brindled mouse contains an in-frame six-nucleotide deletion in the coding region of the murine Menkes ATPase gene resulting in a marked impairment of copper homeostasis in affected males. All mice were housed at the Washington University School of Medicine Vivarium under a 12-hr light/dark cycle. Food and water were provided ad libitum, and all care was given in compliance with National Institutes of Health guidelines on the use of laboratory animals.
Cell Culture.
Rat hippocampal cultures were prepared from 18-day-old embryos as described (12). In all cases, rat neurons were cultured for 2 weeks and then stimulated in extracellular solution (ECS) containing 300 μM glutamate and 30 μM glycine, followed by incubation for 24 hr after insult in conditioned media. Mouse hippocampal cultures were prepared from postnatal day 0 male offspring derived from matings of Mobr heterozygous females with wild-type males. Genotype was determined for the brindled mutation as described previously (12). Mouse neurons were grown on coverslips suspended above a rat glial feeder layer. Hippocampi were dissected and dissociated by using trypsin and trituration through a Pasteur pipette. Neurons were plated on coverslips coated with poly(l-lysine) in MEM with 10% horse serum, allowed to attach for 4 hr, transferred to glial monolayers, and maintained in serum-free MEM (12). Cultures were plated at high density (14,400 cells per cm2), and all analyses were performed between 10 and 14 days in vitro. The chemicals used were APV (Research Biochemicals, Natick, MA), calcein AM (Molecular Probes, Carlsbad, CA), N-acetyl-d-sphingosine (C2 ceramide), CNQX, BCS, bathophenanthroline disulfonate (BPS), S-nitrosoglutathione (GSNO), Nω-nitro-l-arginine, and l-glutathione, reduced (GSH) (Sigma, St. Louis, MO).
Neuronal Survival Assays.
Neurons were rinsed in ECS (150 mM NaCl/4 mM KCl/10 mM Hepes/10 mM d-glucose/2 mM CaCl2) with 2 mM MgCl2, and excitotoxic conditions were then produced by using ECS containing 300 μM glutamate and 30 μM glycine with either CNQX, to elicit NMDA receptor-dependent excitotoxic injury, or APV, to elicit AMPA/kainate receptor-dependent injury. Neurons were stimulated for 5 min, rinsed again, and then incubated for 24 hr after insult in conditioned media. Cells were then incubated with 2 μM calcein AM in essential media for 30 min at 37°C, washed in dye-free ECS, and assayed for viability via epifluorescent microscopy. Percent survival was calculated from the ratio of live neurons on experimentally treated coverslips to live neurons on mock-treated coverslips from the same dish of plated coverslips. Ten random fields of view were counted per coverslip, covering a total of 6 mm2, and two mock-treated and three experimentally treated coverslips from the same dish, per group, were scored and averaged from at least three independent cultures.
In mouse neurons, excitotoxicity was induced through synaptically released glutamate after stimulation with high K+ buffer (25 mM Hepes, pH 7.4/31.5 mM NaCl/90 mM KCl/80 mM d-glucose/2 mM CaCl2/1 mM glycine) with and without APV or copper. Neurons were stimulated for 5 min, rinsed again, and incubated for 24 hr after insult in conditioned media, and viability was assayed as described above. Neuronal apoptosis was induced with C2 ceramide by incubating cultures in conditioned media for 24 hr. Immunofluorescence and confocal analysis were performed as previously described (12).
Calcium Imaging.
Cells were incubated with the fluorescent calcium indicator Fluo-3 AM in essential media for 30 min at 37°C, washed in dye and Ca2+-free ECS, and assayed via confocal microscopy. Images of cells were obtained by using a FluoView microscope (Olympus, Melville, NY) and analyzed by using SigmaScan Pro 5.0 (SPSS, Chicago, IL). All cells in a given field were measured. Before the application of experimental solution, images were obtained for 2–5 min to establish a stable baseline calcium measurement. Experimental solutions were made in ECS with 2 mM Ca2+ and 10 μM CNQX and contained 300 μM glutamate plus 30 μM glycine or an additional 200 μM CuCl2. All solutions were exchanged twice before imaging. The relative Ca2+ signal was calculated by dividing the integrated fluorescent signal from identified neurons by the baseline integrated fluorescent signal and was averaged over all neurons in a field.
Neonatal Hypoxia/Ischemia.
Wild-type males were mated with heterozygous Mobr females, and offspring were screened for the brindled mutation as described (37). On postnatal day 7, littermates underwent hypoxic/ischemic injury as previously described (38, 39). At this age, Mobr mice are viable and without obvious differences from wild-type mice, apart from color. Pups were anesthetized with 5% halothane (balance of room air) for induction and 1.5% halothane for maintenance, and an incision was made on the left side of the neck through which the left carotid artery was isolated and permanently cauterized. After suture and recovery from anesthesia at 37°C for 10 min, pups were returned to the dam for 2 hr to recover from the surgery. Pups were then placed in a hypoxia chamber through which humidified 8% oxygen (balance of nitrogen) flowed for 37 min. At the end of the hypoxic period, pups were returned to the dam and maintained at their regular 12:12 light/dark cycle until killing.
Analysis of Cell Death Markers in Hippocampal Lysates.
Pups were killed by lethal injection of pentobarbital (200 mg/kg) at 24 hr after hypoxic/ischemic injury and perfused with cold PBS containing 3 units per milliliter heparin. Left and right hippocampi were dissected on ice, immediately frozen, and kept at −80°C until lysis. Tissue was lysed in cell lysis buffer (Cell Signaling Technology, Beverly, MA) with a Teflon pestle and cleared by centrifugation at 20,000 × g for 15 min. Protein concentration of the lysate was determined by using the BCA protein assay kit (Pierce, Rockford, IL). Caspase 3 activity was determined in individual lysates as described (38). Briefly, 10 μl of lysate was added to 90 μl of assay buffer (10 mM Hepes, pH 7.4/42 mM KCl/5 mM MgCl2/3 mM DTT/10% sucrose) containing 30 μM 7-amino-4-methylcoumarin, N-acetyl-l-aspartyl-l-glutamyl-l-valyl-l-aspartic acid amide (Ac-DEVD-AMC), and the emitted fluorescence was measured every 5 min for 30 min by using a microplate fluorescence reader (Bio-Tek, Burlington, VT), with an excitation wavelength of 360 ± 40 nm and an emission wavelength of 460 ± 40 nm. When using a standard curve of 7-amino-4-methylcoumarin (AMC), Asp-Glu-Val-Asp (DEVD) cleavage activity was calculated as pmol·min−1 per mg protein.
For immunoblot analysis, lysate pools were created by using equal amounts of protein from the left and right hippocampi of each of three lysates for each genotype. Lysate pools were boiled in SDS sample buffer, and 20 μg of total protein per lane was separated on NuPAGE 3–8% Tris-Acetate gels (Invitrogen, Carlsbad, CA). Proteins were electroblotted onto Immobilon P membranes (Millipore, Billerica, MA) and blocked in ECL Advance blocking reagent (Amersham, Little Chalfont, U.K.). Membranes were probed with primary antibody against spectrin and its cleavage products (MAB1622; Chemicon, Temecula, CA) and visualized by using SuperSignal chemiluminescence (Pierce) on a Kodak (Rochester, NY) Image Station. Membranes were reprobed with antibody against tubulin as loading control (T5168; Sigma).
Acknowledgments
We thank Huaiyang Wu and Fernanda Laezza for help with neuronal cultures and advice and Louis Muglia and Guojun Bu for critical review of the manuscript. This work was supported by a National Science Foundation Graduate Research fellowship (to M.L.S.) and National Institutes of Health Grants NS33184 (to A.M.C.), NS35902 (to D.M.H.), and DK44464 (to J.D.G.).
Abbreviations
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BCS
bathocuproine disulfonate
- APV
2-amino-5-phosphonovalerate
- ECS
extracellular solution.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Culotta VC, Gitlin JD. In: The Molecular and Metabolic Basis of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Vol 3. New York: McGraw–Hill; 2001. pp. 3105–3136. [Google Scholar]
- 2.Prodan CI, Holland NR, Wisdom PJ, Bottomley SS. Neurology. 2004;62:1655–1656. doi: 10.1212/wnl.62.9.1655-a. and author reply (2004) 62:1656. [DOI] [PubMed] [Google Scholar]
- 3.Kumar N, Gross JB, Jr, Ahlskog JE. Neurology. 2004;63:33–39. doi: 10.1212/01.wnl.0000132644.52613.fa. [DOI] [PubMed] [Google Scholar]
- 4.Bruijn LI, Miller TM, Cleveland DW. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 5.Bush AI. Curr Opin Chem Biol. 2000;4:184–191. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 6.Kuper J, Llamas A, Hecht HJ, Mendel RR, Schwarz G. Nature. 2004;430:803–806. doi: 10.1038/nature02681. [DOI] [PubMed] [Google Scholar]
- 7.Keen CL, Uriu-Hare JY, Hawk SN, Jankowski MA, Daston GP, Kwik-Uribe CL, Rucker RB. Am J Clin Nutr. 1998;67:1003S–1011S. doi: 10.1093/ajcn/67.5.1003S. [DOI] [PubMed] [Google Scholar]
- 8.Lutsenko S, Petris MJ. J Membr Biol. 2003;191:1–12. doi: 10.1007/s00232-002-1040-6. [DOI] [PubMed] [Google Scholar]
- 9.Iwase T, Nishimura M, Sugimura H, Igarashi H, Ozawa F, Shinmura K, Suzuki M, Tanaka M, Kino I. Acta Neuropathol (Berlin) 1996;91:482–488. doi: 10.1007/s004010050455. [DOI] [PubMed] [Google Scholar]
- 10.Murata Y, Kodama H, Abe T, Ishida N, Nishimura M, Levinson B, Gitschier J, Packman S. Pediatr Res. 1997;42:436–442. doi: 10.1203/00006450-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 11.El Meskini R, Cline LB, Eipper BA, Ronnett GV. Dev Neurosci. 2005;27:333–348. doi: 10.1159/000086713. [DOI] [PubMed] [Google Scholar]
- 12.Schlief ML, Craig AM, Gitlin JD. J Neurosci. 2005;25:239–246. doi: 10.1523/JNEUROSCI.3699-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niciu MJ, Ma XM, El Meskini R, Ronnett GV, Mains RE, Eipper BA. Neuroscience. 2006;139:947–964. doi: 10.1016/j.neuroscience.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 14.Okeda R, Gei S, Chen I, Okaniwa M, Shinomiya M, Matsubara O. Acta Neuropathol (Berlin) 1991;81:450–457. doi: 10.1007/BF00293467. [DOI] [PubMed] [Google Scholar]
- 15.Brown DR, Qin K, Herms JW, Madlung A, Manson J, Strome R, Fraser PE, Kruck T, von Bohlen A, Schulz-Schaeffer W, et al. Nature. 1997;390:684–687. doi: 10.1038/37783. [DOI] [PubMed] [Google Scholar]
- 16.Kardos J, Kovacs I, Hajos F, Kalman M, Simonyi M. Neurosci Lett. 1989;103:139–144. doi: 10.1016/0304-3940(89)90565-x. [DOI] [PubMed] [Google Scholar]
- 17.Crump FT, Dillman KS, Craig AM. J Neurosci. 2001;21:5079–5088. doi: 10.1523/JNEUROSCI.21-14-05079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boehning D, Snyder SH. Annu Rev Neurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- 19.Lipton SA, Choi YB, Takahashi H, Zhang D, Li W, Godzik A, Bankston LA. Trends Neurosci. 2002;25:474–480. doi: 10.1016/s0166-2236(02)02245-2. [DOI] [PubMed] [Google Scholar]
- 20.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 21.Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West T, Atzeva M, Holtzman DM. Neurobiol Dis. 2006;22:523–537. doi: 10.1016/j.nbd.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Han BH, Xu D, Choi J, Han Y, Xanthoudakis S, Roy S, Tam J, Vaillancourt J, Colucci J, Siman R, et al. J Biol Chem. 2002;277:30128–30136. doi: 10.1074/jbc.M202931200. [DOI] [PubMed] [Google Scholar]
- 24.Trombley PQ, Shepherd GM. J Neurophysiol. 1996;76:2536–2546. doi: 10.1152/jn.1996.76.4.2536. [DOI] [PubMed] [Google Scholar]
- 25.Vlachova V, Zemkova H, Vyklicky L., Jr Eur J Neurosci. 1996;8:2257–2264. doi: 10.1111/j.1460-9568.1996.tb01189.x. [DOI] [PubMed] [Google Scholar]
- 26.Weiser T, Wienrich M. Brain Res. 1996;742:211–218. doi: 10.1016/s0006-8993(96)01009-8. [DOI] [PubMed] [Google Scholar]
- 27.Doreulee N, Yanovsky Y, Haas HL. Hippocampus. 1997;7:666–669. doi: 10.1002/(SICI)1098-1063(1997)7:6<666::AID-HIPO8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Choi YB, Tenneti L, Le DA, Ortiz J, Bai G, Chen HS, Lipton SA. Nat Neurosci. 2000;3:15–21. doi: 10.1038/71090. [DOI] [PubMed] [Google Scholar]
- 29.Paoletti P, Perin-Dureau F, Fayyazuddin A, Le Goff A, Callebaut I, Neyton J. Neuron. 2000;28:911–925. doi: 10.1016/s0896-6273(00)00163-x. [DOI] [PubMed] [Google Scholar]
- 30.Peters S, Koh J, Choi DW. Science. 1987;236:589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- 31.Li YV, Hough CJ, Sarvey JM. Sci STKE. 2003 May 13; doi: 10.1126/stke.2003.182.pe19. [DOI] [PubMed] [Google Scholar]
- 32.Kaler SG. Pediatr Dev Pathol. 1998;1:85–98. doi: 10.1007/s100249900011. [DOI] [PubMed] [Google Scholar]
- 33.Hardingham GE, Bading H. Trends Neurosci. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 34.Choi DW, Koh JY. Annu Rev Neurosci. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 35.Ueno S, Tsukamoto M, Hirano T, Kikuchi K, Yamada MK, Nishiyama N, Nagano T, Matsuki N, Ikegaya Y. J Cell Biol. 2002;158:215–220. doi: 10.1083/jcb.200204066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnston MV. Ment Retard Dev Disabil Res Rev. 2001;7:229–234. doi: 10.1002/mrdd.1032. [DOI] [PubMed] [Google Scholar]
- 37.Grimes A, Hearn CJ, Lockhart P, Newgreen DF, Mercer JF. Hum Mol Genet. 1997;6:1037–1042. doi: 10.1093/hmg/6.7.1037. [DOI] [PubMed] [Google Scholar]
- 38.Han BH, DeMattos RB, Dugan LL, Kim-Han JS, Brendza RP, Fryer JD, Kierson M, Cirrito J, Quick K, Harmony JA, et al. Nat Med. 2001;7:338–343. doi: 10.1038/85487. [DOI] [PubMed] [Google Scholar]
- 39.Parsadanian AS, Cheng Y, Keller-Peck CR, Holtzman DM, Snider WD. J Neurosci. 1998;18:1009–1019. doi: 10.1523/JNEUROSCI.18-03-01009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]