Abstract
The c-KIT proto-oncogene has been implicated in the pathogenesis of several neoplastic diseases, including gastrointestinal stromal tumors and mastocytosis in humans, and mast cell tumors (MCTs) in canines. Cutaneous MCTs are common neoplasms in dogs and have a variable biologic behavior. The goal of this study was to define the prognostic significance of c-KIT mutations identified in canine MCTs and the associations between c-KIT mutations, KIT localization, and KIT expression levels. Microdissection and polymerase chain reaction were performed on 60 MCTs to identify c-KIT mutations. Anti-KIT antibodies were used for immunohistochemical evaluation of KIT localization. Forty-two MCTs were included in a tissue microarray, and KIT expression was quantified using immunofluorescence. Canine MCTs with c-KIT mutations were significantly associated with an increased incidence of recurrent disease and death. c-KIT mutations were also significantly associated with aberrant protein localization; however, the level of KIT expression did not correlate with either c-KIT mutations or changes in protein localization. Considering the high prevalence of canine MCTs and the central role of c-KIT in the tumorigenesis of certain tumors, canine MCTs are an excellent model for characterizing the role of c-KIT in neoplastic diseases and is a potential target for novel therapeutic agents in clinical trials.
Keywords: c-KIT, KIT, mutation, animal model, canine
Introduction
The c-KIT proto-oncogene encodes the receptor tyrosine kinase KIT, which consists of an extracellular ligand binding domain composed of five immunoglobulin-like loops, a transmembrane domain, a negative regulatory juxtamembrane domain, and a split cytoplasmic kinase domain [1–3]. The ligand for KIT is stem cell factor, which is also known as steel factor, KIT ligand, or mast cell growth factor [4–7]. The receptor tyrosine kinase KIT is expressed by multiple cell types, including hematopoietic progenitor cells, germ cells, interstitial cells of Cajal, melanocytes, and mast cells, where it has been associated with cell survival, proliferation, and differentiation [8–14]. In addition to these functions, in mast cells, KIT has been shown to be important for fibronectin adhesion, chemotaxis, and degranulation [5,15–19].
Recently, c-KIT has been implicated in the pathogenesis of multiple human neoplastic diseases. c-KIT mutations, which lead to a constitutively activated KIT product in the absence of ligand, have been identified in the juxtamembrane domain of gastrointestinal stromal tumors in humans [20] and in the kinase domain at codon 816 of human mastocytosis patients [21–23]. Additionally, aberrant KIT expression is increasingly being identified in multiple neoplasms, including small cell lung cancer, prostate cancer, and acute myeloblastic leukemia [24–29]. The significance of this aberrant expression has been determined for some of these cancers, such as small cell lung cancer, where autocrine and paracrine signaling loops have been identified [24,26], and in prostate cancer, where truncated isoforms of KIT that signal through phospholipase C-γ1 have been characterized [27,30]. However, for several other cancers, the significance of this aberrant expression has not been elucidated.
Activating c-KIT mutations [31–34] and aberrant KIT expression has also been described in canine cutaneous mast cell tumors (MCTs) [35–39], therefore implicating c-KIT in their pathogenesis. Unlike mastocytosis in humans, which is a rather rare condition and usually has a positive prognosis [40–42], canine cutaneous MCTs are one of the most common neoplastic diseases in dogs (accounting for 7–21% of all cutaneous neoplasms) [43–46] and have an extremely variable biologic behavior ranging from a benign mass to a fatal metastatic disease [34,47,48]. Canine cutaneous MCTs commonly present as a solitary neoplastic mass in the skin and/or subcutaneous tissue of older dogs, with mean age of onset of approximately 9 years of age. There is no reported sex predilection [49,50]. All breeds of dogs are affected by MCTs, but several breeds, such as the boxer, Boston terrier, bulldog, Weimaraner, and Labrador retriever, have been suggested to have an increased incidence of the disease [45,51]. Prognostic and therapeutic determinations for canine cutaneous MCTs are commonly based on histologic grading. Several histologic grading systems have been developed for the evaluation of canine cutaneous MCTs [47,48]. The most commonly used system is that proposed by Patnaik et al. [48], which defines grade 1 MCTs as being well-differentiated tumors with good prognosis, grade 3 MCTs as being poorly differentiated tumors with poor prognosis, and grade 2 MCTs as being of intermediate differentiation with intermediate prognosis.
c-KIT mutations have been identified in the juxtamembrane domain, primarily in exon 11, of canine MCTs and consist of internal tandem duplications (ITDs) and deletions [31–34,52–55]. ITD c-KIT mutations were identified in 9% of canine MCTs in one study that looked at the mutation status of 88 randomly selected MCTs [33], but these mutations may occur in as many as 30% to 50% of all intermediate- to high-grade MCTs [53]. All, except for one, of the previously described ITDs are in-frame duplications that range from approximately 39 to 69 bp in size [31–34,53–55], and all of the mutations that have been characterized thus far produce a constitutively activated form of KIT in the absence of ligand [31,32,54]. Previous work by our laboratory has shown that c-KIT mutations are significantly associated with histologically higher-grade canine MCTs [33]. Recently, our laboratory has also shown that increased cytoplasmic localization of KIT in canine MCTs is significantly associated with a decreased survival duration and disease-free interval as compared to MCTs with perimembrane KIT localization [39].
The goal of this study was to define the prognostic significance of c-KIT mutations, and the associations between c-KIT mutations, KIT localization, and KIT expression levels in canine MCTs. Mutations in c-KIT's juxtamembrane domain were identified in 15% of the MCTs examined, using laser capture microdissection (LCM) and polymerase chain reaction (PCR) amplification. This is the first study to show that c-KIT mutations in canine MCTs are significantly associated with decreased disease-free and overall survival, and that a significant relationship between KIT protein localization and the presence of c-KIT mutations exists in canine MCTs. These data clearly implicate an important role of c-KIT in the progression of canine cutaneous MCTs. Considering the high prevalence of MCTs in dogs and the central role c-KIT appears to play in the tumorigenesis of many canine MCTs, canine cutaneous MCTs provide an excellent spontaneous in vivo model for studying the molecular biology of c-KIT in human and animal neoplastic diseases. Furthermore, canine cutaneous MCTs are an excellent model for the treatment of cancers that are driven by c-KIT and can be used in clinical trials for testing chemotherapeutics aimed at targeting the c-KIT proto-oncogene.
Materials and Methods
Case Selection, Tissue Samples, and Survival Data
Sixty canine cutaneous MCTs from 60 different dogs submitted to the Michigan State University's Diagnostic Center for Population and Animal Health between 1998 and 2001 were included in this study. Cases were included in this study solely based on the meeting of all inclusion criteria. Inclusion criteria for this study were as follows: 1) all cases were previously diagnosed as canine cutaneous MCT (the diagnosis of canine cutaneous MCT and the histologic grade of each tumor were confirmed by a veterinary pathologist); 2) all cases were treated with surgical excision as the only primary treatment modality (i.e., no chemotherapy or radiation therapy was used); 3) complete follow-up data from the referring veterinarian were available; and 4) adequate formalin-fixed paraffin-embedded tissues for DNA extraction and immunohistochemistry were available. Complete follow-up data for each case included age, sex, breed, weight, number of masses, location of mass, time before excision, medication at the time of surgery, diagnostic tests performed, recurrence, tumor margins, metastasis, survival time, and cause of death. Histologic grading of canine MCTs was performed in conjunction with a multi-institutional review of the current histologic grading system for canine cutaneous MCTs, in which 31 pathologists participated in the histologic grading of 95 canine MCTs [56]. Histologic grades represent a consensus of those results.
LCM and DNA Extraction
LCM was used to isolate neoplastic mast cells for DNA extraction and subsequent PCR amplification of c-KIT exon 11 and intron 11 to identify ITD c-KIT mutations. Five- to 7-µm sections of each formalin-fixed paraffin-embedded MCT were dehydrated and stained with hematoxylin for LCM. A total of 2000 to 4000 neoplastic mast cells was extracted from each tumor sample using the Pixcell LCM system with Macro LCM caps (Arcturus, Mountain View, CA) (Figure 1). Extracted cells that adhered to the Macro LCM caps were incubated overnight in 50 µl of DNA extraction buffer (10 mM Tris, pH 8.0, 1 mM EDTA, and 1% Tween) and 1.5 µl of 15 mg/ml Proteinase K (Roche, Indianapolis, IN) at 37°C. Samples were centrifuged at 4000 rpm for 5 minutes, and Proteinase K was inactivated by heating at 95°C for 8 minutes.
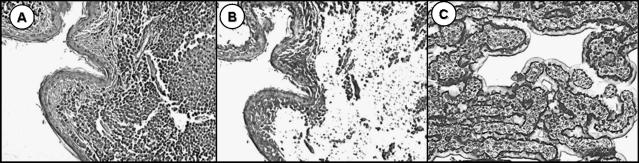
Figure 1.
LCM of neoplastic canine cutaneous MCTs (original magnification, x 10). LCM was performed using archival formalin-fixed paraffin-embedded tissue sections. DNA was extracted from captured cells, and PCR amplification was performed to identify c-KIT mutations. (A) Hematoxylin-stained section of MCT prior to microdissection. (B) Section of MCT following microdissection. (C) Laser capture microdissected cells adhered to cap.
PCR Amplification of c-KIT Exon 11 and Intron 11
PCR amplification was performed using a previously described primer pair that flanks exon 11 and the 5′ end of intron 11 [55], which includes the previously described ITD region of the c-KIT proto-oncogene in canine MCTs [31–34,53–55]. PCRs were prepared in a 25-µl total reaction volume, with 5 µl of LCM-extracted DNA, 5 pmol of each primer, 0.5 U of Taq polymerase (Invitrogen, Carlsbad, CA), and final concentrations of 80 µM deoxynucleoside triphosphate, 2 mM MgCl2, 20 mM Tris-HCl, and 50 µl of KCl. Cycling conditions were as follows: 94°C for 4 minutes; 35 to 45 cycles at 94°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute; 72°C for 5 minutes. Amplified products and ITD mutations were visualized by agarose gel electrophoresis on a 2% agarose gel after ethidium bromide staining (Figure 2).
Figure 2.
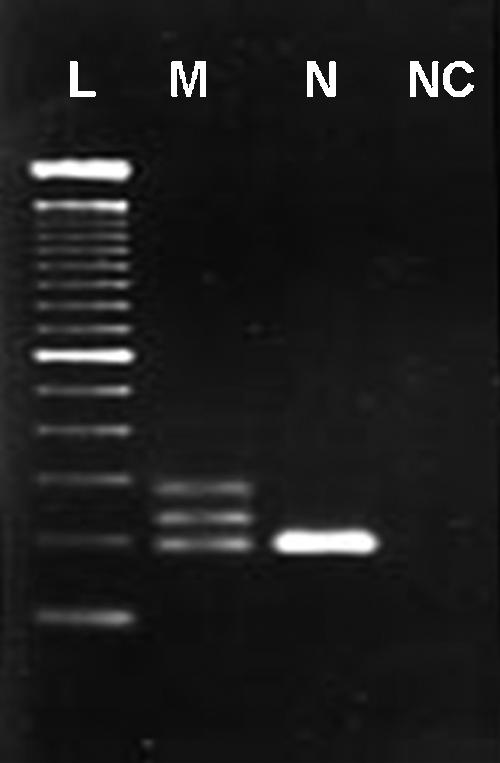
A 2% agarose gel of PCR-amplified c-KIT exon 11 and intron 11 from LCM-extracted DNA from canine MCTs. L: 100-bp ladder; M: heterozygous for normal allele (191 bp) and mutant allele (250 bp), with an upper band representing heterodimerization of normal and mutant alleles; N: 191-bp homozygous normal allele; NC: negative control (no template).
DNA Sequencing
Mutant c-KIT alleles were identified by agarose gel electrophoresis, and DNA fragments were excised for DNA purification. DNA was purified using the Qiaex II gel purification kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. DNA fragments were subcloned into Topo vectors using the Topo cloning kit (Invitrogen) and were subsequently chemically transformed into competent Escherichia coli cells according to the manufacturer's protocol. c-KIT clones were sequenced either through an automated sequencing technique using fluorescently labeled dideoxynucleotides with capillary electrophoresis and detection using an ABI sequence analyzer (Foster City, CA) at Michigan State University's Genomics Technology Support Facility, or by manually sequencing with a Thermo Sequenase Radio-labeled Terminator Cycle Sequencing kit (USB Corporation, Cleveland, OH) and 33P-labeled dideoxynucleotide triphosphates according to the manufacturer's protocol, followed by 48 to 72 hours of exposure to Biomax MR Scientific Imaging Film (Kodak, Rochester, NY).
Immunohistochemistry
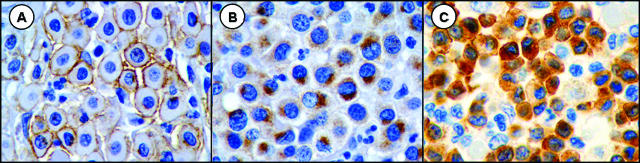
Tissue sections of canine cutaneous MCTs were used for the immunohistochemical evaluation of KIT protein localization, as previously described [39]. In brief, 5-µm sections of formalin-fixed paraffin-embedded tissue were deparaffinized in xylene, rehydrated in graded ethanol, and rinsed in distilled water. Endogenous peroxidase was neutralized with 3% hydrogen peroxide for 5 minutes. Antigen retrieval was achieved by incubating slides in a citric buffer antigen retrieval solution (Dako, Carpinteria, CA) in a steamer (Black and Decker, Towson, MD) for 20 minutes, and nonspecific immunoglobulin binding was blocked by incubation of slides for 10 minutes with a protein-blocking agent (Dako). Using an autostainer, slides were incubated for 30 minutes with a rabbit anti-human c-KIT antibody (Dako) at a dilution of 1:100. A streptavidin immunoperoxidase staining procedure (Dako) was used for immunolabeling. The immunoreaction was visualized with 3,3′-diaminobenzidine substrate (Dako). Sections were counterstained with Mayer's hematoxylin. Positive and negative immunohistochemical controls were included in each run. Known canine MCTs were used as positive controls. Negative controls were canine MCTs that were treated identically as routine sections, except that the 30-minute incubation with primary antibodies was replaced with a 30-minute incubation with the buffer. KIT staining patterns and protein localization for each MCT were characterized as being perimembrane (KIT staining pattern I), focal or stippled cytoplasmic (KIT staining pattern II), or diffuse cytoplasmic protein localization (KIT staining pattern III), as previously described [39] (Figure 3). The evaluation of KIT protein localization was performed by a single investigator (J.D.W.) to eliminate interobserver variability.
Figure 3.
Sections of canine cutaneous MCTs (skin) stained with anti-KIT antibodies and counterstained with hematoxylin (original magnification, x 100, oil) representing three patterns of KIT localization identified in neoplastic canine mast cells. (A) KIT staining pattern I, consisting of perimembrane protein localization. (B) KIT staining pattern II, consisting of focal to stippled cytoplasmic staining. (C) KIT staining pattern III, consisting of diffuse cytoplasmic staining.
Tissue Microarray and Immunofluorescence
One-millimeter cores that were microscopically selected to be representative of each tumor were taken from paraffin-embedded MCT tissue blocks and were placed in a common recipient paraffin block. MCTs included in the tissue array were chosen based on the availability of tissues for transfer to the recipient block. This resulted in 42 MCT samples from 42 cases being represented on the tissue microarray. The recipient block was subsequently heated at 37°C for approximately 1 hour to create a cohesive block. The Five-Micron sections were cut and deparaffinized in xylene, and subsequently dehydrated in graded alcohol with a final rinse in distilled water. Twenty-minute steam retrieval in a citric buffer solution (Dako) was used for antigen retrieval. Non-specific antibody binding was performed with 5% donkey serum with blocking buffer. Slides were incubated with primary rabbit anti-human c-KIT (Dako) antibodies at a dilution of 1:100 overnight in a humidity chamber at 4°C. Sections were then incubated with Cy-3-labeled secondary antibodies, and nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Mean immunofluorescence was quantified for each tumor sample using a Perkin Elmer Scan Array (Perkin Elmer, Wellesley, MA).
Statistics
Univariable analyses. Before developing multivariable models, each risk factor was evaluated for its association with MCT outcomes. Univariable proportional hazards models were developed for each risk factor for each outcome, and the level of association was assessed through the risk factors' P value in the model. Risk factors with P % .20 were considered for inclusion in the multivariable model, which included the two variables c-KIT mutation status and KIT staining pattern.
Multivariable logistic regression models. Logistic regression models were developed for the occurrence of outcomes associated with MCTs, including recurrence of local MCTs, recurrence of distant MCTs, and death associated with MCTs. In addition to risk factors of interest, animal signalment (age, sex, and weight) were included in the multivariable model to account for their effects on model outcome. Results were reported as odds ratio (OR): OR < 1 means that the likelihood of the occurrence of an event is reduced, whereas OR > 1 indicates that the likelihood of an event is increased. OR = 1 indicates that the risk factor neither increases or decreases the likelihood of the outcome.
Multivariable survival analysis models. This study used the Cox proportional hazards models (SAS PROC PHREG) (SAS Version 9.13; SAS Institute, Inc., Cary, NC) for survival analysis, using survival times (time-to-event) as the model outcome, and produced point estimates of the hazard ratio (HR; risk ratio) for risk factors in the model. Proportional hazards regression models were developed for the survival analysis of different outcomes associated with MCTs. These outcomes were days to recurrence of local MCTs, days to recurrence of distant MCTs, and days to death resulting from MCT. In addition to risk factors of interest, animal signalment (age, sex, and weight) was included in the multivariable model to account for their effects on model outcome. The effects of risk factors on days to events were reported as HRs. Comparable to OR, HR < 1 indicates that the risk factor increases time to outcome, whereas HR > 1 indicates that the risk factor decreases time to outcome.
Associations between c-KIT mutation status and KIT staining patterns were tested using Mantel-Hanzel chi-square analysis. Associations between c-KIT mutation status and mean immunofluorescence, and between KIT staining patterns and mean immunofluorescence were tested using Wilcoxon rank sum tests.
Results
Study Population
Sixty canine cutaneous MCTs from 60 dogs that met the inclusion criteria were included in this study. The age of these dogs ranged from 2 to 14 years, with a mean age of 7.84 years. Thirty-six dogs were females and 24 dogs were males. A total of 19 different breeds was represented by the study population. There were 13 mixed-breed dogs, 12 Labrador retrievers, 10 boxers, 6 golden retrievers, 3 pugs, 2 bassett hounds, 2 springer spaniels, and 12 other breeds represented by single dogs. According to the Patnaik histologic grading system for canine MCTs [48], 8 MCTs were grade 1, 45 MCTs were grade 2, and 7 MCTs were grade 3.
c-KIT Mutations in Canine MCTs
DNA fragments representing exon 11 of the c-KIT proto-oncogene were amplified and visualized for each tumor. c-KIT mutations were identified in 9 of 60 MCTs (15%). Mutations in cases 1 to 8 were similar to previously described ITD c-KIT mutations [30–33,52–54]. All of these ITD mutations were in-frame mutations that ranged from 45 to 60 bp in size. In cases 5, 6, 7, and 8, duplications extended by one, two, three, and four nucleotides into intron 11, respectively. The mutation in case 9 was located entirely in intron 11. This mutation was tentatively identified as a duplication based on its banding pattern on agarose gel electrophoresis, but, when sequenced, it was found to consist of a 24-nucleotide poly-T insertion followed by a 15-nucleotide duplication of the sequence preceding the poly-T insertion. Additionally, a G-to-A transition was found in the duplicated sequence that preceded the poly-T insert. Four of the MCTs in which mutations were identified were histologic grade 2 and five were grade 3 (Table 1).
Table 1.
Mutation and Case Description for Cases with ITD c-KIT Mutations.
| Case Number | Duplication Size | Duplication Location | Histologic Grade* | KIT Staining Pattern† | Local Recurrence (months) | Distant Recurrence (months) | MCT-Related Death (months) | Time to Last Follow-Up (If Alive) (months) |
| 1 | 45 | Exon 11 | 3 | 3 | None | None | 0.5 | N/A |
| 2 | 45 | Exon 11 | 2 | 2 | None | None | None | 29.1 |
| 3 | 45 | Exon 11 | 3 | 3 | None | 0.5 | 0.5 | N/A |
| 4 | 45 | Exon 11 | 2 | 2 | 0.5 | 0.5 | 0.5 | N/A |
| 5 | 60 | Exon 11/intron 11 | 3 | 2 | 1 | 1 | 1 | N/A |
| 6 | 54 | Exon 11 /intron 11 | 3 | 3 | 2 | 2 | 3 | N/A |
| 7 | 60 | Exon 11/intron 11 | 3 | 3 | None | 0.6 | 0.6 | N/A |
| 8 | 57 | Exon 11/intron 11 | 2 | 1 | None | None | None | 7.3‡ |
| 9 | 15 | Intron 11§ | 2 | 1 | None | None | None | 20.4 |
Histologic grading was performed based on the Patnaik histologic grading system for canine cutaneous MCTs [48].
KIT staining patterns were classified as described by Webster et al. [39].
Dog 8 died at 7.3 months due to causes unrelated to mast cell disease.
Mutation in dog 9 consisted of a 24-bp poly-T insert with a 15-bp duplication, which was located entirely in intron 11. An additional A-to-G transition was also identified in the duplicated sequence preceding the poly-T insert.
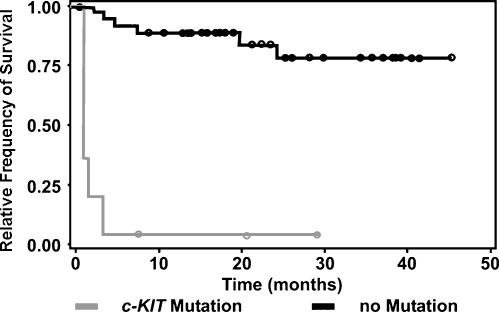
According to multivariable analysis, patients with MCTs containing ITD c-KIT mutations had significantly decreased survival times [P = .0068, HR = 6.23 (1.66–23.4)] and an increased incidence of mortality due to MCT-related disease [P = .0011, OR = 15 (2.95–76.31)] (Figure 4). Additionally, patients with MCTs containing ITD c-KIT mutations were also significantly associated with an increased incidence of recurrence at the original tumor site [P = .0255, OR = 5.4 (1.23–23.75)] and at sites outside of the original tumor margins [P = .0016, OR = 6.13 (1.99–18.92)] and with a decreased disease-free interval both at the site of the original tumor [P = .0157, HR = 5.78 (1.40–23.99)] and at sites outside of the tumor margin [P= .0012, HR = 6.14 (2.06–18.37)].
Figure 4.
Kaplan-Meier survival curve: relative frequency of survival versus time in months for canine cutaneous MCT patients with and without identified c-KIT mutations. The presence of duplication mutation in the c-KIT proto-oncogene was significantly associated with a decreased survival duration [P = .0068, HR = 6.23 (1.66–23.40)].
KIT Protein Localization and c-KIT Mutations
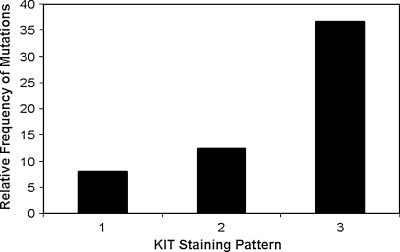
KIT protein localization was examined in each MCT using immunohistochemical staining with anti-KIT antibodies. Twenty-five of the 60 MCTs examined had KIT staining pattern I, which is characterized by perimembrane KIT protein localization, as seen in non-neoplastic (inflammatory) mast cells. Twenty-four of 60 MCTs in this study had KIT staining pattern II, which is characterized by stippled to focal cytoplasmic KIT localization, often with a decrease in perimembrane protein localization; the remaining 11 MCTs had KIT staining pattern III, which is characterized by diffuse cytoplasmic KIT localization. Seven of nine MCTs (77.8%) with ITD c-KIT mutations also had aberrant KIT protein localization (KIT staining pattern II or III). Two of the MCTs with ITD c-KIT mutations had KIT staining pattern I, three cases had KIT staining pattern II, and four cases had KIT staining pattern III. A significant trend was identified between the presence of ITD c-KIT mutations and an increased cytoplasmic localization of KIT (P = .046) (Figure 5), as evidenced by higher KIT staining patterns.
Figure 5.
Correlation between ITD c-KIT mutations and KIT protein localization in canine MCTs. A significant association was found between the presence of c-KIT mutations and the cellular localization of KIT in canine MCTs (P = .046). Seven of nine (77.8%) MCTs with ITD c-KIT mutations had aberrant KIT localization in neoplastic MCTs.
KIT Protein Expression
The tissue microarray representing 42 of 60 samples was used to quantify KIT immunofluorescence. Relationships between immunofluorescence, and c-KIT mutations and KIT protein localization were investigated. No significant relationships were identified (data not shown).
Discussion
The goal of this study was to look at the c-KIT proto-oncogene and its product KIT at both the gene and protein levels to better define the role this gene plays in the pathogenesis of canine cutaneous MCTs. This is the first study to demonstrate a significant association between c-KIT ITD mutations, and an increased rate of recurrent disease and mortality in dogs with canine cutaneous MCTs. Additionally, this is the first study to identify a significant relationship between the presence of ITD c-KIT mutations and the aberrant localization of KIT in canine MCTs. These data document the importance of the c-KIT proto-oncogene in the tumorigenesis of canine cutaneous MCTs and clearly identify the c-KIT proto-oncogene as a potential target for the treatment of canine MCTs.
The c-KIT proto-oncogene was first implicated in the progression of canine cutaneous MCTs when activating mutations were identified in the juxtamembrane domain of c-KIT [31,32]. Following the identification of c-KIT mutations in canine MCTs, work by our laboratory has shown that the presence of c-KIT mutations is significantly associated with higher histologic grade MCTs [33]. The results of this paper further demonstrate the association of c-KIT mutations with higher histologic grade MCTs in dogs. All of the MCTs with c-KIT mutations identified in this study were of histologic grades 2 and 3, whereas no grade 1 MCTs were found to have c-KIT mutations. In this paper, we have further defined the significance of ITD c-KIT mutations in canine MCTs by showing that c-KITITD mutations are significantly associated with an increased incidence of MCT-related death and with an increased occurrence of MCTs at the original or distant cutaneous or extracutaneous locations.
The prognostic value and biologic significance of molecular markers can be confounded by variations in the treatment protocols used in a given study population. To overcome this source of bias, only cases that were treated with surgical excision alone (i.e., no chemotherapy or radiation therapy) were included in this study. This is the only study that has looked at the significance of c-KIT mutations in a population of dogs treated with a single therapeutic protocol.
In this study, ITD c-KIT mutations were found in 15% of the MCTs that were examined. The incidence of ITD c-KIT mutations varied from 9% to 33% in the two previous studies, which consisted of randomly selected and referral high-grade tumors, respectively [33,53]. The predominance of intermediate- and high-grade tumors in the latter study [53] is likely to account for the high incidence of c-KIT mutations in their study population. In the current study, cases were randomly selected and represented the entire spectrum of canine cutaneous MCTs [47,48,51]. Based on the results of this study and previous studies, the true incidence of ITD c-KIT is likely to be between 9% and 15% in all MCTs. However, these mutations may occur in as many as 50% of high-grade canine MCTs [33,53].
Previously, our laboratory has shown that increased cytoplasmic KIT protein localization in neoplastic mast cells is associated with both a decreased disease-free survival and an overall survival of dogs with cutaneous MCTs [39]. In this study, we identified a significant association between the presence of ITD c-KIT mutations and changes in KIT localization in canine cutaneous MCTs. Seven of nine MCTs with c-KIT mutations had aberrant KIT protein localization. Although the significance of this relationship is not currently clear, this may suggest that ITD c-KIT mutations may be responsible for aberrant KIT localization in a subset of canine MCTs. Two cases with c-KIT mutations did not have aberrant KIT localization and remain as outliers to this hypothesis. However, the mutation in one of these MCTs was located within intron 11 only, and therefore could be spliced out during mRNA processing and may not be biologically significant (case 9). It is also important to note that the dog with the intronic c-KIT mutation (case 9) was still alive with no report of local or distant recurrence at 20 months post-surgery. Furthermore, significant statistical relationships between ITD c-KIT mutations and both the incidence of (P = .0052) and time until MCT-related deaths (P = .0267) are preserved when this mutation is not considered as a biologically significant mutation. A potential explanation for the absence of cytoplasmic KIT localization in the other MCT that had an ITD c-KIT mutation may be that this tumor only recently acquired the mutation, and the changes in KIT localization may not have occurred yet at the time of surgical excision. However, ITD c-KIT mutations and changes in KIT localization may represent separate events that occur independent of one another in the progression of canine cutaneous MCTs. This hypothesis is supported by the fact that 28 MCTs included in this study had aberrant KIT localization without the presence of ITD c-KIT mutations. However, these data could also indicate that, in addition to a direct causal relationship between the ITD mutations and aberrant KIT localization, other factors may be responsible for aberrant KIT localization in canine cutaneous MCTs without ITD c-KIT mutations. The primers that were used in this study do not allow for the detection of the previously reported deletions in canine MCTs because the forward primer is located in the region of c-KIT that has been reported to be deleted in a small subset of canine MCTs [32,33]. Therefore, although rare, other c-KIT mutations, such as deletions in the juxtamembrane domain, may be responsible for the aberrant protein localization in those cases in which we did not identify ITD c-KIT mutations. In summary, the correlation between ITD c-KIT mutations and aberrant KIT localization leads to many interesting questions regarding the functional significance of this relationship and the overall functional significance of aberrantly localized KIT when ITD c-KIT mutations are not present. Current work in our laboratory is focused on the further characterization of aberrantly localized KIT and on functional studies to better elucidate the relationship between ITD c-KIT mutations and the aberrant localization of KIT.
No significant relationship was found in this study between the presence of ITD c-KIT mutations or the aberrant localization of KIT and the level of KIT protein expression as measured by mean immunofluorescence in a tissue microarray. These results suggest that constitutive activation of KIT due to ITD mutations or changes in signaling pathways through aberrant KIT localization may be more important in the pathogenesis of canine MCTs than overexpression of KIT and subsequent increases in receptor sensitivity to its ligand. To clarify these observations, these results need to be verified using additional techniques to quantify KIT protein levels in canine MCTs. Additionally, further studies need to be conducted to elucidate the functional significance of aberrantly localized KIT and the effects it has on signaling in neoplastic mast cells.
Spontaneous neoplastic diseases are commonly seen in dogs [43,45] and, in many cases, share morphologic, clinical, and molecular characteristics similar to those of human neoplastic diseases. Therefore, these tumors are an excellent in vivo model of spontaneous neoplasia that may be utilized to better understand the roles of various genes and proteins in the progression of neoplastic diseases, and to serve as model systems for testing the safety and efficacy of novel therapeutic agents [57,58]. Canine cutaneous MCTs are one of the most common neoplasms in dogs and, unlike human mastocytomas, often have an aggressive behavior that can result in death. Due to the high incidence of canine MCTs and the central role that c-KIT plays in MCT tumorigenesis, canine MCTs can serve as an excellent in vivo model for studying its role in the progression of this and other human and animal neoplastic diseases. We propose canine MCTs as a spontaneous in vivo model for clinical trials aimed at determining the safety and efficacy of novel targeted chemotherapeutic agents involving c-KIT signaling pathways.
Acknowledgements
The authors would like to thank the histopathology and immunohistochemistry section at the Diagnostic Center for Population and Animal Health at the Michigan State University, and the Laboratory for Analytical, Cellular, and Molecular Microscopy at the Van Andel Research Institute for their technical assistance.
Abbreviations
- MCT
mast cell tumor
- LCM
laser capture microdissection
- ITD
internal tandem duplication
- HR
hazard ratio
- OR
odds ratio
Footnotes
Funding for this study was provided, in part, by the Companion Animal Fund of the College of Veterinary Medicine, Michigan State University. J.D.W. was funded by a National Institutes of Health T-32 postdoctoral training grant (no. RR17189).
References
- 1.Chan PM, Ilangumaran S, La Rose J, Chakrabartly A, Rottapel R. Autoinhibition of the KIT receptor tyrosine kinase by cystolic juxtamembrane region. Mol Cell Biol. 2003;23:3067–3078. doi: 10.1128/MCB.23.9.3067-3078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yarden Y, Kuang W, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBOJ. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarden Y, Escobedo JA, Kuang WJ, Yang-Feng TL, Daniel TO, Tremble PM, Chen EY, Ando ME, Harkins RN, Francke U, et al. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986;323:226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- 4.Huang E, Nocka K, Beler DR, Chu TY, Buck J, Lahm HW, Wellner D, Leder P, Besmer P. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- 5.Nocka K, Buck J, Levi E, Besmer P. Candidate ligand for the c-kit transmembrane kinase receptor: KL, a fibroblast derived growth factor stimulates mast cells and erythroid progenitors. EMBO J. 1990;9:3287–3294. doi: 10.1002/j.1460-2075.1990.tb07528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zsebo KM, Wypych J, McNiece IK, Lu HS, Smith KA, Karkare SB, Sachdev RK, Yuschenkoff VN, Birkett NC, Williams LR, et al. Identification, purification, and biological characterization of hematopoietic stem cell factor from bull rat liver-conditioned medium. Cell. 1990;63:195–201. doi: 10.1016/0092-8674(90)90300-4. [DOI] [PubMed] [Google Scholar]
- 7.Zsebo KM, Williams DA, Geissler EA, Broudy VC, Martin FH, Atkins HL, Hsu RY, Birkett NC, Okino KH, Murdock DC, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 8.Nocka K, Majumder S, Chabot B, Ray P, Cervone M, Bernstein A, Besmer P. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice—evidence for an impaired c-kit kinase in mutant mice. Genes Dev. 1989;3:816–826. doi: 10.1101/gad.3.6.816. [DOI] [PubMed] [Google Scholar]
- 9.Arber DA, Tamayo R, Weiss LM. Paraffin section detection of the c-kit gene product (CD117) in human tissues: value in the diagnosis of mast cell disorders. Hum Pathol. 1998;29:498–504. doi: 10.1016/s0046-8177(98)90066-1. [DOI] [PubMed] [Google Scholar]
- 10.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa SI. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda R, Takahashi T, Nakamura S, Sekido Y, Nishida K, Seto M, Seito T, Sugiura T, Ariyoshi Y, Takahashi T, et al. Expression of the c-kit protein in human solid tumors and in corresponding fetal and adult normal tissues. Am J Pathol. 1993;142:339–346. [PMC free article] [PubMed] [Google Scholar]
- 12.Natali PG, Nicotra MR, Sures I, Santoro E, Bigotti A, Ullrich A. Expression of c-kit receptor in normal and transformed human nonlymphoid tissues. Cancer Res. 1992;52:6139–6143. [PubMed] [Google Scholar]
- 13.Torihashi S, Ward SM, Nishikawa SI, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electric activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- 14.Tsuura Y, Hiraki H, Watanabe K, Igarashi S, Shimamura K, Fukuda T, Suzuki T, Seito T. Preferential localization of c-kit product in tissue mast cells, basal cells of skin, epithelial cells of breast, small cell lung carcinoma and seminomas/dysgerminoma in human: immunohistochemical study on formalin-fixed, paraffin-embedded tissues. Virchows Arch. 1994;424:135–141. doi: 10.1007/BF00193492. [DOI] [PubMed] [Google Scholar]
- 15.Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, Geissler EN, Galli SJ. Induction of mast cell proliferation, maturation and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci USA. 1991;88:6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yee NS, Paek I, Besmer P. Role of KIT-ligand in proliferation and suppression of apoptosis in mast cells: basis for radiosensitivity of white spotting and steel mutant mice. J Exp Med. 1994;179:1777–1787. doi: 10.1084/jem.179.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dastych J, Metcalfe DD. Stem cell factor induces mast cell adhesion to fibronectin. J Immunol. 1994;152:213–219. [PubMed] [Google Scholar]
- 18.Meininger CJ, Yano H, Rottapel R, Bernstein A, Zsebo KM, Zetter BR. The c-kit receptor ligand functions as a mast cell chemo-attractant. Blood. 1992;79:958–963. [PubMed] [Google Scholar]
- 19.Columbo M, Horowitz EM, Botana LM, MacGlashan DW, Jr, Bochner BS, Gillis S, Zsebo KM, Galli SJ, Lichtenstein LM. The human recombinant c-kit receptor ligand, rhSCF, induces mediator release from human cutaneous mast cells and enhances IgE-dependent mediator release from both skin mast cells and peripheral blood basophils. J Immunol. 1992;149:599–608. [PubMed] [Google Scholar]
- 20.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 21.Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, Sugahara H, Butterfield JH, Ashman LK, Kanayama Y, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, Metcalfe DD. Identification of a point mutation in the catalytic domain of proto-oncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci USA. 1995;92:10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longley BJ, Tyrrell L, Lu SZ, Ma YS, Langley K, Ding T, Duffy T, Jacobs P, Tang LH, Modlin I. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312–314. doi: 10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- 24.Krystal GW, Hines SJ, Organ CP. Autocrine growth of small cell lung cancer mediated by co-expression of c-kit and stem cell factor. Cancer Res. 1996;56:370–376. [PubMed] [Google Scholar]
- 25.Potti A, Moazzam N, Ramar K, Hanekom DS, Kargas S, Koch M. CD117 (c-kit) over expression in patients with extensive-stage small-cell lung carcinoma. Ann Oncol. 2003;14:894–897. doi: 10.1093/annonc/mdg253. [DOI] [PubMed] [Google Scholar]
- 26.Tamborini E, Bonadiman L, Negri T, Bonadiman L, Negri T, Greco A, Staurengo S, Bidoli P, Pastorino U, Pierotti MA, et al. Detection of over-expressed and phosphorylated wild-type KIT receptor in surgical specimens of small cell lung cancer. Clin Cancer Res. 2004;10:8214–8219. doi: 10.1158/1078-0432.CCR-04-1013. [DOI] [PubMed] [Google Scholar]
- 27.Paronetto MP, Farini D, Sammarco I, Maturo G, Vespasiani G, Geremia R, Rossi P, Sette C. Expression of a truncated form of the c-kit tyrosine kinase receptor and activation of src kinase in human prostatic cancer. Am J Pathol. 2004;164:1243–1251. doi: 10.1016/S0002-9440(10)63212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda H, Kanakura Y, Tamaki T, Kuriu A, Kitayama H, Ishikawa J, Kanayama Y, Yonezawa T, Tarui S, Griffin JD. Expression and functional role of the proto-oncogene c-kit in acute myeloblastic leukemia cells. Blood. 1991;78:2962–2968. [PubMed] [Google Scholar]
- 29.Went PT, Dirhofer S, Bundi M, Mirlacher M, Schraml P, Mangialaio S, Dimitrijevic S, Kononen J, Lugli A, Simon R, et al. Prevalence of KIT expression in human tumors. J Clin Oncol. 2004;22:4514–4522. doi: 10.1200/JCO.2004.10.125. [DOI] [PubMed] [Google Scholar]
- 30.Paronetto MP, Venables JP, Elliot DJ, Geremia R, Rossi P, Sette C. TR-KIT promotes the formation of a multi-molecular complex composed by Fyn, PLCγ1 and Sam68. Oncogene. 2003;22:8707–8715. doi: 10.1038/sj.onc.1207016. [DOI] [PubMed] [Google Scholar]
- 31.London CA, Galli SJ, Yuuki T, Hu ZQ, Helfand SC, Geissler EN. Spontaneous canine mast cell tumors express tandem duplications in the proto-oncogene c-KIT. Exp Hematol. 1999;27:689–697. doi: 10.1016/s0301-472x(98)00075-7. [DOI] [PubMed] [Google Scholar]
- 32.Ma Y, Longley BJ, Wang X, Blount JL, Langley K, Caughey GH. Clustering of activating mutations in c-KIT's juxtamembrane coding region in canine mast cell neoplasms. J Invest Dermatol. 1999;112:165–170. doi: 10.1046/j.1523-1747.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- 33.Zemke D, Yamini B, Yuzbasiyan-Gurkan V. Mutations in the juxtamembrane domain of c-KIT are associated with higher grade mast cell tumors in dogs. Vet Pathol. 2002;39:529–535. doi: 10.1354/vp.39-5-529. [DOI] [PubMed] [Google Scholar]
- 34.Zemke D, Yamini B, Yuzbasiyan-Gurkan V. Characterization of an undifferentiated malignancy as a mast cell tumor using mutation analysis in the proto-oncogene c-KIT. J Vet Diagn Invest. 2001;13:341–345. doi: 10.1177/104063870101300411. [DOI] [PubMed] [Google Scholar]
- 35.London CA, Kissenberth WC, Galli SJ, Geissler EN, Helfand SC. Expression of stem cell factor receptor (c-KIT) by malignant mast cells from spontaneous canine mast cell tumours. J Comp Pathol. 1996;113:399–414. doi: 10.1016/s0021-9975(96)80074-0. [DOI] [PubMed] [Google Scholar]
- 36.Morini M, Bettini G, Preziosi R, Mandrioli L. c-KIT gene product (CD117) immunoreactivity in canine and feline paraffin sections. J Histochem Cytochem. 2004;52:705–708. doi: 10.1177/002215540405200515. [DOI] [PubMed] [Google Scholar]
- 37.Preziosi R, Morini M, Sarli G. Expression of the KIT protein (CD117) in primary cutaneous mast cell tumors of the dog. J Vet Diagn Invest. 2004;16:554–561. doi: 10.1177/104063870401600610. [DOI] [PubMed] [Google Scholar]
- 38.Reguera MJ, Rabanal RM, Puigdemont A, Ferrer L. Canine mast cell tumors express stem cell factor receptor. Am J Dermatopathol. 2000;22:49–54. doi: 10.1097/00000372-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Webster JD, Kiupel M, Kaneene JB, Miller R, Yuzbasiyan-Gurkan V. The use of KIT and tryptase expression patterns as prognostic tools for canine cutaneous mast cell tumors. Vet Pathol. 2004;41:371–377. doi: 10.1354/vp.41-4-371. [DOI] [PubMed] [Google Scholar]
- 40.Longley BJ, Duffy TP, Kohn S. The mast cell and mast cell disease. J Am Acad Dermatol. 1995;32:545–561. doi: 10.1016/0190-9622(95)90336-4. [DOI] [PubMed] [Google Scholar]
- 41.Schneider I, Schwartz RA. Mast cell disease. Cutis. 1997;59:63–66. [PubMed] [Google Scholar]
- 42.Longley BJ. What dermatologists need to know about mast cell disease: a dermatopathologist's view. Cutis. 1999;64:281–282. [PubMed] [Google Scholar]
- 43.Brodey RS. Canine and feline neoplasia. Adv Vet Sci Comp Med. 1970;14:309–354. [PubMed] [Google Scholar]
- 44.Finnie JW, Bostock DE. Skin neoplasia in dogs. Aust Vet J. 1979;55:602–604. doi: 10.1111/j.1751-0813.1979.tb07068.x. [DOI] [PubMed] [Google Scholar]
- 45.Preister WA. Skin tumors in domestic animals. Data from 12 United States and Canadian colleges of veterinary medicine. J Natl Cancer Inst. 1973;50:457–466. doi: 10.1093/jnci/50.2.457. [DOI] [PubMed] [Google Scholar]
- 46.Rothwell TLW, Howlett CR, Middleton DJ, Griffiths DA, Duffs BC. Skin neoplasms of dogs in Sydney. Aust Vet J. 1987;64:161–164. doi: 10.1111/j.1751-0813.1987.tb09673.x. [DOI] [PubMed] [Google Scholar]
- 47.Bostock BE. The prognosis following surgical removal of mastocytomas in dogs. J Small Anim Pract. 1973;14:27–40. doi: 10.1111/j.1748-5827.1973.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 48.Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet Pathol. 1984;21:469–474. doi: 10.1177/030098588402100503. [DOI] [PubMed] [Google Scholar]
- 49.Thamm DH, Vail DM. Mast cell tumors. In: Withrow SJ, MacEwen EG, editors. Small Animal Clinical Oncology. Philadelphia, PA: Saunders; 2001. pp. 261–282. [Google Scholar]
- 50.Misdorp W. Mast cells and canine mast cell tumours: a review. Vet Q. 2004;26:156–169. doi: 10.1080/01652176.2004.9695178. [DOI] [PubMed] [Google Scholar]
- 51.Michels GM, Knapp DW, DeNicola DB, Glickman N, Bonney P. Prognosis following surgical excision of canine cutaneous mast cell tumors with histopathologically tumor-free versus nontumor-free margins: a retrospective study of 31 cases. J Am Anim Hosp Assoc. 2002;38:458–466. doi: 10.5326/0380458. [DOI] [PubMed] [Google Scholar]
- 52.Reguera MJ, Ferrer L, Rabanal RM. Evaluation of an intron deletion in the c-KIT gene of canine mast cell tumors. Am J Vet Res. 2002;63:1257–1261. doi: 10.2460/ajvr.2002.63.1257. [DOI] [PubMed] [Google Scholar]
- 53.Downing S, Chien MB, Kaas PH, Moore PE, London CA. Prevalence and importance of internal tandem duplications in exons 11 and 12 of c-KIT in mast cell tumors of dogs. Am J Vet Res. 2002;63:1718–1723. doi: 10.2460/ajvr.2002.63.1718. [DOI] [PubMed] [Google Scholar]
- 54.Pryer NK, Lee LB, Zadovaskaya R, Yu X, Sukbuntherng J, Cherrington JM, London CA. Proof of target for SU11654: inhibition of KIT phosphorylation in canine mast cell tumors. Clin Cancer Res. 2003;9:5729–5734. [PubMed] [Google Scholar]
- 55.Jones CLR, Grahn RA, Chien MB, Lyons LA, London CA. Detection of c-KIT mutations in canine mast cell tumors using fluorescent polyacrylamide gel electrophoresis. J Vet Diagn Invest. 2004;16:95–100. doi: 10.1177/104063870401600201. [DOI] [PubMed] [Google Scholar]
- 56.Kiupel M, Webster JD, Bailey KL, Best S, DeLay J, Detrisac CJ, Gamble D, Ginn PE, Goldschmidt MH, Hendrick MJ, et al. Microscopic grading of canine cutaneous mast cell tumors: a multi-institutional review. Vet Pathol. 2004;41:576–576. [Google Scholar]
- 57.Porrello A, Cardelli P, Spugnini EP. Pet models in cancer research: general principles. J Exp Clin Cancer Res. 2004;23:181–193. [PubMed] [Google Scholar]
- 58.MacEwen EG, Vail DM. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18:781–792. doi: 10.3109/07357900009012210. [DOI] [PubMed] [Google Scholar]