Abstract
The expression and activity of P-glycoprotein (pGP) play a role in the multidrug resistance of tumors. Because solid-growing tumors often show pronounced hypoxia or extracellular acidosis, this study attempted to analyze the impact of an acidic environment on the expression and activity of pGP and on the cytotoxicity of chemotherapeutic agents. For this, prostate carcinoma cells were exposed to an acidic extracellular environment (pH 6.6) for up to 24 hours. pGP activity was more than doubled after 3 to 6 hours of incubation in acidic medium, whereas cellular pGP expression remained constant, indicating that increased transport rate is the result of functional modulation. In parallel, the cytotoxic efficacy of daunorubicin showed pronounced reduction at low pH, an effect that was reversible on coincubation with a pGP inhibitor. A reduction of intracellular Ca2+ concentration by 35% under acidic conditions induced a higher transport rate of pGP, an effect comparable to that found on inhibition of protein kinase C (PKC). These data indicate that pGP activity is increased by acidic pH presumably as a result of lowered intracellular calcium levels and inhibition of PKC. These findings may explain the reduced cytotoxicity of chemotherapeutic agents in hypoxic/acidic tumors.
Keywords: P-glycoprotein, acidity, chemotoxicity, intracellular Ca2+ concentration, protein kinase C
Introduction
Solid-growing tumors differ profoundly from normal tissues with respect to morphologic and histopathological features and also in terms of physiological characteristics at the cellular and tissue levels. These functional parameters include properties of microcirculation, interstitial transport, oxygen and substrate supply, extracellular pH, and bioenergetic status [1]. These factors are closely linked and form a complex network, the so-called metabolic microenvironment. As a result of numerous functional and structural abnormalities in the tumor vascular network, the metabolic microenvironment of tumors is fundamentally different from that found in normal tissues [1]. Consequently, the mean oxygen partial pressure (pO2) in tumors is often considerably lower than that in surrounding normal tissues. Approximately 60% of human tumors show severe hypoxia or even anoxia in vital tumor tissues [2]. Because of insufficient O2 delivery, tumor cells undergo marked changes in their metabolic pathways. The cells switch to glycolysis (a phenomenon that can also occur in tumors even when oxygen supply is sufficient—known as the Warburg effect), leading to the accumulation of high levels of lactate and low glucose concentrations. Simultaneously, many tumors exhibit pronounced extracellular acidity with pH values that are, in some instances, even lower than 6.5 [1].
Data that have been available for several years suggest that hypoxia in solid tumors is one of the major reasons for the limited efficacy of several nonsurgical treatment modalities, such as sparsely ionizing radiation [3,4] or photodynamic therapy [5]. Additionally, several cell culture studies have shown various chemotherapeutic agents to be more effective in the presence of oxygen [6–8]. In in vivo experiments, Teicher et al. [9] showed the antineoplastic activity of several drugs in experimental sarcomas of mice to be correlated with tumor perfusion. The authors assumed that well-perfused tissue areas corresponded to normoxic regions, whereas poorly perfused regions indicated hypoxic tissues. They found that several chemotherapeutic agents (including cyclophosphamide, carboplatin, and melphalan) induced a more pronounced cell kill in “oxygenated” tumor areas than in “hypoxic” regions, indicating possible O2 sensitivity of these drugs. Grau and Overgaard [10], using in vivo experiments, showed that 5-fluorouracil or methotrexate had a cytotoxic effect only on well-oxygenated, but not on hypoxic, tumors. Various possible direct or indirect (through acidification of extracellular space) reasons for these differences have been discussed (for a review, see Ref. [11]): 1) oxygen might directly influence the mechanism of action (pharmacodynamics) of antineoplastic drugs (e.g., alkylating agents) [12]; 2) hypoxia can cause cell cycle arrest and in turn reduce the efficacy of agents acting only on proliferating cells [12,13]; and 3) hypoxic tissues show pronounced extracellular acidity [1], which might influence intracellular/extracellular drug distribution and in turn lead to a reduced intracellular concentration of chemotherapeutic agents [13–15].
In addition, several tumor cell lines (as well as cells from numerous normal tissues) have active transport mechanisms through which drugs are moved out of the cells. Transport proteins, which belong to the ATP-binding cassette (ABC) transporter family, can carry different chemotherapeutic agents [e.g., daunorubicin (DNR), paclitaxel, vincristine, and many others] and may therefore be responsible for chemoresistance seen in a number of tumors (multidrug resistance, MDR) [16,17]. In the field of clinical oncology, one important member of the ABC transporter family is P-glycoprotein (pGP; a product of the MDR1 gene) [18,19]. Inhibition of this transporter has been seen to increase the cytotoxic efficacy of several chemotherapeutic agents [20].
Besides constitutional differences in the pGP expression of tumor entities or cell lines, the expression as well as the functional activity of the transporter have been shown to be regulated. Cytokines [interleukin (IL) 1β, IL-6, and tumor necrosis factor α (TNF-α)] and growth factors (epidermal growth factor, EGF) are known to be capable of altering the functional activity of pGP [21,22].
When these results are considered, a question arises: Do microenvironmental parameters affect the transport efficacy of pGP? Only a small number of studies have analyzed the impact of the microenvironment on pGP expression. Hypoxia [23], glucose depletion [24], and reactive oxygen species [25] have been shown to be capable of regulating the MDR1 gene. However, the impact of the microenvironment on the functional activity of pGP still remains unclear.
The aim of this study was to analyze whether extracellular pH (a parameter of the metabolic microenvironment) in tumors affects the activity or expression of pGP and whether pH has an impact on the cytotoxic efficacy of chemotherapeutic drugs through the modulation of pGP transport rate. In addition, the study attempted to clarify which intracellular mechanisms might be involved in such a regulation of pGP activity by the metabolic microenvironment.
Materials and Methods
Materials
pGP antibody (clone C219) was purchased from Signet Laboratories (Dedham, MA), and the secondary antibody [rabbit anti-mouse, horseradish peroxidase (HRP)-conjugated] was from BioTrend (Cologne, Germany). Ionomycin was obtained from MP-Biomedicals (Irvine, CA). All other chemicals were purchased from Sigma-Aldrich (Taufkirchen, Germany).
Cell Line
The subline AT1 of the rat R-3327 Dunning prostate carcinoma was used in all experiments. This cell line is known to functionally express pGP [26]. Cells were grown in RPMI medium supplemented with 10% fetal calf serum (FCS) at 37°C under a humidified 5% CO2 atmosphere and passaged once per week. For the experiments, cells grew either in Petri dishes (2.5 x 106 to 3.0 x 106 cells) or in 96-well plates (4 x 104 to 5 x 104 cells). For the experiments, cells were transferred to RPMI medium without additional FCS supplementation 24 hours prior to the measurements. For control experiments, the medium was buffered with 20 mM HEPES adjusted to pH 7.4. For acidic conditions, cells were incubated in a medium buffered with 20 mM morpholinoethane-sulfonic acid + 4.51 mM NaHCO3, which resulted in pH 6.6 for up to 24 hours.
Rhodamine-123 Efflux Assay
To assess the activity of pGP, the efflux rate of rhodamine-123 in the presence or absence of a specific pGP inhibitor (verapamil, VPL) was measured. For this, cells were incubated with rhodamine (0.5 µM, dissolved in Ringer solution) for 30 minutes at 37°C. Subsequently, the rhodamine-containing solution was removed, and the cells were rapidly washed with phosphate-buffered saline (PBS) at 4°C and then incubated with fresh rhodamine-free Ringer solution. Samples (100 µl) of the supernatant were taken at 0, 5, 15, and 45 minutes after medium exchange. Rhodamine-123 efflux rate could then be calculated from the increase in rhodamine-123 concentration in the Ringer solution, as determined by fluorimetric measurements using a fluorescence microplate reader (Victor2; Wallac, Turku, Finland) with excitation/emission wavelengths of 485/535 nm. To determine pGP-mediated efflux rate, a second set of cells was primarily incubated with rhodamine-123, as described above; however, thereafter, the solution was replaced by rhodamine-free Ringer solution containing VPL (10 µM, dissolved in EtOH). Due to the inhibition of pGP by VPL, the rhodamine efflux rate was slower. The ratio of the efflux rate with and without VPL was used as a measure of the activity of pGP-mediated efflux [21].
As a second measure of pGP activity, intracellular rhodamine-123 concentration in the presence or absence of VPL was determined. For this, cells were treated as described above (30 minutes of incubation with 0.5 µM rhodamine solution, then the medium was changed to rhodamine-free solution with and without 10 µM VPL). After 45 minutes, cells were washed and lysed with Triton X-100. The intracellular rhodamine-123 concentration of the lysate was determined fluorimetrically at excitation/emission wavelengths of 485/535 nm. The ratio of the intracellular rhodamine concentration with and without VPL was used as a measure of pGP activity.
All measurements were normalized to the protein content in each Petri dish determined with bicinchoninic acid (BCA) assay (Pierce, KMF Laborchemie, SanktAugustin, Germany), as described previously [27].
pGP Expression
The cellular expression of pGP was determined in whole-cell enzyme-linked immunosorbent assay, as described previously [28]. In brief, after cell fixation with 4% paraformaldehyde for 60 minutes, cells were washed with a permeabilizing buffer containing 0.1% Triton X-100 and then incubated for 20 minutes with this buffer, to which 0.6% H2O2 had been added. After incubation with the primary anti-pGP antibody (diluted 1:1000) at 4°C overnight, cells were washed and incubated with a secondary anti-mouse peroxidase antibody (diluted 1:1000) for 1 hour. Thereafter, cells were incubated with an HRP substrate (containing 0.5 mg/ml o-phenylenediamine, 11.8 mg/ml Na2HPO4-2H2O, 7.3 mg/ml citric acid, and 0.015% H2O2) for 15 minutes and measured photometrically at a wavelength of 490 nm using a microplate reader (Victor2; Wallac). To normalize pGP expression for the number of cells in each well, permeabilized cells were subsequently incubated with 0.2% trypan blue solution for 5 minutes, washed with PBS, and dissolved with 1% sodium dodecyl sulfate, and the trypan blue concentration as a measure of cell number was determined photometrically.
Intracellular pH Measurement
Intracellular pH was determined using the pH-sensitive fluorescent dye 2′,7′-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF), as described previously by Weiner and Hamm [29]. In brief, 3.0 x 105 to 3.5 x 105 cells were incubated on coverslips with BCECF (final concentration, approximately 2 µM) for 5 minutes at 37°C. Subsequently, the coverslips were transferred to an inverse microscope (Axiovert 100 TV; Zeiss, Oberkochen, Germany). Excitation wave-length was switched between 460 and 488 nm. Emission fluorescence was monitored at 535 nm using a charge-coupled device (CCD) camera system (C4742-95; Hamamatsu Photonics, Herrsching, Germany) and image analysis software (Aquacosmos ver. 1.3; Hamamatsu Photonics). After subtraction of background levels, the fluorescence intensity ratio at both excitation wavelengths was calculated every 10 seconds. At the end of each measurement period, pH calibration was performed. For this, two calibration solutions containing 132 mM KCl, 10 mM NaCl, 1 mM CaCl, 1 mM MgCl, 10 mM HEPES, and 10 µM nigericin were prepared and adjusted to pH 6.8 and 7.6, respectively. The cells were superfused with these solutions, and fluorescence ratio was measured. The intracellular pH of each cell was calculated from linear interpolation of calibration points.
Intracellular Ca2+ Concentration
Cytosolic free Ca2+ ([Ca2+]in) was determined using the Ca2+-sensitive fluorescent dye Fura-2, as described by Grynkiewicz et al. [30]. In brief, 3.0 x 105 to 3.5 x 105 cells were incubated on coverslips with Fura-2 (final concentration, 5 µM) for 15 minutes at 37°C. Thereafter, the coverslips were transferred to an inverse microscope, and fluorescence was measured at 535 nm whereas excitation wavelength was switched between 334 and 380 nm. The fluorescence intensity of each cell was monitored with a CCD camera system, and the fluorescence intensity ratio at both excitation wavelengths (FL340/FL380) was calculated every 5 seconds. [Ca2+]in was calculated according to Grynkiewicz et al. [30], assuming a dissociation constant (Kd) of 225 nM. At the end of each measurement period, Ca2+ calibration was performed with two calibration solutions, each containing 141 mM NaCl, 0.8 mM NaH2PO4, 3.2 mM Na2HPO4, 4 mM KCl, and 1 µM ionomycin. One calibration solution additionally contained 1 µM CaCl2, whereas the other one was rendered Ca2+-free by the addition of 1 mM EGTA. The cells were superfused with these solutions, and fluorescence ratios (Rmin and Rmax) were measured. With these values, intracellular Ca2+ concentration was calculated according to the formula described in Grynkiewicz et al. [30].
Caspase 3 Activity and Cell Toxicity Assay
To assess the cytotoxic activity of DNR (which is a substrate of pGP) and cisplatin (which is not transported by pGP) [19,26] under different conditions, the relative caspase 3 activity normalized with respect to untreated control cells and the repopulation of cells 42 hours after chemotherapy were measured. For these experiments, cells were grown as a confluent monolayer (2.5 x 106 to 3.0 x 106 cells per Petri dish). Cells were incubated with serum-free medium 24 hours prior to commencement of chemotherapy. Three hours before the application of chemotherapeutic drugs, cells were exposed to either acidic (pH 6.6) or control medium (pH 7.4). Subsequently, cells were incubated with DNR (final concentration, 10 µM) or cisplatin (final concentration, 150 µM) for 3 hours at 37°C, whereby acidic or control conditions were maintained. After this period of time, the medium was replaced again. For caspase 3 activity measurements, cells were exposed to serum-free control medium (pH 7.4) for a further 3 hours, after which caspase 3 activity was measured as described below. For repopulation experiments, the medium was replaced following incubation with chemotherapeutic drugs with FCS-containing control medium, and cells were maintained under normal cell culture conditions for 42 hours. Thereafter, the cell concentration in the Petri dishes was determined using an automatic cell counter (Z2; Coulter, Krefeld, Germany). In a second series of experiments designed to assess the impact of pGP activity on DNR toxicity, cells were incubated with DNR solution additionally containing the pGP inhibitor VPL (final concentration, 10 µM; dissolved in EtOH). Caspase 3 activity and repopulation were performed analogously to the first series, as described above.
Caspase 3 activity was determined as described previously [31] using a caspase 3 activity kit (Clontech Laboratories, Heidelberg, Germany), in accordance with the manufacturer's instructions. For this, cells were incubated with a lysis buffer (100 µl) for 20 minutes on1 ice, harvested, and centrifuged at 16,000g for 10 minutes at 4°C. The supernatant (60 µl) was incubated with the caspase 3 substrate DEVD-AFC (60 µl; to give a final concentration of 40 µM) at 37°C in a 96-well plate. The fluorescence of the cleaved product AFC was measured at 405 nm excitation wavelength and 535 nm emission wavelength using a fluorescence microplate reader (Victor2; Wallac). Cleaved AFC was quantified with a calibration curve using known AFC concentrations. All measurements were normalized with respect to the protein content in each probe.
Necrosis formation was quantified by the assessment of lactate dehydrogenase (LDH) release from cells into the medium. The activity of LDH in media and cell lysates was determined in an automatic analyzer (Cobas-Mira; Roche Diagnostics, Mannheim, Germany).
Statistical Analysis
Results are expressed as mean ± standard error of the mean (SEM). Differences between groups were assessed by two-tailed Wilcoxon test for unpaired samples. The significance level was set at α = 5% for all comparisons.
Results
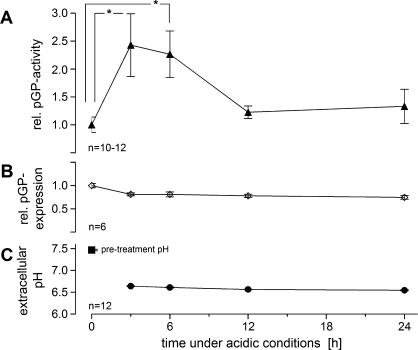
In rat R-3327-AT1 prostate carcinoma cells, a reduction of extracellular pH to a value of 6.6 (Figure 1C) for up to 6 hours resulted in a significant increase in pGP-mediated rhodamine-123 efflux rate as measured by the ratio of the efflux rates in the absence and presence of the inhibitor VPL (Figure 1A). When cells were kept under acidic environmental conditions for 3 to 6 hours, relative pGP activity was more than doubled. After this time, the activity returned to control levels. This has also been shown by measurements of intracellular rhodamine-123 concentration in the presence or absence of the inhibitor VPL. Under control conditions (pH 7.4), intracellular concentration was approximately 1.7-fold higher when pGP transport was inhibited compared to values obtained without VPL (Table 1). Maintaining the cells in an acidic environment for 3 to 6 hours led to a significant increase in this concentration ratio (Table 1), indicating a higher pGP activity. Because the cellular expression of pGP protein was not markedly altered by an acidic environment (Figure 1B), the increase in the rhodamine efflux rate must result from either an increase in the functional activity of pGP or a translocation of preformed transporters to the cell membrane. Due to the increased activity of the pGP transporter, it seems likely that the intracellular concentration of chemotherapeutic drugs is reduced (which, in rhodamine efflux experiments, resulted in a lower intracellular rhodamine concentration; data not shown).
Figure 1.
(A) Activity of pGP (described by the ratio of rhodamine-123 efflux rates in the presence or absence of VPL, respectively) and (B) relative pGP expression in AT1 cells kept under acidic conditions for up to 24 hours. (C) Extracellular pH during this period. Values at t = 0 hour were measured at pH 7.47 (pretreatment pH of normal cell culture medium) after which the medium was changed to pH 6.6. Values are expressed as mean ± SEM. *P < .05; n, number of experiments.
Table 1.
Ratio of Intracellular Rhodamine-123 Concentration in the Presence and Absence of the pGP Inhibitor VPL (10 µM) in AT1 Cells Maintained under Acidic Conditions for Up to 24 Hours.
| Time under Acidic Conditions (h) | n | Rhodamine-123 Concentration Ratio |
| 0 | 12 | 1.72 ± 0.16 |
| 3 | 12 | 2.97 ± 0.28** |
| 6 | 12 | 2.43 ± 0.26* |
| 12 | 12 | 1.82 ± 0.15 |
| 24 | 12 | 1.81 ± 0.27 |
Values are expressed as mean ± SEM.
n, number of experiments.
P < .05 vs t = 0 hour.
P < .01 vs t = 0 hour.
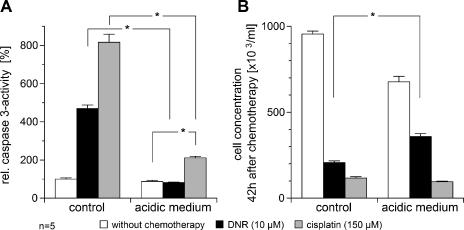
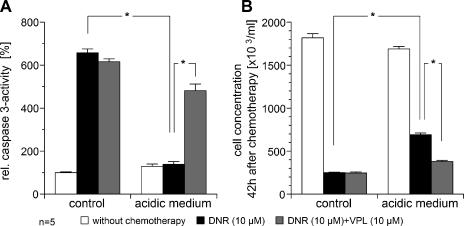
The cytotoxicity of DNR (which is a substrate of pGP) and cisplatin (which is known not to be transported by this pump) [19] was reduced by the acidic environment. With cisplatin, caspase 3 activity was significantly reduced compared to normal pH (Figure 2A). However, even though cisplatin-induced caspase 3 activation was markedly reduced in the acidic environment, overall cell kill (as measured by the number of surviving tumor cells) was not significantly different in cells incubated with cisplatin at different pH levels. These results indicate that the cytotoxicity of cisplatin was only marginally affected by the acidic environment. This may be the result of an increase in necrosis formation measured by LDH release into the medium. With cisplatin treatment at pH 7.4, 5 ± 2% of the total LDH was found in the medium, whereas at pH 6.6, this fraction increased significantly to 33 ± 12%. DNR-induced caspase 3 activation was markedly reduced by the acidic environment, reaching approximately the same level as that found in nontreated cells (Figures 2A and 3A). In parallel, the number of surviving tumor cells 42 hours after chemotherapy was significantly higher (Figures 2B and 3B). The results presented in Figures 2 and 3 show differences between individual absolute values for both caspase 3 activity and cell concentration. Because the results were obtained in two independent experimental series, different measured values reflect interexperimental variation. To determine whether the effect seen with DNR was the result of increased pGP activity, VPL was added. Under acidic conditions, caspase 3 activity was significantly increased compared to DNR treatment alone (Figure 3A). A higher caspase 3 activity (indicating a higher apoptosis rate) was also evident in the lower number of surviving tumor cells (Figure 3B).
Figure 2.
Cytotoxic activity of DNR (10 µM) and cisplatin (150 µM) measured by (A) the drug-induced caspase 3 activity and (B) the repopulation of tumor cells 42 hours after chemotherapy under acidic (pH 6.6) and control (pH 7.4) conditions. Values are expressed as mean ± SEM of five experiments. *P < .05.
Figure 3.
Cytotoxic activity of DNR (10 µM) measured by (A) the DNR-induced caspase 3 activity and (B) the repopulation of tumor cells 42 hours after chemotherapy under acidic (pH 6.6) and control (pH 7.4) conditions. In addition, the impact of pGP inhibition by VPL (10 µM) on DNR cytotoxicity under these conditions is shown. Values are expressed as mean ± SEM of five experiments. *P < .05.
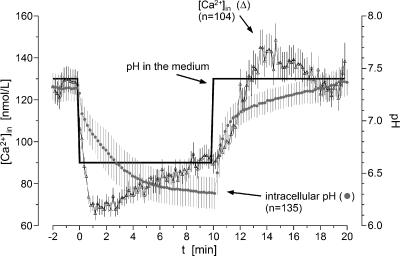
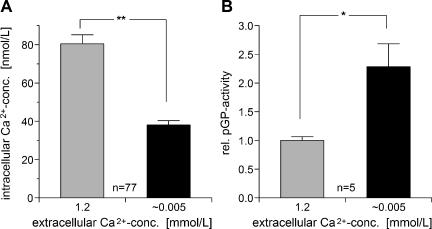
To study the underlying mechanisms by which extracellular pH may influence pGP activity, changes in intracellular pH and Ca2+ concentration were measured (Figure 4). On exposure of the cells to an acidic medium (i.e., a decline in extracellular pH from 7.4 to 6.6), intracellular pH fell to 6.3 within 10 minutes. After the return of the medium back to pH 7.4, intracellular pH also returned to control values. Intracellular Ca2+ concentration also changed markedly when the extracellular pH was lowered. Within the first minute, [Ca2+]in declined by approximately 45% of the initial value and recovered partially by 10% within 10 minutes. When the pH of the medium returned to normal, the Ca2+ concentration also increased, returning to control levels within a few minutes (Figure 4). Similar changes in intracellular Ca2+ concentration can also be obtained by reducing extracellular Ca2+ from its control level (1.2 mM) to low values (0.005 mM), whereby intracellular Ca2+ concentration was reduced by approximately 50% (Figure 5A). Under these environmental conditions, relative pGP activity was more than doubled (Figure 5B) and is comparable to levels obtained under an acidic extracellular environment (Figure 1A). These results indicate that intracellular Ca2+ level may play an important role in the regulation of pGP activity. Under acidic conditions, [Ca2+]in is markedly reduced so that a subsequent increase in pGP activity is observed.
Figure 4.
Time course of intracellular pH (●) and intracellular Ca2+ concentration (Δ) after a rapid change of extracellular pH in the medium. Values are expressed as mean ± SEM (n, number of cells investigated).
Figure 5.
Impact of extracellular Ca2+ concentration on (A) intracellular Ca2+ concentration [Ca2+]in (n, number of cells investigated) and (B) on the activity of pGP (described by the ratio of rhodamine-123 efflux rates in the presence or absence of VPL, respectively; n, number of experiments). Values are expressed as mean ± SEM. *P < .05; **P < .001.
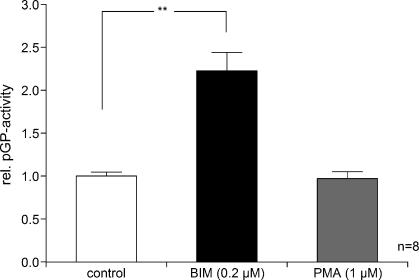
One possible mechanism by which intracellular Ca2+ concentration may affect pGP-mediated drug efflux is through an alteration of protein kinase C (PKC) activity. To test this hypothesis, the impact of the inhibition or activation of PKC on pGP activity was assessed. Inhibition of the PKC by bisindolylmaleimide I (BIM) led to doubling of the pGP-mediated rhodamine-123 efflux, whereas activation of the PKC by phorbol 12-myristate 13-acetate (PMA) practically had no effect (Figure 6).
Figure 6.
Impact of PKC inhibition by BIM (0.2 µM) or activation by PMA (1 µM) on the activity ofpGP (described by the ratio of rhodamine-123 efflux rates in the presence or absence of VPL). Values are expressed as mean ± SEM. *P < .05; n, number of experiments.
Discussion
The impact of an acidic extracellular environment on the drug transport rate of pGP and the consequences of such conditions on the cytotoxic efficacy of chemotherapeutic drugs were investigated in this study. For this, R3327-AT1 prostate carcinoma was used, a cell line that shows a strong expression of pGP, predominantly on the cell membrane [32], indicating that pGP in this cell line is functional [26,32]. These cells were exposed to an extracellular pH of 6.6, a value that has been reported to prevail in many human tumors [1]. As a measure of transport activity, rhodamine-123 efflux was measured at various times during incubation in an acidic culture medium. A significant increase in transport rate (a factor of 2–2.5) was observed when cells were exposed to an acidic environment for 3 to 6 hours (Figure 1A). Thereafter, pGP activity returned to control levels most probably as a result of cellular damage incurred by low pH. After 24 hours of incubation under acidic conditions, many cells became detached from the Petri dish. In vivo, tumor oxygenation shows pronounced temporal fluctuations due to temporal changes in tumor blood flow [33]. This results in periods during which the extracellular space becomes acidic, which alternate with periods during which a normal pH prevails. For this reason, an elevation of pGP activity over even longer times can be expected in vivo.
The higher efflux rate of pGP under acidic conditions leads to a lower concentration of chemotherapeutic drugs within the cell, as indicated by a higher fraction of intracellular rhodamine-123 concentration in the presence or absence of VPL (Table 1). Because cytotoxic efficacy is usually directly correlated with intracellular drug concentration, a reduction in the efficacy of chemotherapeutic agents that are substrates of pGP is to be expected under acidic conditions. In the present study, DNR-induced cytotoxicity was found to be significantly reduced after cell incubation at pH 6.6, whereas the cytotoxicity of cisplatin, which is not a pGP substrate, was affected only to a minor extent (Figure 2A). The overall cell kill with cisplatin was independent of extracellular pH (Figure 2B). Herman etal. [34] found that, with decreasing pH (from 7.4 to 6.45), cisplatin-induced cytotoxicity was markedly increased in EMT6 mouse mammary carcinoma cells. One possible explanation for the discrepancy between the latter and the present study may be differences in the sensitivity of the cell lines to cisplatin. A further reason could be the high cisplatin dose used in the present study, which may induce an almost maximal cytotoxic effect that cannot be further amplified by low pH. However, in contrast to experiments with DNR, a reduction of cell kill was not observed.
When DNR treatment was combined with VPL (a known inhibitor of pGP), the cytotoxic effect under acidic conditions was found to be restored almost to that of nonacidic cells (Figure 3). These results support the notion of an increased pGP activity being responsible for the reduced cytotoxicity of DNR at acidic pH. The lack of impact of VPL on DNR cytotoxicity under nonacidic control conditions (Figure 3) might be due to the fact that, at normal pH, DNR leads to an almost maximal caspase 3 activation (possibly as a result of an already low pGP activity), which in turn results in a cell kill that cannot be further enhanced by pGP inhibition.
Because anthracyclines are eliminated from the cells by other transporters such as multidrug resistance-associated protein 1 (MRP1), the observed effects of the acidic environment may be the result of altered MRP1 activity or expression. To measure MRP1 activity, a similar efflux study was performed as described for pGP. In this case, however, calcein (0.1 µM) was used as a fluorescent MRP1 substrate, and the transporter was inhibited by probenecid (2 mM). The activity of the MRP1 was, however, found to be only marginally increased (by a factor of 1.4 ± 0.2) when the cells were incubated at an acidic pH for 3 to 6 hours (data not shown). These results indicated that modulation of pGP activity plays a much more important role in the effect of acidosis on DNR cytotoxicity than modulation of MRP1 activity.
A higher transport rate could be: 1) due to an increase in cellular pGP expression; 2) a higher activity of pre-existing transporters; or 3) a translocation of preformed transporters to the outer cell membrane (which might be the case with pGP) [35,36]. Several parameters causing changes in the tumor micromilieu, such as hyperthermia, glucose depletion, reactive oxygen species, or hypoxia, have also been shown to cause changes in pGP expression (with hypoxia possibly resulting from an upregulation of the hypoxia-inducible factor HIF-1) [23–25,37,38]. In addition, exposure to chemotherapeutic drugs that are inhibitors of pGP or MRP1 (e.g., VPL, rifampicin) has been shown to induce pGP expression [39]. Finally, cytokines (such as IL-6, IL-1β, or TNF-α) have been shown to modulate pGP expression [21]. Even so, the acidic extracellular pH applied in the present study had almost no effect on cellular pGP concentration (Figure 1B), indicating that a higher transport rate seems to be predominantly the result of increased pump activity. Functional modulation of pGP activity by metabolic parameters has been discussed by other groups. Because the ABC transporter actively pumps substrates out of the cell, activity may depend on the cellular ATP level, which serves as an energy source. Because many solid-growing tumors show pronounced hypoxia, tumor cells have to switch to the less effective metabolic pathway of glycolysis, which results in a reduced yield of ATP [1,40]. The inhibition of glycolysis has been proposed as a possible therapeutic strategy to overcome drug resistance. Indeed, it has been shown that inhibition of mitochondrial respiration leads to an increase in the cytotoxic efficacy of various chemotherapeutic drugs probably due to a reduced pGP activity, which in turn can be attributed to a depletion of ATP [41]. In the present study, where cells were exposed to acidic conditions under normoxia, glucose concentration in the medium was high even after 24 hours of incubation (9.31 ± 0.15 mM; data not shown). For this reason, depletion of ATP and subsequent inhibition of pGP activity are not to be expected over the time span of the experiment. In contrast, the rhodamine-123 efflux rate was more than doubled when the cells were incubated at pH 6.6 (Figure 1A).
Besides microenvironmental factors, cytokines have also been found to modulate pGP function. In a chemoresistant cell line (MCF-7/AdrR) functionally expressing pGP, Yang et al. [22] found that EGF increased pGP activity within 30 minutes, probably by phospholipase C activation. However, pGP phosphorylation by PKC has also been discussed as a possibility of modulating pGP activity at a posttranslational level [42,43]. Tsuruoka et al. [44] reported that activation of PKC by PMA can reduce the pGP-mediated drug efflux rate. In the present study, PMA had almost no effect on rhodamine-123 efflux, whereas the inhibition of PKC by BIM led to a doubling of transport rate (Figure 6). PKC thus obviously appears to play a role in the regulation of pGP activity. PKC inhibition increases transport rate, whereas PKC activation reduces it. The question arises as to whether extracellular pH is able to modulate PKC activity and thereby exert its influence on the pGP transport rate. Various membrane receptors [e.g., ovarian cancer G-protein-coupled receptor 1 (OGR1) [45] or T-cell death-associated gene 8 (TDAG8) [46]] have been shown to be capable of acting as membrane-located proton sensors that use inositol phosphate as a second messenger. Through these receptors, extracellular pH can directly affect intracellular signaling pathways. Because such a direct pH-sensing mechanism has not been described for PKC, other possible signaling pathways also need to be considered. Several PKC isoforms are Ca2+-dependent, so that one possibility would be a change in the intracellular free calcium concentration ([Ca2+]in). In the present study, [Ca2+]in was found to be reduced by approximately 35% when cells were exposed to an acidic medium (Figure 4). A comparable reduction in intracellular calcium can be obtained by reducing the extracellular Ca2+ concentration to approximately 5 µM (Figure 5A). When this had been done (pH 7.4, extracellular Ca2+ = 5 µM), pGP activity was more than doubled, revealing the important role that intracellular calcium concentration may play in the regulation of the pGP transport rate. Low intracellular Ca2+ levels that have been found in chemoresistant cell lines may also be indicative of the impact of calcium in this context [47,48]. Grabowski et al. [49] described the accumulation of etoposide in pGP-overexpressing cells as being independent of intracellular free calcium levels. However, the study only looked at the effects of transient calcium changes and their blockage using a BAPTA-AM pretreatment, whereas the impact of basal concentration was not examined.
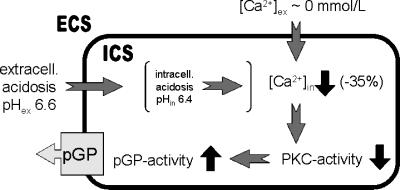
From the findings of the present study, it seems probable that intracellular Ca2+ concentration affects the pGP transport rate, possibly through an alteration of PKC activity. Based on our findings, we propose a model of mechanisms by which an acidic extracellular environment may affect pGP activity (Figure 7): extracellular acidosis leads to a decrease of intracellular calcium concentration by approximately 35%. This decrease is comparable to that found when extracellular calcium level is reduced to almost 0 mM. As a result of reduced intracellular Ca2+, PKC activity will be reduced, leading in turn to an increase in pGP activity. These mechanisms may explain the decrease of DNR-induced cytotoxicity, which can be restored by VPL through pGP inhibition (Figure 3).
Figure 7.
Model of the mechanisms by which an acidic extracellular microenvironment may affect pGP activity. ECS, extracellular space; ICS, intracellular space (for details, see text).
Nevertheless, the question as to how extracellular pH can influence intracellular Ca2+ level has not been answered conclusively in this study. In Figure 4, intracellular pH can be seen to decrease within 5 to 10 minutes following a lowering of extracellular pH. Such intracellular acidosis has not been described in vivo. Several studies have demonstrated that the intracellular pH of solid-growing tumors remains almost constant even if the extracellular pH becomes strongly acidic (for a review, see Ref. [1]). In cell culture experiments, however, a decrease in cellular pH parallel to the medium pH similar to that found in the present study has been described [50,51]. The discrepancy between in vivo and in vitro findings is still not fully understood. The much larger interstitial space present in the cell culture (compared to solid tissues) represents a much larger H+ pool, which leads to a pronounced H+ influx into the cells that cannot be compensated by the cells' active proton pumps. In solid tissues, the extracellular H+ pool is smaller so that the cells may be capable of maintaining their intracellular pH at a constant level.
Whether intracellular pH plays any role at all in the acidosis-induced decrease of calcium level also needs to be considered. As shown in Figure 4, intracellular free Ca2+ changes occurring in response to an alteration in extracellular pH take place over a time span that is shorter than that required for changes in intracellular pH to be seen. For this reason, it seems unlikely that Ca2+ changes are mediated by alterations in intracellular pH. If intracellular Ca2+ level is independent of intracellular pH (but nevertheless dependent on extracellular H+ level), the mechanism shown in Figure 7 may still be valid in vivo. To test such a hypothesis, however, in vivo experiments would be necessary.
In conclusion, the present study clearly shows that an acidic extracellular microenvironment (which is commonly found in human tumors due to their obligatory glycolytic metabolism) has an impact on the functional activity of pGP. Under these conditions, the pGP transport rate is significantly increased, thus leading to a lower concentration of chemotherapeutic drugs in tumor cells and subsequently to reduced cytotoxic efficacy. These findings may be of significance for clinical oncology because an alleviation of extracellular acidity by supportive treatments could provide a means of increasing the chemosensitivity of tumor cells.
Abbreviations
- BCECF
2′,7′-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein
- BIM
bisindolylmaleimide I
- LDH
lactate dehydrogenase
- MRP1
multidrug resistance-associated protein 1
- pGP
P-glycoprotein
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
Footnotes
This study was supported by the Deutsche Krebshilfe (grant 106774/106906).
References
- 1.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 2.Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biological, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 3.Bush RS, Jenkin RD, Allt WE, Beale FA, Bean H, Dembo AJ, Pringle JF. Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer Suppl. 1978;37:302–306. [PMC free article] [PubMed] [Google Scholar]
- 4.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OCA. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 5.Henderson BW, Fingar VH. Relationship of tumor hypoxia and response to photodynamic treatment in an experimental mouse tumor. Cancer Res. 1987;47:3110–3114. [PubMed] [Google Scholar]
- 6.Liang BC. Effects of hypoxia on drug resistance phenotype and genotype in human glioma cell lines. J Neuro-Oncol. 1996;29:149–155. doi: 10.1007/BF00182138. [DOI] [PubMed] [Google Scholar]
- 7.Sanna K, Rofstad EK. Hypoxia-induced resistance to doxorubicin and methotrexate in human melanoma cell lines in vitro. Int J Cancer. 1994;58:258–262. doi: 10.1002/ijc.2910580219. [DOI] [PubMed] [Google Scholar]
- 8.Teicher BA, Lazo JS, Sartorelli AC. Classification of antidoxorubicinneoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981;41:73–81. [PubMed] [Google Scholar]
- 9.Teicher BA, Holden SA, al Achi A, Herman TS. Classification of antineoplastic treatments by their differential toxicity toward putative oxygenated and hypoxic tumor subpopulations in vivo in the FSaIIC murine fibrosarcoma. Cancer Res. 1990;50:3339–3344. [PubMed] [Google Scholar]
- 10.Grau C, Overgaard J. Effect of etoposide, carmustine, vincristine, 5-fluorouracil, or methotrexate on radiobiologically oxic and hypoxic cells in a C3H mouse mammary carcinoma in situ. Cancer Chemother Pharmacol. 1992;30:277–280. doi: 10.1007/BF00686295. [DOI] [PubMed] [Google Scholar]
- 11.Durand RE. Keynote address: the influence of microenvironmental factors on the activity of radiation and drugs. Int J Radiat Oncol Biol Phys. 1991;20:253–258. doi: 10.1016/0360-3016(91)90100-i. [DOI] [PubMed] [Google Scholar]
- 12.Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev. 1994;13:139–168. doi: 10.1007/BF00689633. [DOI] [PubMed] [Google Scholar]
- 13.Chaplin DJ, Horsman MR, Trotter MJ, Siemann DW. Therapeutic significance of microenvironmental factors. In: Molls M, Vaupel P, editors. Blood Perfusion and Microenvironment of Human Tumors. Springer: Berlin; 1998. pp. 131–143. [Google Scholar]
- 14.Parkins CS, Chadwick JA, Chaplin DJ. Inhibition of intracellular pH control and relationship to cytotoxicity of chlorambucil and vinblastine. Br J Cancer Suppl. 1996;27:S75–S77. [PMC free article] [PubMed] [Google Scholar]
- 15.Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics: I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol. 2003;66:1207–1218. doi: 10.1016/s0006-2952(03)00467-2. [DOI] [PubMed] [Google Scholar]
- 16.Chang G. Multidrug resistance ABC transporters. FEBS Lett. 2003;555:102–105. doi: 10.1016/s0014-5793(03)01085-8. [DOI] [PubMed] [Google Scholar]
- 17.Higgins CF. ABC transporters: physiology, structure and mechanism—an overview. Res Microbiol. 2001;152:205–210. doi: 10.1016/s0923-2508(01)01193-7. [DOI] [PubMed] [Google Scholar]
- 18.Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–7485. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- 19.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 20.Fojo T, Bates S. Strategies for reversing drug resistance. Oncogene. 2003;22:7512–7523. doi: 10.1038/sj.onc.1206951. [DOI] [PubMed] [Google Scholar]
- 21.Lee G, Piquette-Miller M. Cytokines alter the expression and activity of the multidrug resistance transporters in human hepatoma cell lines; analysis using RT-PCR and cDNA microarrays. J Pharm Sci. 2003;92:2152–2163. doi: 10.1002/jps.10493. [DOI] [PubMed] [Google Scholar]
- 22.Yang JM, Sullivan GF, Hait WN. Regulation of the function of P-glycoprotein by epidermal growth factor through phospholipase C. Biochem Pharmacol. 1997;53:1597–1604. doi: 10.1016/s0006-2952(97)82451-3. [DOI] [PubMed] [Google Scholar]
- 23.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-induciblefactor-1 -dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- 24.Ledoux S, Yang R, Friedlander G, Laouari D. Glucose depletion enhances P-glycoprotein expression in hepatoma cells: role of endoplasmic reticulum stress response. Cancer Res. 2003;63:7284–7290. [PubMed] [Google Scholar]
- 25.Wartenberg M, Gronczynska S, Bekhite MM, Saric T, Niedermeier W, Hescheler J, Sauer H. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular prostate tumor spheroids by hyperthermia and reactive oxygen species. Int J Cancer. 2005;113:229–240. doi: 10.1002/ijc.20596. [DOI] [PubMed] [Google Scholar]
- 26.Siegsmund MJ, Kreukler C, Steidler A, Nebe T, Kohrmann KU, Alken P. Multidrug resistance in androgen-independent growing rat prostate carcinoma cells is mediated by P-glycoprotein. Urol Res. 1997;25:35–41. doi: 10.1007/BF00941904. [DOI] [PubMed] [Google Scholar]
- 27.Weber F, Freudinger R, Schwerdt G, Gekle M. A rapid screening method to test apoptotic synergisms of ochratoxin A with other nephrotoxic substances. Toxicol In Vitro. 2005;19:135–143. doi: 10.1016/j.tiv.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Versteeg HH, Nijhuis E, van den Brink GR, Evertzen M, Pynaert GN, van Deventer SJ, Coffer PJ, Peppelenbosch MP. A new phosphospecific cell-based ELISA for p42/p44 mitogen-activated protein kinase (MAPK), p38 MAPK, protein kinase B and cAMP-response-element-binding protein. Biochem J. 2000;350:717–722. [PMC free article] [PubMed] [Google Scholar]
- 29.Weiner ID, Hamm LL. Use of fluorescent dye BCECF to measure intracellular pH in cortical collecting tubule. Am J Physiol. 1989;256:F957–F964. doi: 10.1152/ajprenal.1989.256.5.F957. [DOI] [PubMed] [Google Scholar]
- 30.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescent properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 31.Schwerdt G, Freudinger R, Schuster C, Silbernagl S, Gekle M. Inhibition of mitochondria prevents cell death in kidney epithelial cells by intra- and extracellular acidification. Kidney Int. 2003;63:1725–1735. doi: 10.1046/j.1523-1755.2003.00934.x. [DOI] [PubMed] [Google Scholar]
- 32.Batra S, Karlsson R, Witt L. Potentiation by estramustine of the cytotoxic effect of vinblastine and doxorubicin in prostatic tumor cells. Int J Cancer. 1996;68:644–649. doi: 10.1002/(SICI)1097-0215(19961127)68:5<644::AID-IJC15>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Chaplin DJ, Hill SA. Temporal heterogeneity in micro-regional erythrocyte flux in experimental solid tumours. Br J Cancer. 1995;71:1210–1213. doi: 10.1038/bjc.1995.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herman TS, Teicher BA, Collins LS. Effect of hypoxia and acidosis on the cytotoxicity of four platinum complexes at normal and hyperthermic temperatures. Cancer Res. 1988;48:2342–2347. [PubMed] [Google Scholar]
- 35.Ferrao P, Sincock P, Cole S, Ashman L. Intracellular P-gp contributes to functional drug efflux and resistance in acute myeloid leukaemia. Leuk Res. 2001;25:395–405. doi: 10.1016/s0145-2126(00)00156-9. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Barroso M, Samanta R, Greenberger L, Sztul E. Experimentally induced changes in the endocytic traffic of P-glycoprotein alter drug resistance of cancer cells. Am J Physiol. 1997;273:C687–C702. doi: 10.1152/ajpcell.1997.273.2.C687. [DOI] [PubMed] [Google Scholar]
- 37.Felix RA, Barrand MA. P-glycoprotein expression in rat brain endothelial cells: evidence for regulation by transient oxidative stress. J Neurochem. 2002;80:64–72. doi: 10.1046/j.0022-3042.2001.00660.x. [DOI] [PubMed] [Google Scholar]
- 38.Wartenberg M, Fischer K, Hescheler J, Sauer H. Redox regulation of P-glycoprotein-mediated multidrug resistance in multicellular prostate tumor spheroids. Int J Cancer. 2000;85:267–274. [PubMed] [Google Scholar]
- 39.Granzotto M, Drigo I, Candussio L, Rosati A, Bartoli F, Giraldi T, Decorti G. Rifampicin and verapamil induce the expression of P-glycoprotein in vivo in Ehrlich ascites tumor cells. Cancer Lett. 2004;205:107–115. doi: 10.1016/j.canlet.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 40.Vaupel P, Schaefer C, Okunieff P. Intracellular acidosis in murine fibrosarcomas coincides with ATP depletion, hypoxia, and high levels of lactate and total Pi. NMR Biomed. 1994;7:128–136. doi: 10.1002/nbm.1940070305. [DOI] [PubMed] [Google Scholar]
- 41.Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65:613–621. [PubMed] [Google Scholar]
- 42.Fine RL, Chambers TC, Sachs CW. P-glycoprotein, multidrug resistance and protein kinase C. Oncologist. 1996;1:261–268. [PubMed] [Google Scholar]
- 43.Chambers TC, Pohl J, Raynor RL, Kuo JF. Identification of specific sites in human P-glycoprotein phosphorylated by protein kinase C. J Biol Chem. 1993;268:4592–4595. [PubMed] [Google Scholar]
- 44.Tsuruoka S, Sugimoto K, Fujimura A, Imai M, Asano Y, Muto S. Protein kinase C and phosphatidylinositol 3-kinase independently contribute to P-glycoprotein-mediated drug secretion in the mouse proximal tubule. Pflugers Arch. 2001;442:321–328. doi: 10.1007/s004240100542. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 46.Ishii S, Kihara Y, Shimizu T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J Biol Chem. 2005;280:9083–9087. doi: 10.1074/jbc.M407832200. [DOI] [PubMed] [Google Scholar]
- 47.Chen JS, Agarwal N, Mehta K. Multidrug-resistant MCF-7 breast cancer cells contain deficient intracellular calcium pools. Breast Cancer Res Treat. 2002;71:237–247. doi: 10.1023/a:1014461832403. [DOI] [PubMed] [Google Scholar]
- 48.Liang X, Huang Y. Intracellularfree calcium concentration and cisplatin resistance in human lung adenocarcinoma A549 cells. Biosci Rep. 2000;20:129–138. doi: 10.1023/a:1005530501137. [DOI] [PubMed] [Google Scholar]
- 49.Grabowski DR, Dubyak GR, Rybicki L, Hidaka H, Ganapathi R. Tumor cell resistance to topoisomerase II poisons: role for intracellular free calcium in the sensitization by inhibitors or calcium-calmodulin-dependent enzymes. Biochem Pharmacol. 1998;56:345–349. doi: 10.1016/s0006-2952(98)00159-2. [DOI] [PubMed] [Google Scholar]
- 50.Glunde K, Düßmann H, Juretschke HP, Leibfritz D. Na+/H+ exchange subtype 1 inhibition during extracellular acidification and hypoxia in glioma cells. J Neurochem. 2002;80:36–44. doi: 10.1046/j.0022-3042.2001.00661.x. [DOI] [PubMed] [Google Scholar]
- 51.Wahl ML, Owen JA, Burd R, Herlands RA, Nogami SS, Rodeck U, Berd D, Leeper DB, Owen CS. Regulation of intracellular pH in human melanoma: potential therapeutic implications. Mol Cancer Ther. 2002;1:617–628. [PubMed] [Google Scholar]