Abstract
This study uses an operant, behavioral model to assess the daily changes in the decay rate of short-term memory, motivation, and motor ability in rats exposed to chronic restraint. Restraint decreased reward-related motivation by 50% without altering memory decay rate or motor ability. Moreover, chronic restraint impaired hippocampal-dependent spatial memory on the Y maze (4-hr delay) and produced CA3 dendritic retraction without altering hippocampal-independent maze navigation (1-min delay) or locomotion. Thus, mechanisms underlying motivation for food reward differ from those underlying Y maze exploration, and neurobiological substrates of spatial memory, such as the hippocampus, differ from those that underlie short-term memory. Chronic restraint produces functional, neuromorphological, and physiological alterations that parallel symptoms of depression in humans.
Keywords: stress, motivation spatial memory, hippocampus, metabolism, operant conditioning
Chronic stress shapes neuronal dendritic morphology, which is consistent with its influence on cognitive ability. Rodents exposed to chronic stress for weeks or months exhibit decreased dendritic complexity within the hippocampus, which is typically associated with impaired spatial memory, a hippocampal function (O’Keefe & Nadel, 1978). As examples, daily tianeptine injections that facilitate serotonin reuptake to prevent stress-induced hippocampal CA3 dendritic retraction (Magariños, Deslandes, & McEwen, 1999; Watanabe, Gould, Daniels, Cameron, & McEwen, 1992b) block stress-induced spatial memory deficits (Conrad, Galea, Kuroda, & McEwen, 1996). CA3 dendritic retraction recovers within 10 days following the end of chronic restraint (Conrad, Magariños, LeDoux, & McEwen, 1999), which corresponds to the return of functional spatial memory following chronic stress (Luine, Villegas, Martinez, & McEwen, 1994). Likewise, chronic stress decreases dendritic arbors within the medial prefrontal cortex (mPFC; S. M. Brown, Henning, & Wellman, 2005; Cook & Wellman, 2004; Radley, Sisti, Hao, Rocher, McCall, Hof, McEwen, & Morrison, 2004), which also impairs recall of conditioned fear extinction, a function of the mPFC (Miracle, Brace, Huyck, Singler, & Wellman, 2006). Finally, chronic stress enhances dendritic complexity within the amygdala (Vyas, Mitra, Rao, & Chattarji, 2002), and a similar chronic stress paradigm facilitates amygdalar-dependent acquisition of conditioned fear (Conrad et al., 1999). These studies indicate that dendritic structural changes appear to govern cognitive ability.
The variety of morphological and behavioral changes produced by chronic stress raises several questions:
1. Does chronic stress have selective effects on mnemonic ability or is cognition shaped indirectly through nonmnemonic factors that include motivation and motor ability?
Rodents exposed to chronic stress for weeks or months exhibit anhedonic behaviors (Katz, Roth, & Schmaltz, 1981; Katz & Schmaltz, 1980; Willner, 1997) and show decreased motivation (Barr & Phillips, 1998; Konkle, Baker, Kentner, Barbagallo, Merali, & Bielajew, 2003), which are hallmark features of depression that may interfere with performance on tasks that are based upon reward and motivation.
2. Do such well-documented effects account for what appear to be memory problems, or are those factors independent sequelae of chronic stress?
Motivation and locomotion are often confounded, with locomotion typically used as a motivational index (Conrad et al., 1996; Negroni et al., 2004). However, motivation and locomotion are separate constructs, which may be independently influenced by chronic stress.
3. Might chronic stress affect cognition, motivation and motor ability along different time-courses?
In the present experiments, memory, motivation, and motor ability were determined on a daily basis during the course of chronic restraint to assess their development by using a mathematical model of behavior in an appetitive, operant conditioning paradigm. Mathematical principles of reinforcement (MPR; Killeen, 1994; Killeen & Sitomer, 2003) characterizes response rates on various schedules of reinforcement as a function of three parameters: memory decay rate (λ), motivation (a), and motor ability (δ). MPR has been applied previously in behavioral neuroscience research, to analyze the effects of brain lesions or drugs (Kheramin, Body, Herrera, Bradshaw, Szabadi, Deakin, & Anderson, 2005; Reilly, 2003). At the conclusion of this phase, the influence of chronic stress on spatial memory was assessed using a hippocampal-dependent, spatial recognition Y maze. We hypothesized that chronic stress would alter reward-related behavior by decreasing motivation (a) as restraint treatment persisted by decreasing response rates on higher fixed ratio requirements. Whereas some reports have indicated that motor ability is unaltered by chronic stress (Conrad et al., 1996), others have found impairments (Mizugochi, Yuzurihara, Ishige, Sasaki, & Tabira, 2002). Consequently, chronic stress may disrupt motor ability on a slower time scale than is observed with motivation. No specific effects were hypothesized for memory decay rate as measured by appetitive, operant conditioning because it was unknown whether restraint stress influences the rate at which the memory of a response will decay.
Experiment 1
Method
All procedures are in accordance with federal and institutional guidelines set forth by the Institutional Animal Care and Use Committee for animals in research at Arizona State University.
Subjects
Eighteen male Sprague–Dawley rats (Rattus norvegicus, Charles River Laboratories, Kingston, NY) were single-housed in a colony room with a 12:12-hr light/dark cycle, lights off at 6 a.m. Rats were allowed tap water and food (Rodent Block 8604) ad libitum for 65 days, and subsequently they were food-restricted and maintained at 85% of their ad libitum weight to ensure performance in the operant chambers for food reinforcers. Following operant training, the 3 highest responding rats and 3 lowest responding rats were removed from the experiment to reduce variance in the experimental data. The remaining 12 rats were match-paired for performance level (maximum response rate) and randomly assigned to control or stress conditions. The control and stress groups were housed in separate chambers within the colony room to prevent interaction.
Apparatus
Operant chamber
Six operant conditioning chambers (Med Associates Incorporated, Georgia, VT) were equipped with single retractable levers, positioned on the intelligence panel 6.0 cm above the floor and 1.0 cm from the left side of the panel. The lever was an aluminum plate 2.0 cm long × 5.0 cm wide × 0.2 cm high, which retracted completely upon completion of the required number of responses. Three lights were positioned 4.0 cm above the lever (red, yellow, green). Access to the food hopper was through an aperture (5.0 cm high × 5.0 cm wide) located 4.0 cm to the right of the lever and 2.0 cm above the floor. The experimental chambers consisted of three clear Plexiglas walls on the front, rear, and ceiling sides, and two aluminum panels on the lateral sides, including the intelligence panel. The floor consisted of parallel aluminum bars 0.5 cm wide and spaced 1.1 cm apart. The chambers were 24.0 cm long × 30.5 cm wide × 29.5 cm high. A white incandescent house light bulb was positioned in the center of the side opposite the lever 1.5 cm from the ceiling and was hemi-encased by an aluminum covering that allowed light through an opening at the top. A Pentium 133 via Med PC software controlled data collection and stimuli presentation.
Y maze
The Y maze was used to assess spatial memory, as described by Conrad et al. (1996), and consisted of three equilaterally intersecting Plexiglas arms (58 cm long × 19 cm wide × 38 cm high). Spatial cues included vertical metal poles positioned around the outside perimeter of the maze and above the black Plexiglas sides and patterned construction paper and posters attached to surrounding walls. The floor of the Y maze was covered with corncob bedding and mixed between trials to prevent use of odor cues.
Procedure
Food allotment
Following the establishment of 85% weights, rats were allocated food according to a growth equation to maintain target weights. In both experiments, food was placed on top of the cage each day at approximately 3 p.m. During the treatment period, control rats could therefore consume allocated food immediately, whereas stressed rats were required to wait until they were taken out of restraints at 6 p.m. However, this difference did not likely influence operant performance the subsequent day between groups, due to the temporal remoteness of feeding time to operant testing the following day (≥16 hr).
Operant behavior
Days 1–2
The rats had no prior experience with operant conditioning, therefore preliminary training in the operant chambers was necessary after the rats had stabilized at their 85% weights. A preliminary autoshaping procedure (P. L. Brown & Jenkins, 1968) presented the lever and green light for 5 s, which then retracted while a food pellet was delivered, followed by a 60-s intertrial interval. If a leverpress response occurred during this 5-s interval, a food pellet was delivered, the lever was retracted, and the stimuli turned off. Reinforcement was delivery of a 45-mg Noyes food pellet, formula A/I (Research Diets, Incorporated, New Brunswick, NJ). Rats experienced 2 days of autoshaping (60 trials/day).
Days 3–4
Following autoshaping, the rats were exposed to 2 days of a fixed ratio (FR) 1 schedule consisting of 45 trials, followed by 2 days of increasing FR schedules, and then (Days 5–7) increasing variable ratio (VR) schedules, during which food was provided after random numbers of responses averaging up to 30.
Days 8–90
The testing procedure involved distinctive tones and combinations of lights above the lever, which functioned as discriminative stimuli for the corresponding ratio. Upon reinforcement, discriminative stimuli were extinguished and the lever was retracted for 10 s during the intertrial intervals, and for 60 s during the intercomponent intervals. Collection of 10 reinforcers or the dissipation of 20 min resulted in advancement to the next schedule component. The component ratio schedules (4, 15, 40, 75, and 150) appeared just once during each session, in random order from one session to the next.
MPR testing for the control group of rats commenced at 8 a.m. each day and testing for the stress group commenced at approximately 10 a.m. Behavioral testing analyses were within-subjects comparisons of treatment to baseline, which prevented confounds resulting from circadian rhythms. Additionally, waste trays were washed before every behavioral testing trial to avoid any influences of olfactory stimuli.
Chronic stress (Days 69–89)
Rats in the stress group were restrained in cylindrical wire mesh restrainers (25 cm long × 8 cm diameter) in their home cages 6 hours per day for 21 consecutive days. This method reliably produces hippocampal dendritic atrophy (Conrad et al., 1999; Magariños & McEwen, 1995a) and spatial memory deficits (Conrad et al., 1996; Luine et al., 1994). Restraint commenced at 1 p.m. each day, approximately 1 hour after completion of operant testing so as to avoid any pairing of the operant chambers with stress treatment. To reduce negative associations between the restraint process and the experimenter, researchers who assisted with the restraint procedure or did not assist with the operant and Y maze testing procedures.
Y maze (Days 91–92)
On the second day following the termination of stress, rats were run in the Y maze with a 1-min delay between training and testing. Rats were chosen in random order and placed in an arm of the Y maze (start arm) with one of the arms blocked off (novel arm) and allowed to explore the start arm and remaining arm (alternate arm) for 15 min. Rats were then removed from the maze, the novel arm unblocked, and the bedding mixed. After a 1-min delay, rats were placed back in the start arm and allowed to explore all arms freely for 5 min. Because rats tend to explore novel environments, functional memory was measured as the number of entries into the novel arm compared with the alternate arm. Poor memory would lead to relatively equal entries into the novel and alternate arms, indicating that the animals had forgotten the experience in the alternate arm and would view it as an unexplored arm.
On the 3rd day following stress, rats were run in the Y maze in a different room containing novel spatial cues, with a 4-hr delay between training and testing. The 1-min and 4-hr versions of the Y maze were used to assess function of spatial working memory and long-term spatial memory, respectively.
Statistical Analyses
MPR trend analysis
The parametersλ, δ, and a were obtained from response rates in a multiple FR schedule by using the rate equation (Equation 1) for FR schedules (Killeen & Sitomer, 2003):
| (1) |
where N is FR schedule value, and BFR is response rate for that schedule. The Appendix describes how variations of each of the different behavioral characteristics can produce variations in the regression line given by the above equation.
The memory decay rate parameter, lambda (λ), is the rate of decay of the delay of reinforcement gradient. According to MPR, responses are strengthened in proportion to the amount of memory available to them, with those that are proximal in time to the reinforcer getting strengthened the most. If memory decay rate (λ) is fast, then the responses closest in time to the reinforcer become strengthened, whereas those further away do not, increasing the likelihood that only the target responses (lever pressing) will be paired with the reinforcer. If the memory decay rate is slow, as inferred by a smaller value of λ, then nontarget behaviors (superstitious behaviors) may become strengthened in addition to the required responses. A long series of target responses will saturate memory, causing the parenthetical numerator in Equation 1 to approach a value of 1 for large ratio schedules, indexed by N.
The motor ability parameter, delta (δ), measures the time required for the animal to emit a response. Animals that are less agile (or under more physical constraints) respond more slowly, reflected in a higher value of δ (Mobini, Chiang, Ho, Bradshaw, & Szabadi, 2000; Reilly, 2003).
The motivation parameter, specific activation (a), measures an animal’s arousal level induced by a given reinforcer, and depends on the quality of the reinforcer (incentive effects) and the deprivation level of the organism (drive effects). An animal that is more motivated will maintain high responding when confronted with higher work requirements, reflected through MPR as a higher value of a (Killeen, 1995).
The 20% trimmed means (Wilcox, 1998) of the response rates for each FR schedule were taken over blocks of 3–6 days and plotted as a function of FR schedule. Least squares regression to Equation 1 identified the parameter values that characterized behavior in each block. Logarithmic transforms of λ and reciprocal transforms of a, which approximately normalized their distributions, were used for data analysis.
To highlight the changes due to the stress, parameter values for the trimmed means in the treatment period were transformed into percentage differences from baseline. The baseline values for each rat were created by averaging the daily parameter values for the 15 days preceding the commencement of stress treatment. A two-way analysis of variance (ANOVA) was performed on the percentage differences for each of the parameters (λ, δ, and a) with treatment (control, stress) as the between-groups variable and consecutive blocks as a repeated measure. Four nonoverlapping blocks were chosen for statistical analysis (Days 1–5, 6–11, 12–17, and 18–21; see Figure 1). When an interaction was discovered, post hoc Newman–Keuls tests were performed.
Figure 1.

Examples of mathematical principles of reinforcement regression curves for control and stress groups, depicting response rates as a function of fixed ratio (FR) schedule (work requirement) on selected blocks of the treatment period. Control rats maintained stable response rates overall, whereas the rates of stressed rats were attenuated on higher FR schedules as the treatment progressed, indicating a decrease in motivation.
Y maze
An entry was determined when the two front paws were placed into an arm. The percentage of entries into each arm over Min 1–5 was obtained for each rat, and these values were used to assess spatial memory ability. To determine whether rats entered the novel arm significantly more often than the alternate arm in each condition, nonparametric analyses were performed on the percentage of entries in each by using a Wilcoxon’s matched-pairs signed-ranks test. To compare whether stressed rats and control rats differed in spatial memory ability, the percentage of entries in the alternate arm was subtracted from the percentage of entries in the novel arm, and this difference score was analyzed by using a mixed factors ANOVA with treatment (stress, control) as the between-subjects variable and delay (1-min, 4-hr) as the repeated variable. The start arm was not included in spatial memory analysis because of a potential bias from placement by the experimenter (Conrad, Grote, Hobbs, & Ferayorni, 2003; Conrad et al., 2004).
Food intake
To determine whether restraint stress affected food metabolism, a two-way ANOVA was performed on food allotment data. Treatment (control, stress) was the between-subjects variable and week was a within-subjects repeated variable. The week variable consisted of four 7-day blocks: 7 days prior to stress treatment (BL), stress Days 1–7 (Week 1), Days 8–14 (Week 2), and Days 15–21 (Week 3). The dependent variable was the average daily amount of food required to maintain their 85% ad libitum body weight (see Food allotment section).
Results
Parameter Trends
Stressed rats exhibited significant decreases in response rates on higher value schedules as restraint treatment continued, without significant changes in the memory available for responses or in the duration of responses. MPR reflected these changes, with chronic restraint influencing a (motivation), without altering λ (memory decay rate), and δ (motor ability). A two-way repeated measures ANOVA performed on the percentage change from baseline values of a (motivation) revealed a significant interaction between treatment and days of stress, F(1, 5) = 3.937, p < .01 (main effects p > .1). Post hoc Newman–Keuls tests indicated that values of a continually decreased in stressed rats as restraint treatment progressed, whereas control rats maintained relatively stable performance, Figure 2A. For both λ andδ, a two-way repeated measures ANOVA performed on the percentage change from baseline values revealed no significant main effects of treatment, consecutive blocks, or interactions (all ps > .4; see Figure 2B and 2C).
Figure 2.
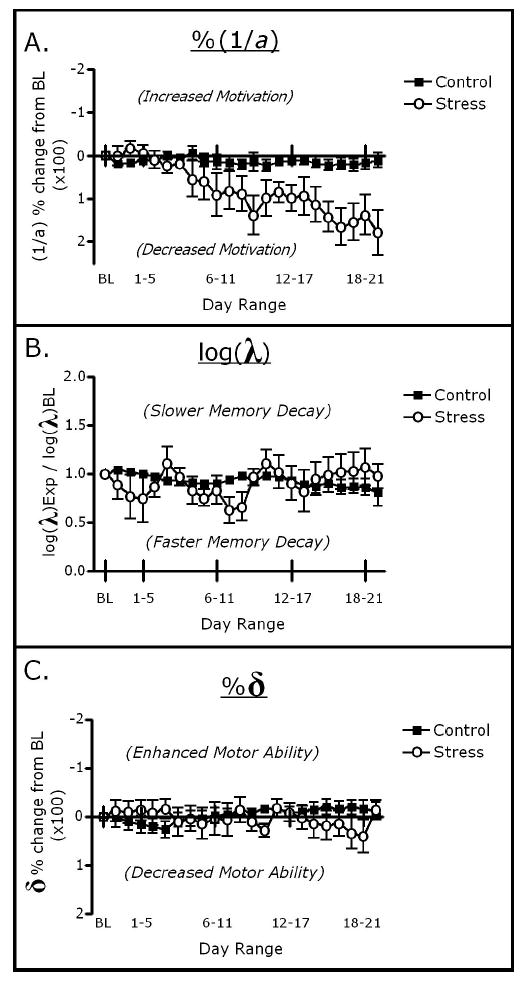
Influence of chronic restraint on changes in parameters from baseline levels. Y-axes are inverted on Panels A and C to facilitate interpretation. Control and stressed rats remained relatively similar in λ (B) and δ (C) throughout the treatment period. Stressed rats showed decreases in a during the treatment period (A), becoming approximately 50% less motivated after stress treatment compared with during pretreatment conditions, as measured by mathematical principles of reinforcement .
Y maze
Chronically stressed rats performed worse on the Y maze than did controls, and performance improved for both control and stressed rats when the delay interval was shortened from 4 hr to 1 min (Figure 3A). This finding was supported by a 2 × 2 mixed factor ANOVA (Treatment × Delay) on Y maze difference scores, which indicated a significant main effect of treatment, F(1, 8) = 4.847, p = .05, and a significant effect of delay interval, F(1, 8) = 28.214, p < .001, without a significant interaction ( p > .50). Wilcoxon’s nonparametric tests were performed to determine whether each group entered the novel arm more than the alternate arm. Control rats entered the novel arm more than the alternate arm for both the 4-hr and 1-min delays ( p < .05). In contrast, stressed rats entered the novel and alternate arms similarly during the 4-hr delay ( p > .1), but had a tendency to enter the novel arm more than the alternate arm during the 1-min delay ( p = .07; see Figure 3B).
Figure 3.
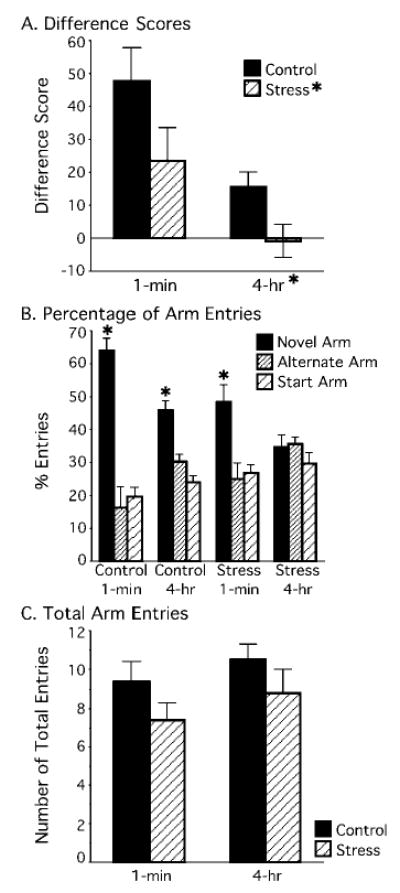
Influence of stress and delay on spatial memory ability. A: Difference scores (percentage of entries into novel arm – percentage of entries into alternate arm) indicated that stressed rats performed worse on the Y maze than did controls and entered the novel and alternate arms equally (at chance) after a 4-hr delay. B: Control rats significantly entered the novel arm more than the alternate arm in both the 1-min and 4-hr delay Y maze versions ( p < .05). Stressed rats in Experiment 1 had a tendency to enter the novel arm in the 1-min delay Y maze version ( p = .07), which reached significance (p < .01) when combined with the 5 stressed rats from the pilot study. Stressed rats were impaired on the 4-hr delay version ( p > .1). C: Control and stress rats had similar numbers of total entries after a 1-min or 4-hr delay.
To determine whether this tendency at the 1-min delay would reach significance with additional subjects, we compared pilot data from 5 rats that were behaviorally tested and chronically stressed under the same conditions (same duration of MPR and chronic restraint stress treatment followed by Y maze testing) as the Experiment 1 stressed rats. The earlier pilot data had no controls, necessitating that a mixed factors ANOVA be performed on stressed rats only, comparing experiment (pilot, current) and delay (1 min, 4 hr). Indeed, chronically stressed rats performed poorly on the 4-hr delay and well on the 1-min delay, as revealed by a significant main effect of delay, F(1, 8) = 14.507, p < .01, without a significant effect of experiment ( p > .3), nor significant interaction ( p > .6). Moreover, Wilcoxon’s tests showed that stressed rats tested at the 4-hr delay entered the novel and alternate arms similarly ( p > .1), but when the delay was shortened to 1-min, they entered the novel arm more than the alternate arm ( p < .01).
Motivation to explore the Y maze was determined from total entries made. The analysis showed that control and stressed rats made a similar number of entries during maze exploration. A 2 × 2 mixed factors ANOVA (Treatment × Delay) revealed that the total number of entries into all arms in Min 1–5 were statistically similar between control and stressed rats on either the 4-hr or 1-min Y maze versions (all ps > .1; see Figure 3C).
Food Intake
Rats were allotted food as a function of the deviation of their daily body weights from their 85% weights. Stressed rats required increased food amounts as stress persisted to maintain their weights compared with control rats (Figure 4A). This finding was supported by a significant interaction of treatment across weeks in Experiment 1, F(1, 3) = 8.35, p < .001, by using a 2 × 4 repeated measures ANOVA (Treatment × Week) and Newman–Keuls post hoc tests.
Figure 4.
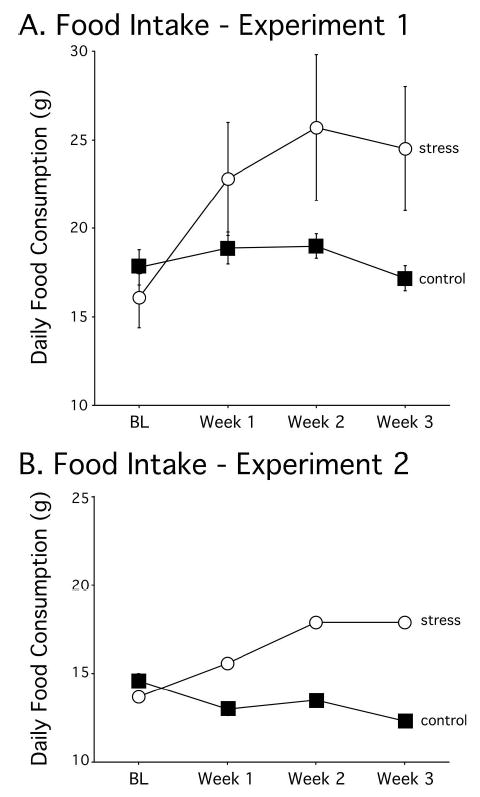
Food consumption during the treatment period for Experiments 1 and 2. Data points indicate average food allotted (g) for four 7-day blocks: 7 days preceding treatment (BL), Days 1–7 of treatment (Week 1), Days 8–14 of treatment (Week 2), and Days 15–21 of treatment (Week 3). Stressed rats required more food to maintain body weight as restraint treatment persisted, indicating stress-induced hypermetabolism. The error bars in Panel B are too small to see.
Experiment 2
Method
Subjects
A separate study with 10 male Sprague–Dawley rats was performed to verify the physiological effectiveness of the experimental paradigm in changing brain morphology and adrenal weights. These 10 rats were match paired and randomly assigned to groups, as done in Experiment 1.
Apparatus and Procedure
Rats in Experiment 2 were run by using the same apparatus and protocol as in Experiment 1 (restraint, operant behavior, Y maze, food allotment, etc.). Various postmortem measures were measured to evaluate the effectiveness of the paradigm in Experiment 1 in producing physiological and neuromorphological changes that are characteristic of chronic stress.
Physiological Measures
Rats were given an overdose of Nembutal (ip 100 mg/kg) immediately following Y maze testing, and their adrenal glands were removed, isolated, and weighed for analysis. Concurrently, the rats’ brains were extracted and processed according to the directions of a rapid Golgi staining kit (FD Neurotechnologies Consulting & Services, Incorporated, Ellicott City, MD) for evaluation of dendritic morphology. Apical and basal dendritic branch points of CA3 neurons were examined to verify stress-induced dendritic atrophy (Galea et al., 1997; Magariños & McEwen, 1995b; McLaughlin, Baran, Wright, & Conrad, 2005; Watanabe, Gould, Cameron, Daniels, & McEwen, 1992a; Watanabe et al., 1992b; Watanabe, Gould, & McEwen, 1992c).
Statistics
Adrenal weight
The right and left adrenal gland weights of each rat were averaged, and a one-way ANOVA was performed on these values with treatment group (control, stress) as the between-subjects variable.
Food intake
A two-way ANOVA was performed on food allotment data.
Histology
The numbers of dendritic branch points in hippocampal CA3 neurons have been shown to decrease after 21 days of restraint stress treatment (Conrad et al., 1999; Galea et al., 1997; Sousa, Lukoyanov, Madeira, Almeida, & Paula-Barbosa, 2000; Vyas et al., 2002; Watanabe et al., 1992a). To verify this morphological outcome in this study, CA3 dendritic branch points were quantified by using a total of 60 neurons. Three short-shaft type neurons and three long-shaft type neurons (Fitch, Juraska, & Washington, 1989; McLaughlin et al., 2005) were selected for each rat, provided they were (a) stained in their entirety, (b) located within the CA3 region, and (c) relatively isolated from neighboring neurons. The average number of branch points was determined for each rat, and a one-way ANOVA was performed on these values.
Results
Food Intake
As observed in Experiment 1, stressed rats required increased food amounts as stress persisted to maintain their weights, whereas food requirements did not change for control rats (Figure 4B; significant interaction of treatment across weeks in Experiment 2, F(1, 3) = 50.73, p < .001).
Adrenal Weight
Average adrenal weights for the restrained rats at the end of 21 days of restraint treatment were significantly greater than for controls, F(1, 8) = 25.8, p < .001 (control = 19.5 mg ± 0.5 mg; stress = 26.2 mg ± 1.2 mg).
Hippocampal Morphology
Chronic stress decreased CA3 neuronal dendritic branch points in the apical region and had a tendency to decrease branch points in the basal region (Figure 5; one-way ANOVA for apical region, F[1, 9] = 17.5, p < .01; basal region, F[1, 9] = 4.79, p = .06). The number of CA3 dendritic branch points was 18.8 ± 0.7 (control) and 13.7 ± 0.9 (stress) for the apical regions, and 16.3 ± 0.8 (control) and 14.0 ± 0.5 (stress) for the basal regions.
Figure 5.
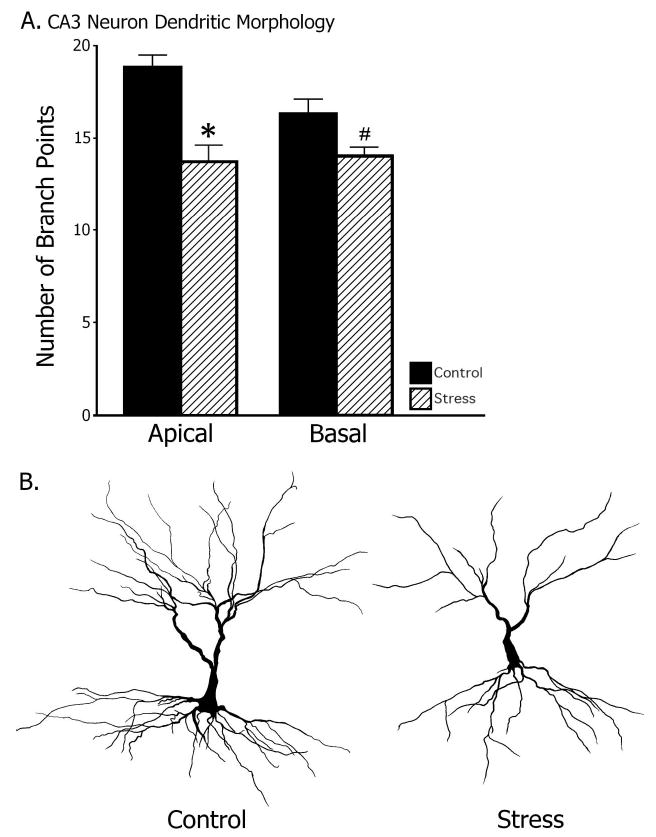
Dendritic branch points of hippocampal CA3 pyramidal neurons. Stressed rats had significantly less apical dendritic branch points than did control rats ( p < .01) and exhibited a nearly significant abbreviation in basal branch points ( p = .06).
General Discussion
During exposure to chronic restraint, daily behavioral assessment of response rates allowed continual observation of potential changes in three characteristics of operant performance: motivation (a), memory decay rate (λ), and motor ability (δ). Response rates in higher fixed-ratio requirements began to decrease among stressed rats during the second week of restraint and continued to decrease until restraint treatment ended. Indeed, when response rates were reflected through MPR, levels of motivation for reward (a) gradually fell over 21 days of restraint, culminating in an approximate 50% reduction from baseline levels. There were minimal changes in the duration of responses and in the memory available for responses among stressed rats during restraint, which suggests that motor ability (δ) and the rate of memory decay (λ), respectively, remained consistent among both stressed and control rats. In summary, as stress persisted, motivation decreased, whereas motor ability and memory decay rate (determined by MPR) remained relatively unaffected.
Chronic stress impaired discrimination between the novel and alternate arm following a 4-hr delay on the Y maze but failed to alter MPR memory decay rate, λ. Although chronic stress may alter certain behaviors to influence Y maze performance, several findings suggest that spatial memory was specifically influenced by chronic stress. For instance, potential negative associations between the investigator and the restraint process could have influenced a rat’s Y maze performance because an investigator and handling were part of Y maze testing. However, different investigators participated in the restraint and testing procedures to reduce negative associations between the investigator and the testing process. Moreover, if the rats had associated handling with an aversive experience, then performance on both Y maze versions would likely have been more similar. But, chronic stress did not affect the total entries made and discrimination following a 1-min delay, indicating that 21 days of restraint treatment specifically impaired hippocampal-dependent spatial memory, while leaving a separate spatial memory system intact. Previous studies have reported spatial memory deficits following chronic stress (Conrad et al., 1996; Luine et al., 1994; Mizoguchi et al., 2000; Nishimura, Endo, Endo, & Kimura, 1999), which were prevented when testing conditions were made less challenging (Beck & Luine, 1999; Bellani, Luecken, & Conrad, 2006) or when salient intramaze cues were provided (Wright & Conrad, 2005). The 1-min delay testing condition more closely paralleled the steadiness of MPR memory decay rate and indicates that the neurobiological substrates such as the hippocampus that underlie spatial memory, as determined by the Y maze, differ from those that underlie MPR memory decay rate.
The hypothesis that chronic stress would decrease motivation (a) as assessed by MPR was supported and consistent with how chronic stress can diminish motivation in a variety of tasks (Nestler, 2002). In the current study, response rates in higher fixed-ratio requirements began to decrease in the second week of chronic restraint and continued to decline throughout the testing period, without a clear plateau. These observations indicate that decreased motivation does not necessarily interfere with motor ability because a decreased while δ remained steady. But, whether more drastic reductions in motivation that exceed the 50% reduction observed in this study could have interfered with motor ability remains to be tested. Moreover, this study showed that decreased motivation develops over time, and differences among studies may hinge upon when motivation is assessed with respect to the start of chronic stress. Chronically stressed rats that exhibited decreases in motivation showed locomotor levels that were similar to those displayed by the controls on the Y maze, indicating that motivation for reward and motivation to explore in the Y maze may have separate underlying neural substrates.
Although decreases in motivation were predicted following chronic stress, the effects on motor ability were anticipated with less certainty. Both motivation and motor ability have been considered components of psychomotor activity (Simpson, 1972), which can be impaired by chronic stress in rats (Mizugochi et al., 2002), but are not always impaired (Conrad et al., 1996; Wright & Conrad, 2005). The findings from MPR that chronic stress decreased response rates (a) without differences in the duration of responses (δ) suggest that motivation and motor ability are independent. Moreover, the invariance of δ is consistent with the locomotor data from the Y maze, in which chronically stressed rats entered as many arms as did controls. Therefore, chronic stress decreased the desire but not the physical ability to perform.
Restraint stress produced a variety of physiological effects. Chronic stress increased adrenal gland weight, which likely accommodated an augmented glucocorticoid requisite and concurs with research on structural changes in the adrenal cortex due to elevated levels of stress hormones (Bornstein, Ehrhart-Bornstein, Güse-Behling, & Scherbaum, 1992). Stressed rats showed decreases in body weight as restraint persisted, and more food was subsequently allotted, tailored for each rat, to accommodate this change. The plateau of food allotted at the second week of stress treatment indicates that stressed rats did not continually lose weight, but rather their weights stabilized once a sufficient amount of food was allotted to accommodate a higher overall rate of metabolism. Therefore, we conclude that stressed rats required more food to maintain their weights than did controls as restraint persisted. This is in agreement with research on glucocorticoid-driven cellular resistance to insulin, whereby decreases in weight gain among stressed rats fed ad libitum are indeed produced by restraint-induced changes in metabolism, as opposed to food inaccessibility during restraint (Amrani, Chaouloff, Mormede, & Dandenne, 1991; Chaouloff & Zamfir, 1993; Kai et al., 2000). These results also illustrate a method of confirming the efficacy of stress treatment in future studies in which rats are food-restricted and stable body weights are a requirement of the experimental protocol.
Potential confounding issues in this study may arise from food restriction and the operant testing schedule. First, increases in food consumption are not likely linked to decreases in motivation for reward exhibited by stressed rats. Food consumption increased in Week 1 of treatment, which maintained 85% weights for behavioral testing; whereas decreases in motivation began during Week 2, indicating that the beginning of increased food consumption did not coincide with the motivational decline in stressed rats. Furthermore, stressed rats were fed approximately 16 hr prior to behavioral testing, preventing potential satiation confounds. Second, controls were subjected to the same, potentially stressful conditions of food restriction and serve as the proper control for that source of stress. The data clearly show that cognitive (spatial memory, motivation) and physiological (metabolism, adrenal weight) changes that are characteristic of chronic stress were not observed in controls. Hence, food restriction does not appear to have been as stressful as chronic restraint. Third, the timing of operant testing is another concern because the control and stressed rats were tested at 8 and 10 a.m., respectively. Testing stressed subjects separately from controls was necessary to reduce the potential exchange of odors and/or vocalizations. Although circadian rhythms can potentially influence performance, this 2-hr window was reasonably narrow to mitigate this concern. Moreover, behavioral testing analyses were within-subject comparisons to reduce confounds from circadian rhythms. Therefore, the issues raised by food deprivation and operant schedules were not likely critical issues in the outcome of these findings.
Restraint effectively produced decreases in branch point numbers among hippocampal CA3 neurons. This concurs with a multitude of other studies showing that chronic stress causes CA3 apical dendritic atrophy (Conrad et al., 1999; Galea et al., 1997; Lambert et al., 1998; Magariños & McEwen, 1995a; McKittrick et al., 2000; McLaughlin et al., 2005; Vyas et al., 2002; Watanabe et al., 1992c). However, the current study observed CA3 dendritic retraction in both apical and basal regions, whereas studies that use males typically observe dendritic retraction within the apical arbors only (Conrad et al., 1999; Galea et al., 1997; Magariños & McEwen, 1995a; McKittrick et al., 2000; Watanabe et al., 1992c). Food restriction increases serum corticosterone levels in rats (Heiderstadt, McLaughlin, Wright, Walker, & Gomez-Sanchez, 2000), and the combination of restraint and food restriction may have compounded the influence of glucocorticoids on dendritic arborization patterns of the male rats in this study, producing noticeable reductions in basal dendritic arborization. Chronic exposure to a predator failed to produce CA3 dendritic retraction, but the combination of predator exposure and high fat diet caused CA3 dendritic retraction (Baran et al., 2005). The present data show that daily operant training did not interfere with the manifestation of CA3 dendritic retraction following chronic stress.
Chronic stress for weeks to months can be used as an animal model of depression (Katz et al., 1981; Katz & Schmaltz, 1980; Willner, 1997), and the current restraint procedures produced many symptoms that are similar to major depression in humans. Chronic restraint decreased motivation on the appetitive operant conditioning paradigm, which is consistent with anhedonia and decreased motivation (Barr & Phillips, 1998; Konkle et al., 2003), hallmark features of depression (Frazer & Morilak, 2005; Henn & Vollmayr, 2005). Chronic restraint increases adrenal size, which coincides with adrenal hypertrophy evident in major depression (Nemeroff, Krishnan, Reed, Leder, Beam, & Dunnick, 1992). CA3 dendritic retraction produced by chronic restraint mirrors the reduced hippocampal volume of clinically depressed patients (Sheline, Sanghavi, Mintun, & Gado, 1999; Sheline, Wang, Gado, Csernansky, & Vannier, 1996; Videbech & Ravnkilde, 2004). Finally, chronic restraint impaired long-term (4-hr delay) spatial recognition memory, which is consistent with the memory decline of clinically depressed patients (Seeman, McEwen, Singer, Albert, & Rowe, 1997). Changes in hippocampal structure, either directly or indirectly, may contribute to these cognitive deficits because chronic stress impaired hippocampal-dependent spatial memory (4-hr delay) without altering short-term (1-min delay) spatial memory or memory decay rate (as assessed by MPR), which reflect cognitive functions that are not likely mediated by the hippocampus. These findings provide a time line for the development of motivational decreases and target putative neurological substrates that may underlie some of the cognitive changes.
Acknowledgments
We thank Sarah Baran, Rudy Bellani, Cainan Foltz, Juan Gomez, Ron Goode, Gillian Hamilton, James Harman, Jamie Jackson, Neeley John, Elizabeth Lightner, Katie McLaughlin, Joe Nguyen, Diana Posadas-Sanchez, Rebecca Rich, Federico Sanabria, Sergey Tsekhanov, Jacqueline Vollmer, Lindsay Wieczorek, and Ryan Wright for their contributions to this project. We especially thank Clark Presson and the ASU Honor’s College.
Appendix
Variations in Behavioral Characteristics Produce Variations in the Regression Line

Footnotes
Jonathan K. Kleen, Matthew T. Sitomer, Peter R. Killeen, and Cheryl D. Conrad, Department of Psychology, Arizona State University.
References
- Amrani A, Chaouloff F, Mormede P, Dandenne M. Glucose, insulin, and open field responses to immobilization in nonobese (NOD) mice. Physiology & Behavior. 1991;56:241–246. doi: 10.1016/0031-9384(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Baran SE, Campbell AM, Kleen JK, Foltz CH, Wright RL, Diamond DM, Conrad CD. Synergy between high fat diet and chronic stress retracts apical dendrites in CA3. NeuroReport. 2005;16:39–43. doi: 10.1097/00001756-200501190-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Chronic mild stress has no effect on responding by rats for sucrose under a progressive ratio schedule. Physiology & Behavior. 1998;64:591–597. doi: 10.1016/s0031-9384(98)00060-2. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: Role of monoamines and amino acids. Brain Research. 1999;830:56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- Bellani R, Luecken L, Conrad CD. Peripubertal anxiety profile can predict spatial memory impairments following chronic stress. Behavioural Brain Research. 2006;166:263–270. doi: 10.1016/j.bbr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Bornstein S, Ehrhart-Bornstein M, Güse-Behling H, Scherbaum W. Structure and dynamics of adrenal mitochondria following stimulation with corticotropin releasing hormone (CRH) Anatomical Record. 1992;234:255–262. doi: 10.1002/ar.1092340212. [DOI] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM. Auto-shaping of the pigeon’s key-peck. Journal of the Experimental Analysis of Behavior. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Short-term, mild stress alters dendritic morphology in rat medial prefrontal cortex. Cerebral Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Zamfir O. Psychoneuroendocrine outcomes of short-term crowding stress. Physiology & Behavior. 1993;54:767–770. doi: 10.1016/0031-9384(93)90089-x. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LAM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y-Maze, and this effect is blocked by tianeptine pretreatment. Behavioral Neuroscience. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. The Neurobiology of Learning and Memory. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute restraint stress impairs spatial memory in male but not female rats: Influence of estrous cycle. Pharmacology Biochemistry and Behavior. 2004;78:569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Magariños AM, LeDoux JE, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behavioral Neuroscience. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Fitch JM, Juraska JM, Washington LW. The dendritic morphology of pyramidal neurons in the rat hippocampal CA3 area. I. Cell types. Brain Research. 1989;479:105–114. doi: 10.1016/0006-8993(89)91340-1. [DOI] [PubMed] [Google Scholar]
- Frazer A, Morilak DA. What should animal models of depression model? Neuroscience and Biobehavioral Reviews. 2005;29:515–523. doi: 10.1016/j.neubiorev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Heiderstadt KM, McLaughlin RM, Wright DC, Walker SE, Gomez-Sanchez CE. The effect of chronic food restriction and water restriction on open-field behaviour and serum corticosterone levels in rats. Lab Animal. 2000;34:20–28. doi: 10.1258/002367700780578028. [DOI] [PubMed] [Google Scholar]
- Henn FA, Vollmayr B. Stress models of depression: Forming genetically vulnerable strains. Neuroscience and Biobehavioral Reviews. 2005;29:799–804. doi: 10.1016/j.neubiorev.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Kai K, Morimoto K, Morita E, Okada Y, Yamamoto S, Kanda K, et al. Environmental stress modifies glycemic control and diabetes onset in Type 2 diabetes prone Otsuka Long Evans Tokushima Fatty (OLETF) rats. Physiology & Behavior. 2000;68:445–452. doi: 10.1016/s0031-9384(99)00187-0. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Roth KA, Schmaltz K. Amphetamine and tranylcypromine in an animal model of depression: Pharmacological specificity of the reversal effect. Neuroscience and Biobehavioral Reviews. 1981;5:259–264. doi: 10.1016/0149-7634(81)90007-5. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Schmaltz K. Dopaminergic involvement in attention: A novel animal model. Progress in Neuropsychopharmacology. 1980;4:585–590. doi: 10.1016/0364-7722(81)90099-0. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Herrera FM, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: Implications for models of inter-temporal choice. Behavioural Brain Research. 2005;156:145–152. doi: 10.1016/j.bbr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Killeen PR. Mathematical principles of reinforcement. Behavioral & Brain Sciences. 1994;17:105–172. [Google Scholar]
- Killeen PR. Economics, ecologics, and mechanics - the dynamics of responding under conditions of varying motivation. Journal of the Experimental Analysis of Behavior. 1995;64:405–431. doi: 10.1901/jeab.1995.64-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen PR, Sitomer MT. MPR. Behavioral Processes. 2003;62:49–64. doi: 10.1016/S0376-6357(03)00017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: Sex and strain compared. Brain Research. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Buckelew SK, Staffiso-Sandoz G, Gaffga S, Carpenter W, Fisher J, Kinsely CH. Activity-stress induces atrophy of apical dendrites of hippocampal pyramidal neurons in male rats. Physiology & Behavior. 1998;65:43–49. doi: 10.1016/s0031-9384(98)00114-0. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Research. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Magariños AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. European Journal of Pharmacology. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors. Neuroscience. 1995a;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995b;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Magariños AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- McLaughlin K, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: Possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiology of Learning and Memory. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. Journal of Neuroscience. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizugochi K, Yuzurihara M, Ishige A, Sasaki H, Tabira T. Chronic stress impairs rotarod performance in rats: Implications for a depressive state. Pharmacology, Biochemistry and Behavior. 2002;71:79–84. doi: 10.1016/s0091-3057(01)00636-0. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Comparison of the effects of clozapine, haloperidol, chlorpromazine and d-amphetamine on the performance on a time-constrained progressive ratio schedule and on locomotor behavior in the rat. Psychopharmacology. 2000;152:47–54. doi: 10.1007/s002130000486. [DOI] [PubMed] [Google Scholar]
- Negroni J, Venault P, Pardon MC, Pérez-Diaz F, Chapouthier G, Cohen-Salmon C. Chronic ultra-mild stress improves locomotor performance of B6D2F1 mice in a motor risk situation. Behavioural Brain Research. 2004;155:265–273. doi: 10.1016/j.bbr.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Krishnan KRR, Reed D, Leder R, Beam C, Dunnick NR. Adrenal gland enlargement in major depression. A computerized tomographic study. Archives of General Psychiatry. 1992;49:384–387. doi: 10.1001/archpsyc.1992.01820050048008. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nishimura JI, Endo Y, Endo Y, Kimura F. A long-term stress exposure impairs maze learning performance in rats. Neuroscience Letters. 1999;273:125–128. doi: 10.1016/s0304-3940(99)00645-x. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. Oxford, England: Clarendon Press; 1978. The hippocampus as a cognitive map. [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Reilly MP. Extending mathematical principles of reinforcement into the domain of behavioral pharmacology. Behavioural Processes. 2003;62:75–88. doi: 10.1016/s0376-6357(03)00027-5. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory decline: McArthur studies of successful aging. Journal of Clinical Endocrinology and Metabolism. 1997;82:2458–2465. doi: 10.1210/jcem.82.8.4173. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. Journal of Neuroscience Methods. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JC, Vannier MW. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences, USA. 1996;93:3908 –3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EJ. 3. Washington, DC: Gryphon House; 1972. The classification of educational objectives in the psychomotor domain: The psychomotor domain. [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OFX, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: A meta-anlaysis of MRI studies. American Journal of Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992a;2:431–436. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. European Journal of Pharmacology. 1992b;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research. 1992c;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. How many discoveries have been lost by ignoring modern statistical methods? American Psychologist. 1998;53:300–314. [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8:151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]


