Abstract
GrB/scFvMEL, a fusion protein composed of human granzyme B (GrB) and the single-chain antibody scFvMEL, targets melanoma gp240 antigen and exerts impressive cytotoxic effects by inducing apoptosis. We evaluated the effects of GrB/scFvMEL on chemotherapy, radiation therapy, metastasis in vitro, and the growth of human melanoma A375 xenograft tumors in nude mice. GrB/scFvMEL showed synergistic cytotoxicity when coadministered with doxorubicin, vincristine or cisplatin, and additive effects, in combination with etoposide or cytarabine. Optimal cytotoxic effects were obtained when cells were treated first with GrB/scFvMEL followed by exposure to the agent (rather than the reverse). Pretreatment of A375 cells with GrB/scFvMEL significantly sensitized melanoma cells to ionizing radiation assessed using a clonogenic survival assay. Subtoxic doses of GrB/scFvMEL inhibited the invasion of A375 cells into Matrigel. GrB/scFvMEL (37.5 mg/kg) was administered intravenously to nude mice bearing A375 tumors. Saline-treated tumors increased 24-fold, whereas tumors treated with GrB/scFvMEL showed a significant tumor growth delay increasing four-fold. Tumor tissue displayed an increase in apoptotic nuclei compared to control. Thus, the targeted delivery of GrB to tumors may have a significant potential for cancer treatment. Targeted therapeutic agents specifically designed to impact cellular apoptotic pathways may represent a novel class of therapeutic agents.
Keywords: Granzyme B, single-chain Fv antibody, immunotoxin, apoptosis, melanoma
Introduction
Disseminated melanoma is presently not curable [1] using current clinical tools, and the metastatic spread of melanoma appears to directly involve alterations in cellular apoptotic pathways [2,3]. Traditional chemotherapy is an extremely ineffective means of treating malignant melanoma due to the inherent drug-resistant characteristic of this disease. The reasons for the chemoresistant phenotype in human melanoma are not well understood and are probably multifactorial. However, a growing understanding of the molecular events that mediate tumor growth and metastatic spread has led to the development of rationally designed targeted therapeutics that offer a dual hope of maximizing antitumor efficacy and minimizing toxicity to normal tissues [4]. Approaches to directly modulate cellular apoptosis pathways are of particular interest in terms of new drug developments in melanoma. The activation of apoptotic mechanisms in melanoma cells has been directly implicated in the response of patient tumors to chemotherapy, response to radiotherapy, and in cells' propensity to metastasize [5–8].
The high-molecular-weight melanoma-associated glycoprotein gp240 has previously been demonstrated in a majority (80%) of melanoma cell lines and fresh tumor samples [9]. Antibodies targeting the gp240 antigen, such as ZME-018 and 9.2.27, have been extensively studied in melanoma patients and have demonstrated an impressive ability to localize in metastatic tumors after systemic administration [10–13]. ZME-018 antibody possesses high specificity for melanoma and is minimally reactive with a variety of normal tissues, making it a promising candidate for further study [11,12,14,15].
Recombinant single-chain Fv antibody (scFv)-based agents have been used in preclinical studies for cell-targeted delivery of cytokines [16] and intracellular delivery of highly cytotoxic n-glycosidases such as recombinant gelonin (rGel) toxin [17]. The smaller size of these antibody fragments may allow better penetration into tumor tissues, improved pharmacokinetics, and reduction in the immunogenicity observed with intravenously administered murine antibodies.
Initially, to target melanoma cells, we chose a recombinant single-chain antibody scFvMEL, which retains the same binding affinity and specificity of the parental ZME-018 antibody recognizing the surface domain of the gp240 antigen. The scFvMEL antibody has been used extensively in our laboratory to target gp240-bearing cells in vitro and using xenograft models [13,15,17–21]. This antibody binds to target cells and is efficiently internalized, making this an excellent carrier for the delivery of toxins or other cytotoxic payloads.
Cytotoxic T lymphocyte (CTL) and natural killer (NK) cell types destroy tumor cells by direct physical delivery of human granzyme B (GrB), a serine protease, thereby inducing apoptosis. This is one of the major mechanisms in cellular immune response [22]. CTL granules contain perforin (PFN), a pore-forming protein, and a family of serine proteases termed granzymes. In CTL-mediated cytolysis, PFN is initially released and inserts into the target cell membranes, where it polymerizes to form transmembrane pores [23], which facilitates the access of NK- or CTL-released GrB to the target cell cytoplasm. Once delivered to the cytoplasm, GrB induces apoptosis by directly activating caspases and inducing rapid DNA fragmentation [24]. GrB can cleave many procaspases in vitro including caspases 3, 7, 6, 8, and 9 [25]. Some studies have shown that GrB activated cell death pathways through cleavage of Bid and activation of the mitochondrial death pathway in intact cells [26]. In addition to caspase-mediated cytotoxic events, GrB can also rapidly translocate to the nucleus and cleave poly(ADP-ribose) polymerase (PARP) and nuclear matrix [27], subsequently inducing cell death through caspase-independent pathway.
Because almost all cells contain mechanisms responsible for mediating cell death (apoptosis), we propose that the targeted delivery of GrB protein to the interior of cells will result in cell death through apoptotic mechanisms, assuming that sufficient quantities of active enzyme are successfully delivered to the appropriate subcellular compartment.
We previously described [18] a novel recombinant fusion construct designated as GrB/scFvMEL, composed of human GrB and the anti-gp240 single-chain antibody scFvMEL. This construct was shown to contain enzymatically active GrB, and we demonstrated that the construct specifically bound to human A375-M melanoma cells. In addition, we demonstrated that this agent efficiently delivered GrB to the cytoplasm of melanoma target cells. The cytotoxic effects of the fusion construct on A375-M cells were impressive, and the observed apoptotic effects were shown to be mediated by caspase-dependent and caspase-independent pathways. In the current study, we further investigated the proapoptotic effects of GrB/scFvMEL on different melanoma cell lines, and we examined the impact of targeted apoptosis on the response of tumor cells to chemotherapeutic agents, ionizing radiation, and metastatic potential. In addition, we examined the in vivo antitumor activity of this novel fusion construct against A375 melanoma tumor xenografts. Our data strongly indicate that GrB/scFvMEL demonstrates impressive antitumor activity in vitro and in vivo, enhances the sensitivity of human melanoma cells to various chemotherapeutic agents, sensitizes tumor cells to ionizing radiation, and inhibits tumor cell invasion of Matrigel. The targeted delivery of human proapoptotic GrB to tumor cells has a significant potential for cancer treatment.
Materials and Methods
Cell Culture
Human melanoma cells were obtained from Dr. I. J. Fidler (M. D. Anderson Cancer Center, Houston, TX). TXM-1 and TXM-18L cells were cultured in minimum essential medium (MEM), and A375-M cells were cultured in Dulbecco's MEM containing 10% fetal bovine serum (FBS), with added sodium pyruvate (100 mM), nonessential amino acids (10 mM), glutamine (200 nM), and MEM vitamins. MEL-526 cells were cultured in RPMI 1640 containing 10% FBS. All cells were routinely grown at a density of 7 x 106 cells per T-75 flask, subcultured twice per week, and were routinely tested and found to be free of Mycoplasma contamination using the Gen-Probe assay kit (Gen-Probe, Inc., San Diego, CA).
Expression and Purification of GrB/scFvMEL
The construction, expression, and purification of GrB/scFvMEL have been previously described [18]. The fusion protein was stored in sterile 150 mM NaCl at -20°C.
Antigen gp240 Staining and Fluorescence-Activated Cell Sorter (FACS) Analysis
Samples consisting of 1 x 106 cells were first treated with ZME-018 IgG2a for 20 minutes at 4°C, then stained with allophycocyanin (APC)-conjugated goat anti-mouse antibody (BD Immunocytometry System, San Jose, CA) for another 20 minutes at 4°C, both resuspended in 100 µl of FACS staining buffer [2% fetal calf serum/Dulbecco's phosphate-buffered saline (DPBS)]. As negative staining control, cells were stained with an isotype-matched control antibody of irrelevant specificity (mouse IgG2a; PharMingen, San Diego, CA) at the same concentration as that of the antibody against gp240. Following staining, cells were washed twice with DPBS, resuspended in 500 µl of 1% paraformaldehyde solution, and stored on ice in the dark. FACS analysis was performed immediately thereafter on a FACS Caliber cytometer (Becton Dickinson, San Jose, CA). APC fluorescence was detected in an FL-4 channel. For each cell line, 10,000 events were acquired. Analysis was performed with the CellQuest Pro software (Becton Dickinson).
Enzyme-Linked Immunosorbent Assay (ELISA) Assays
Ninety-six-well ELISA plates containing adherent melanoma cells (5 x 104 cells/well) were used as described previously [19]. To detect the binding activity of GrB/scFvMEL, cells were incubated with purified GrB/scFvMEL at various concentrations for 1 hour at room temperature (RT). After they were washed, the cells were incubated with rabbit anti-scFvMEL antibody, followed by the addition of goat anti-rabbit/HRP conjugate (HRP-GAR) antibody. Finally, the substrate (2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid, ABTS) solution containing 1 µl/ml 30% H2O2 was added to the wells. Absorbance at 405 nm was measured after 30 minutes.
Internalization Analysis by Immunofluorescence
Cells were plated into 16-well chamber slides (Nalge Nunc International, Naperville, IL) at a density of 1 x 104 cells/well. Cells were treated with GrB/scFvMEL (40 nM) for 1 hour. Proteins binding to the cell surface were removed by brief incubation with glycine buffer (0.5 M NaCl and 0.1 M glycine, pH 2.5) followed by immunofluorescent staining, as described previously [18]. Briefly, cells were fixed in 3.7% formaldehyde and permeabilized in 0.2% Triton X-100. Samples were blocked with 3% bovine serum albumin (BSA), incubated with goat anti-GrB monoclonal antibody (mAb), and then incubated with FITC-coupled anti-goat IgG and propidium iodide (PI; 2.5 µg/ml). The slides were mounted with DABCO containing 1 µg/ml PI and analyzed under a Nikon Eclipse TS-100 fluorescence microscope (Tokyo, Japan). Photographs were taken with a scope-mounted camera.
In Vitro Cytotoxicity Assays
To examine the cytotoxicity of GrB/scFvMEL or MEL/sFv/rGel, melanoma cells were plated on 96-well plates at a density of 4 x 103 cells/well and allowed to adhere for 24 hours at 37°C in 5% CO2. After 24 hours, the medium was replaced with one that contained various concentrations of fusion proteins. The effects on the growth of tumor cells in culture were determined by crystal violet (0.5% in 20% methanol) staining and solubilized with Sorenson's buffer (0.1 M sodium citrate, pH 4.2, in 50% ethanol), as described previously [19]. Percent control refers to the percentage of cells in drug-treated wells compared to that of control (untreated) wells.
Combination Studies of GrB/scFvMEL with Chemotherapeutic Agents
Cells in exponential growth phase were plated into 96-well plates. After 24 hours, the cells were treated with a drug-containing medium. At the end of the indicated incubation period, growth inhibition was assessed by crystal violet staining. IC25 doses of either GrB/scFvMEL or chemotherapeutic agents have been used in combination studies. To determine the effects of sequencing, cells were treated with two different sequences: sequence I—cells were pretreated with a chemotherapeutic agent for 6 hours, followed by coadministration with GrB/scFvMEL for 72 hours; sequence II—cells were pretreated with GrB/scFvMEL for 6 hours, followed by coadministration with a chemotherapeutic agent for 72 hours. Chemotherapeutic agents include doxorubicin (DOX), vincristine (VCR), etoposide (VP-16), cisplatin (CDDP), cytarabine (Ara C), and 5-fluorouracil (5-FU).
Clonogenic Survival Assay
The effectiveness of the combination of GrB/scFvMEL and ionizing radiation was assessed by clonogenic assay. Melanoma cells were either treated with PBS or pretreated with GrB/scFvMEL (10 nM) for 16 hours. Then, cells were irradiated with various doses of ionizing radiation and then processed for clonogenic cell survival assay. Following treatment, cells were trypsinized and counted. Known numbers were replated in triplicate and returned to the incubator to allow macroscopic colony development. Colonies were stained with crystal violet solution and counted after ∼ 14 days. The percentage of plating efficiency and the fraction surviving a given treatment were calculated based on the survival of nonirradiated cells treated with the agent in question.
Tumor Cell Invasion (Matrigel) Assay
Subconfluent A375DR cells were collected by trypsinization, resuspended in culture medium, and seeded in 20 µl (100,000 cells) on the lid of a culture dish. The lid was then placed on a dish filled with 2 ml of culture medium and incubated at 37°C for 48 hours. Matrigel solution (100 µl, 2.7 mg/ml) was pipetted onto the bottom of a 24-well culture dish and left to set at 37°C. Cell aggregates were transferred over the cushion and then overlaid with an additional 100 µl of Matrigel. The aggregates into Matrigel were covered with 400 µl of culture medium in the absence or in the presence of GrB/scFvMEL (50 nM). The aggregates were then observed daily under a light microscope; at the end of the incubation time, pictures of the aggregates were taken. The densities of cells invading into Matrigel surrounding the aggregates were analyzed by AlphaEaseFC software (Alpha Innotech, San Leandro, CA), and percent invasion was calculated based on the cell densities of two groups and standardized by the value of the nontreatment control group as 100% invasion.
Animal Model Studies
A375-M xenograft model and therapy study design Athymic (nu/nu) mice, 4 to 6 weeks old, were obtained from Harlan Sprague Dawley (Indianapolis. IN). The animals were maintained under specific pathogen-free conditions and were used at 6 to 8 weeks of age. Animals were injected subcutaneously (right flank) with 3 x 106 log-phase A375-M melanoma cells, and tumors were allowed to establish. Once tumors had reached a measurable size (∼30–50 mm3), animals were treated through the intravenous tail vein with either saline (control) or GrB/scFvMEL fusion construct. Fusion protein GrB/scFvMEL was given intravenously in a 0.2-ml volume. Doses mentioned in this paper are total doses administered once every other day for five doses. The doses are reported as milligrams per kilogram based on a mouse average weight of 20 g. For example, a 20-g mouse given 750 µg of GrB/scFvMEL would receive a dose of 37.5 mg/kg.
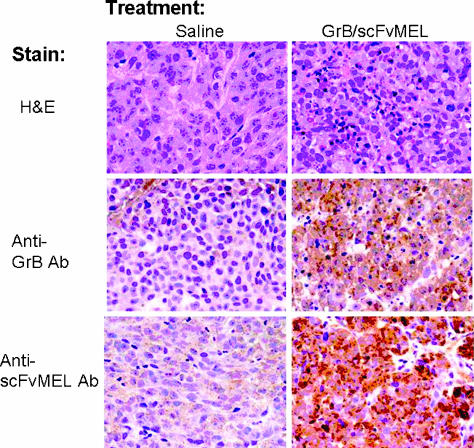
Localization of GrB/scFvMEL after systematic administration Mice bearing A375-M xenograft tumors were administered GrB/scFvMEL (37.5 mg/kg). Twenty-four hours later, animals were sacrificed; representative tissue sections were removed; formalin was fixed and stained by hematoxylin and eosin (H&E); and immunohistochemical staining for GrB/scFvMEL was detected by either anti-GrB or anti-scFvMEL antibody.
Terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) assay to detect apoptosis Tumor tissue sections were stained by TUNEL using an in situ cell death detection kit (Roche Molecular Biochemicals, Mannheim, Germany). Briefly, pretreatment of paraffin-embedded tissue was performed to dewax, rehydrate, and then incubate with proteinase K followed by fixation and permeabilization. The tissue sections were incubated with a TUNEL reaction mixture in a humidified chamber for 60 minutes at 37°C, and then the slides were rinsed thrice with PBS. Samples were analyzed under a Nikon Eclipse TS100 fluorescent microscope (Tokyo, Japan), and photographs were taken with a scope-mounted Nikon digital camera.
In vivo cytotoxicity studies
Once tumors' measurable sizes (∼ 30–50 mm3) have been established, animals were treated (intravenously through the tail vein) every other day (five times, 37.5 mg/kg) with either saline (control) or the GrB/scFvMEL fusion construct. Tumor growth was monitored by measuring (by caliper) two perpendicular tumor diameters every 2 or 3 days for an additional 28 days, and the volume of the tumor was calculated from the formula: tumor volume = (width)2 x length/2 [28].
Statistical Analysis
Data were analyzed using paired t test (Prism 3.0). Data are presented as mean ± SE. A difference was regarded as significant if P < .05.
Results
Antigen gp240 Expression on Melanoma Cells, the Cell-Binding Activity of the scFvMEL Component of GrB/scFvMEL, and Internalization of GrB/scFvMEL
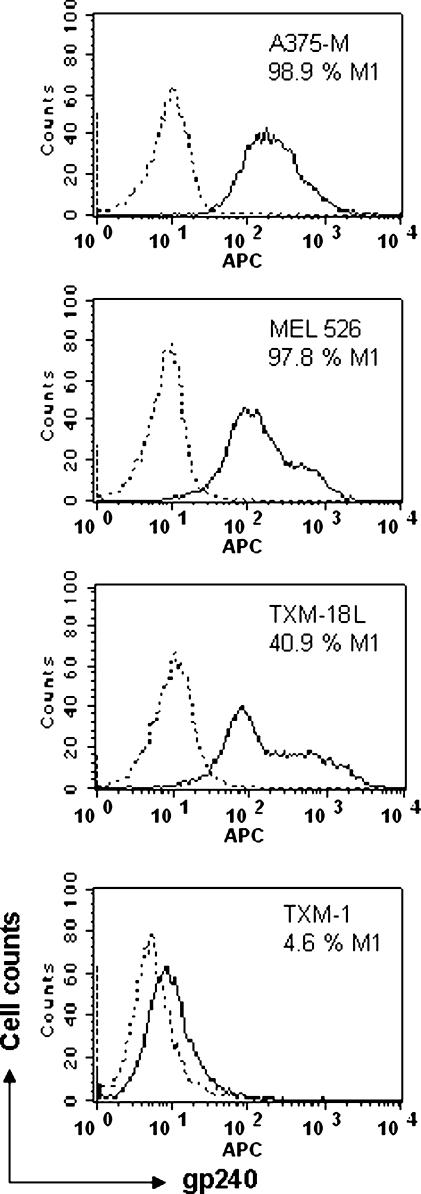
To examine the expression of the gp240 antigen on melanoma cell lines, melanoma cells consisting of 1 x 106 were stained with parental mAb ZME-018 IgG2a that specifically binds to the gp240 antigen (for 20 minutes) and APC-conjugated goat anti-mouse antibody (for another 20 minutes) at 4°C. As negative staining control, cells were stained with an isotype-matched control antibody of irrelevant specificity (mouse IgG2a) at the same concentration as that of the antibody against gp240. The expression levels of gp240 were indicated as solid lines (by FACS assay) for melanoma cell lines A375-M, MEL-526, TXM-18, and TXM-1, on which percent gp240-positive antigens were 98.9%, 97.8%, 40.9%, and 4.6%, respectively. These data demonstrated that the gp240 antigen was more highly expressed on A375-M, MEL-526, and TXM-18 cells; however, there was a very low-level expression of the gp240 antigen on TXM-1 cells, showing that the solid lines were overlaid by the dotted lines representing isotype control (Figure 1).
Figure 1.
The gp240 antigen is expressed in human melanoma cell lines by FACS analysis. Melanoma cells consisting of 1 x 106 cells were first incubated with mAb ZME-018 IgG2a then stained with APC-conjugated goat anti-mouse antibody, as described under Materials and Methods. As negative staining control, cells were stained with an isotype-matched control antibody of irrelevant specificity (mouse IgG2a). Cells were fixed in 1% paraformaldehyde solution and stored on ice in the dark after washing. FACS analysis was carried out, and APC fluorescence was detected by a FACS Caliber cytometer. For each cell line, 10,000 events were acquired. Analysis was performed with the CellQuest Pro software. Expression of gp240 was indicated as solid lines for all five melanoma cell lines, which were overlaid by dotted lines representing isotype control. High-level expression of the gp240 antigen was demonstrated in all human melanoma cell lines, except TXM-1 cells.
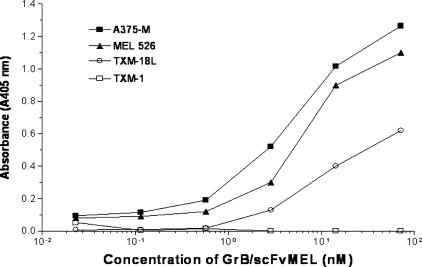
Because the gp240 antigen highly presents in melanoma A375-M, MEL-526, and TXM-18L cells, but not in TXM-1 cells, we further examined if the GrB/scFvMEL fusion construct could bind to antigen-positive cells. ELISA was performed to determine the binding activity of the GrB/scFvMEL fusion construct to melanoma cells. The results demonstrated that GrB/scFvMEL bound to high-level gp240 antigen-expressing melanoma A375-M, MEL-526, and TXM-18L cells. Moreover, the binding activity was stronger in A375-M and MEL-526 cells, followed by TXM-18L cells. However, the protein did not bind to TXM-1, in which very low-level expression of the gp240 antigen was detected by an anti-scFvMEL rabbit mAb (Figure 2).
Figure 2.
GrB/scFvMEL specifically binds to the gp240 antigen-positive melanoma cell lines, as assessed by ELISA. Ninety-six-well ELISA plates containing adherent melanoma cells (5 x 104 cells/well) were blocked by addition of a solution containing 5% BSA for 1 hour. To detect the binding activity of GrB/scFvMEL, cells were incubated with purified GrB/scFvMEL at various concentrations for 1 hour at RT. After washing, the cells were incubated with rabbit anti-scFvMEL antibody, followed by the addition of HRP-GAR antibody. Finally, the substrate solution ABTS containing 1 µl/ml 30% H2O2 was added to the wells. Absorbance at 405 nm was measured after 30 minutes. GrB/scFvMEL bound to high-level gp240 antigen-expressing melanoma A375-M, MEL-526, and TXM-18 cells, but not to TXM-1 cells, which has a very low-level expression of the gp240 antigen.
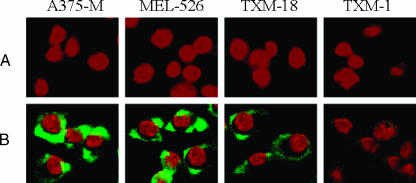
The GrB moiety of the fusion construct was efficiently delivered into the cytosol of gp240 antigen-positive A375-M, MEL-526, and TXM-18 melanoma cells after treatment with GrB/scFvMEL for 1 hour, as assessed by immunofluorescent staining detected using goat anti-GrB antibody (Figure 3). This effectively demonstrates that the gp240 expression on the tumor cell surface and the binding of the construct to the gp240 antigen are responsible for the internalization of the GrB/scFvMEL fusion construct.
Figure 3.
Internalization of GrB/scFvMEL into A375-M, MEL-526, TXM-18, and TXM-1 cells, as assessed by immunofluorescent staining. Cells were treated with 40 nM GrB/scFvMEL for 1 hour. Molecules bound to the cell surface were removed by brief treatment with glycine buffer (pH 2.5). Cells were fixed in 3.7% formaldehyde and permeabilized in 0.2% Triton X-100. Samples were blocked with 3% BSA, incubated with goat anti-GrB mAb, and then incubated with FITC-coupled anti-goat IgG and PI. The slides were mounted with DABCO containing 1 µg/ml PI and analyzed under a Nikon Eclipse TS-100 fluorescence microscope. Internalization of GrB/scFvMEL was observed in the gp240 antigen -positive A375-M, MEL-526, and TXM-18 cells, but not in the gp240 antigen-negative TXM-1 cells. (A) No GrB/scFvMEL treatment control. (B) GrB/scFvMEL treatment for 1 hour.
In Vitro Cytotoxicity of GrB/scFvMEL against Various Melanoma Cell Lines
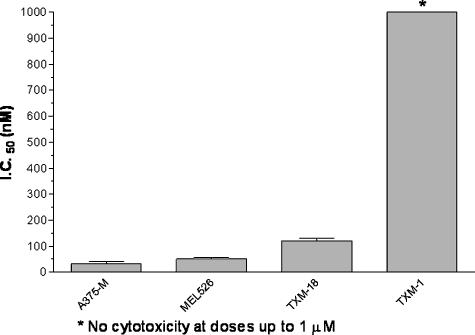
The cytotoxicity of GrB/scFvMEL was assessed against log-phase A375-M, MEL-526, TXM-18L, and TXM-1 cells in culture. Fifty-percent growth-inhibitory effects were found at concentrations of ∼ 30 nM on A375-M cells, of ∼ 50 nM on MEL-526 cells, and of ∼ 150 nM on TXM-18L, respectively. However, no cytotoxic effects were found on TXM-1 cells at doses of up to 1 µM (Figure 4, Table 1). By comparison, the cytotoxic effects of GrB/scFvMEL on target cells were similar to those of the previously described MEL sFv/rGel [17] (Table 1). These data suggest that there appears to be a correlation between a high expression of gp240 and an improved cellular binding of GrB/scFvMEL, resulting in a comparatively greater cytotoxicity of the fusion protein. In addition, studies in our laboratory demonstrate that a companion construct MEL sFv/rGel generates a cytotoxic effect against cells through a necrotic process rather than an apoptotic process. These comparative studies demonstrate that the cytotoxic/apoptotic effects of the GrB pay load can match the robust cytotoxic/necrotic effects of rGel-containing fusion toxins.
Figure 4.
The cytotoxic effects of GrB/scFvMEL on the gp240 antigen-positive melanoma cell lines. Samples (GrB/scFvMEL or MEL sFv/rGel) were assayed using a standard 72-hour cell proliferation assay with melanoma cell monolayer and using crystal violet staining, as described under Materials and Methods. Percent control refers to the percentage of cells in drug-treated wells compared to that of control (untreated) wells. A 50% growth-inhibitory effect (IC50) was calculated. The bar chart showed the IC50 value of GrB/scFvMEL in different melanoma cell lines. GrB/scFvMEL demonstrated cytotoxic effects on A375-M, MEL526, and TXM-18L, which expressed high-level gp240 antigen. However, no cytotoxic effects on TXM-1 cells were found at doses of up to 1 µM.
Table 1.
Cytotoxicitiy of GrB/scFvMEL Versus MEL sFv/rGel against Various Human Melanoma Cell Lines*.
| Cell Line | gp240 Antigen (% Positive)† | IC50 (nM) | |
| GrB/scFvMEL | scFvMEL/rGel | ||
| A375-M | 98.9 | 32.1 ± 7.95 | 22.03 ± 3.08 |
| MEL-526 | 97.8 | 50.0 ± 4.32 | 40.0 ± 5.55 |
| TXM-18L | 40.9 | 158.3 ± 12.45 | 144.3 ± 9.56 |
| TXM-1‡ | 4.6 | >1000 | >1000 |
Samples (GrB/scFvMEL and MEL sFv/rGel) were assayed using a standard 72-hour cell proliferation assay and using crystal violet staining. IC50 values were calculated and demonstrated as mean ± SEM (nM).
Percent positive FACS analysis.
No cytotoxic effect was observed at doses of up to 1000 nM.
Combination Studies of GrB/scFvMEL with Conventional Chemotherapeutic Agents on A375-M
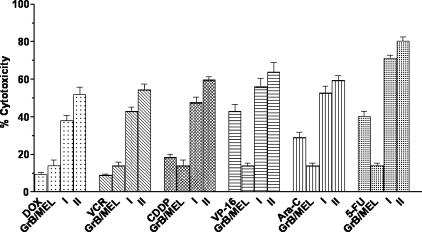
Because cellular apoptotic processes appear to mediate response/resistance to chemotherapy [8,29], we next examined the effects of GrB/scFvMEL in combination with various chemotherapeutic agents on target cells. Coadministration of GrB/scFvMEL and various classes of chemotherapeutic agents to A375-M cells for 72 hours demonstrated synergistic antitumor activity with adriamycin (DOX), VCR or CDDP, and additive effects, in combination with VP-16, Ara C, or 5-FU. Pretreatment with GrB/scFvMEL for 6 hours, followed by coexposure to these chemotherapeutic agents for 72 hours (sequence II), showed significantly inhibited growth compared to pretreatment with drugs followed by coexposure of the fusion construct (sequence I) (Figure 5). The results indicated that the cytotoxic effects of chemotherapeutic agents could be markedly enhanced by pretreatment with GrB/scFvMEL for 6 hours on gp240 antigen-positive melanoma cells. GrB/scFvMEL was not cytotoxic to antigen-negative TXM-1 cells at concentrations of up to 1 µM. We pretreated TXM-1 cells with GrB/scFvMEL at 1 µM for 6 hours, then coadministered various chemotherapeutic agents at IC50 or IC25 doses. As expected, the cytotoxic effects of various chemotherapeutic agents were not enhanced by pretreatment with GrB/scFvMEL for 6 hours.
Figure 5.
Pretreatment with GrB/scFvMEL sensitizes various chemotherapeutic agents. The log-phase gp240 antigen-positive (A375-M) cells were pretreated (at IC25 doses) with various chemotherapeutic agents for 6 hours, followed by the addition of GrB/scFvMEL (IC25). The cells were then incubated for a total of 72 hours (sequence I). Alternatively, cells were first treated with GrB/scFvMEL for 6 hours, and then various chemotherapeutic agents were added for 72 hours (sequence II). Chemotherapeutic agents include DOX, VCR, VP-16, cisplantin (CDDP), Ara C, and 5-FU. The coadministration GrB/scFvMEL and chemotherapeutic agents to A375 cells for 72 hours demonstrated synergistic antitumor activity with DOX, VCR or CDDP, and additive effects, in combination with VP-16 or Ara C or 5-FU. Pretreatment with GrB/scFvMEL for 6 hours, followed by coexposure to these chemotherapeutic agents for 72 hours (II), showed significantly inhibited growth as compared to pretreatment with drugs followed by coexposure to the fusion construct (I).
Cytotoxicity of GrB/scFvMEL Against A375 Doxorubicin-Resistant (A375DR) Subline
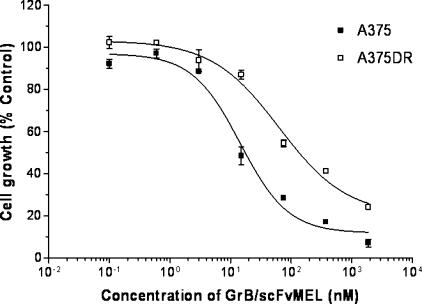
To examine if cellular resistance to DOX also causes cross-resistance to the GrB/scFvMEL fusion construct, an A375DR cell line was first established by continuous exposure of log-phase parental A375-M cells to increasing concentrations of DOX, followed by serial dilution and clonal selection of colonies. One clone designated as A375DR was 400-fold resistant to DOX compared to the parental A375 cell line (IC50 = 200 nM for A375DR versus IC50 = 0.5 nM for the parental A375 cell line). Treatment of the A375DR cells demonstrated an IC50 that was only 4.5-fold higher compared to parental A375 cells (IC50 = 63.6 nM for A375DR versus IC50 = 14.5 nM for A375) (Figure 6, Table 2). Expression of the gp240 antigen was assessed by ELISA using the ZME-018 antibody specifically recognizing the gp240 antigen. This study demonstrated that relative gp240 expression was virtually identical on parental A375 and A375DR cells (data not shown). The data demonstrated that cellular resistance to DOX is associated with marginal cross-resistance to GrB/scFvMEL.
Figure 6.
Cellular resistance to DOX is associated with marginal cross-resistance to GrB/scFvMEL. A375DR cells are 400-fold resistant to DOX compared to parental A375 cells. Log-phase A375DR cells were treated with various doses of GrB/scFvMEL for 72 hours. Percent control refers to the percentage of cells in drug-treated wells compared to that of control (untreated) wells. A375DR cells demonstrated only a 4.5-fold resistance to GrB/scFvMEL compared to parental A375 cells (IC50 = 63.6 vs 14.5 nM).
Table 2.
Comparison of the Cytotoxic Effects of GrB/scFvMEL and DOX on Parental A375 Cells and A375DR Sublines.
| Cells | IC50 (nM) | |
| DOX | GrB/scFvMEL | |
| A375 | 0.5 ± 0.19 | 14.5 ± 3.93 |
| A375DR | 200.0 ± 16.28 | 63.6 ± 4.01 |
| Resistance index* | 400.0 | 4.5 |
Resistance index = (IC50 on A375DR) / (IC50 on A375).
Effects of Pretreatment with GrB/scFvMEL on Radiosensitivity in Human Melanoma A375-M Cells
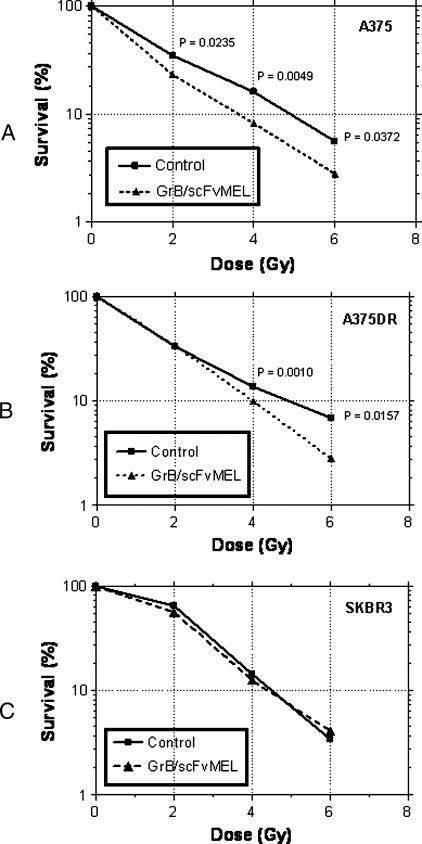
To determine if induction of apoptosis by GrB/scFvMEL can impact the sensitivity of melanoma cells to external beam ionizing radiation, A375, A375DR, and antigen-negative SKBR3 cells were first pretreated with 10 nM GrB/scFvMEL for 16 hours; the cells were then irradiated and plated for clonogenic cell survival. Figure 7 shows that GrB/scFvMEL treatment suppressed the clonogenic survival of both A375 and A375DR cells. The number of surviving colonies at radiation doses of 2, 4, and 6 Gy was reduced from approximately 34.8 ± 0.23%, 16.0 ± 0.06%, and 5.6 ± 0.55%, respectively, in the A375 radiation-alone control group to 23.1 ± 1.59%, 8.2 ± 0.49%, and 2.8 ± 0.52% in the GrB/scFvMEL plus radiation treatment group, respectively (Figure 7A, Table 3A). The observed sensitization was statistically significant (P < .05) at 2, 4, and 6 Gy dosage groups. After the treatment of A375DR cells with irradiation alone, colony survival at doses of 4 and 6 Gy was 13.8 ± 0.32% and 6.9 ± 0.64%, respectively, compared to 9.4 ± 0.29% and 2.8 ± 0.12%, respectively, in the groups cotreated with GrB/scFvMEL and radiation (Figure 7B, Table 3B). The observed sensitization in the A375DR groups was statistically significant with P < .05 at 4 and 6 Gy, respectively. However, no statistically significant sensitization was observed in antigen-negativeSKBR3 cells treated with irradiation compared with GrB/scFvMEL-treated cells followed by irradiation at 2, 4, and 6 Gy (P > .05) (Figure 7C, Table 3C). Therefore, treatment with GrB/scFvMEL sensitizes both antigen-positive A375 and A375DR cells that are resistant to ionizing radiation. This radiosensitization appears to be dependent on the expression of the gp240 antigen because we did not observe radiation sensitization with antigen-negative cells.
Figure 7.
Treatment with GrB/scFvMEL sensitizes melanoma cells to ionizing radiation. Radiosensitization by GrB/scFvMEL was based on clonogenic cell survival assays. A375 (A), A375DR (B), and SKBR3-HP (C) cells were pretreated with GrB/scFvMEL (10 nM for 16 hours), the drug was washed off, and cells were irradiated at various doses and plated for clonogenic cell survival assay. Observed sensitizations were statistically significant at 2, 4, and 6 Gy dosage groups on A375 cells (P < .05) and at 4 and 6 Gy dosage groups on A375DR cells (P < .05 and .005, respectively). No statistically significant sensitization was observed in gp240 antigen-negative SKBR3 cells (P > 0.05).
Table 3A.
Clonogenic Survival (% Survival) of A375 Cells Treated with Irradiation Alone (Control) Versus Irradiation and Treatment with GrB/scFvMEL.
| 0 Gy | 2 Gy | 4 Gy | 6 Gy | |
| Control | 100 | 34.8 ± 0.23 | 16.0 ± 0.06 | 5.6 ± 0.55 |
| GrB/scFvMEL | 100 | 23.1 ± 1.59 | 8.2 ± 0.49 | 2.8 ± 0.52 |
| P | >.05 | <.05* | <.05* | <.05* |
Significant difference.
Table 3B.
Clonogenic Survival (% Survival) of A375DR Cells Treated with Irradiation Alone (Control) Versus Irradiation and Treatment with GrB/scFvMEL.
| 0 Gy | 2 Gy | 4 Gy | 6 Gy | |
| Control | 100 | 33.4 ± 0.09 | 13.8 ± 0.32 | 6.9 ± 0.64 |
| GrB/scFvMEL | 100 | 34.0 ± 1.07 | 9.4 ± 0.29 | 2.8 ± 0.12 |
| P | >.05 | >.05 | <.05* | <.05* |
Significant difference.
Table 3C.
Clonogenic Survival (% Survival) of SKBR3-HP Cells Treated with Irradiation Alone (Control) Versus Irradiation and Treatment with GrB/scFvMEL.
| 0 Gy | 2 Gy | 4 Gy | 6 Gy | |
| Control | 100 | 59.8 ± 6.90 | 12.5 ± 4.45 | 3.4 ± 0.13 |
| GrB/scFvMEL | 100 | 55.9 ± 0.27 | 12.2 ± 1.43 | 4.1 ± 0.23 |
| P | >.05 | >.05 | >.05 | >.05 |
Effect of GrB/scFvMEL on the Metastatic Potential of A375DR Cells
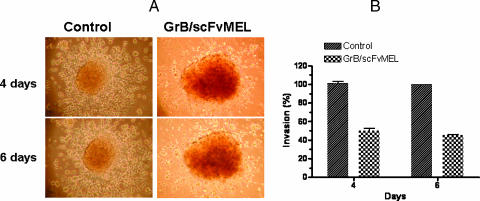
We then examined whether the GrB/scFvMEL fusion construct might affect the metastatic potential of melanoma cells because cellular apoptotic posture is one of the factors that is known to be responsible for mediating cellular invasion and metastatic potential [2,3,29,30]. We studied the effects of GrB/scFvMEL on the ability of A375DR cells to invade a matrix of a reconstituted basement membrane (Matrigel). A375DR cells spontaneously form cell aggregates in Matrigel when prepared by the hanging-drop technique. A375DR cells actively leave the aggregate and invade Matrigel prepation at 4 and 6 days (Figure 8A). The densities of cells invading into Matrigel surrounding the aggregates were analyzed by AlphaEaseFC software (Alpha Innotech), and percent invasion was calculated based on the cell densities of two groups and standardized by the value of the nontreatment control group as 100% invasion. The treatment of A375DR cells with GrB/scFvMEL inhibits the A375DR invasion of Matrigel. The capability of the invasion of cells significantly decreased to 50.0 ± 2.89% at 4 days and to 45.0 ± 1.16% at 6 days (Figure 8, A and B).
Figure 8.
GrB/scFvMEL inhibits A375DR cell invasion of Matrigel. A375DR cell aggregates were prepared as described under Materials and Methods. Cell aggregates were transferred over the Matrigel cushion and then overlaid with an additional 100 µl of Matrigel. The aggregates into Matrigel were covered with 400 µl of culture medium in the absence or presence of GrB/scFvMEL (50 nM). The aggregates were then observed daily under a light microscope (A). A375DR cells actively leave the aggregate and invade Matrigel preparation at 4 and 6 days. The treatment of A375DR cells with GrB/scFvMEL inhibits the A375DR invasion of Matrigel at 4 and 6 days. The densities of cells invading into Matrigel surrounding the aggregates were analyzed by AlphaEaseFC software, and percent invasion was calculated based on the cell densities of the two groups and standardized by the value of the nontreatment control group as 100% invasion (B).
In Vivo Animal Studies of GrB/scFvMEL (A375-M Xenograft Model)
We first examined the internalization or localization of the GrB/scFvMEL fusion construct in mice bearing A375-M xenograft tumors. After GrB/scFvMEL administration 24 hours later, mice were sacrificed, and representative tissue sections were removed, formalin-fixed and stained by H&E, and detected by either anti-GrB antibody or anti-scFvMEL antibody. Localization or internalization of GrB/scFvMEL was observed in tumor tissues (Figure 9).
Figure 9.
GrB/scFvMEL is localized or internalized in tumor tissues. Mice bearing A375-M xenograft tumors were administered intravenously GrB/scFvMEL (37.5 mg/kg). Twenty-four hours after the last dose, animals were sacrificed, and tumor tissues were removed, fixed, and stained by immuno-histochemical staining for GrB/scFvMEL detected by either anti-GrB or anti-scFvMEL antibody. Localization and internalization of GrB/scFvMEL were observed in tumor tissues in the treatment group, but not in the control group.
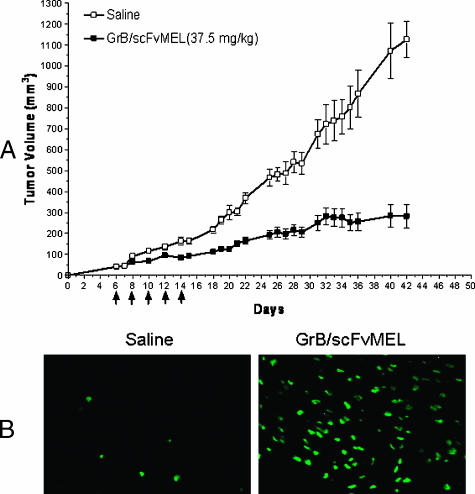
To examine the in vivo antitumor effects of GrB/scFvMEL, studies were performed on A375-M human melanoma tumor xenografts. Mice bearing the tumors were treated (intravenously through the tail vein) five times, every other day, with either GrB/scFvMEL or saline. Saline treatment control group tumors increased 24-fold (from 50 to 1200 mm3) over 28 days. In contrast, GrB/scFvMEL-treated tumors increased four-fold (from 50 to 200 mm3) (Figure 10A). The GrB/scFvMEL fusion construct demonstrated impressive antitumor activity. Tumor tissue nuclei were stained by TUNEL assay. The results clearly demonstrated that tumor tissues displayed apoptotic nuclei in the GrB/scFvMEL treatment group (Figure 10B).
Figure 10.
GrB/scFvMEL demonstrates antitumor activity in a xenograft melanoma model by inducing apoptosis in tumor tissues. (A) Antitumor effect of GrB/scFvMEL on A375-M xenograft tumors. Female athymic (nu/nu) mice, 6 to 8 weeks of age, were injected subcutaneously on the right flank with 3 x 106 log-phase A375-M cells, and tumors were allowed to establish. Once tumors had reached a measurable size (∼ 50 mm3), animals were administered intravenously with either saline (control) or the GrB/scFvMEL fusion construct five times, every other day (37.5 mg/kg, total dose). Animals were monitored and tumors were measured for an additional 28 days. Saline-treated control tumors increased 24-fold (from 50 to 1200 mm3) over 28 days. In contrast, GrB/scFvMEL-treated tumors increased four-fold (from 50 to 200 mm3). (B) Apoptosis detection in tumor tissue by TUNEL assay. Mice bearing A375-M xenograft tumors were administered intravenously GrB/scFvMEL (37.5 mg/kg). Twenty-four hours after the last dose, a group of animals was sacrificed, and tumor tissues were removed and fixed. Tumor tissue sections were stained by TUNEL and analyzed under a Nikon Eclipse TS 100 fluorescent microscope. Tumor tissues displayed highly apoptotic nuclei in the treatment group compared to the control group.
Discussion
The human serine protease granzyme B (GrB) is a powerful intracellular mediator of cellular apoptotic cascades and has been identified as one of the primary methods that regulatory immune cells (macrophages, NK cells, cytotoxic T cells, and dendritic cells) employ to destroy target cells [31–38]. This enzyme provides multimodal apoptosis activation through both caspase-dependent and caspasen-dependent pathways [39]. Our study was the first to describe a novel fusion construct of either an antibody [18] or a growth factor [40] containing GrB, which redirects this enzyme specifically to antigen- or receptor-expressing target cells and potentially describes a new class of targeted therapeutic agents composed of human cytotoxic proteins fused to tumor-targeting molecules.
The salient finding of this study is that the antimelanoma GrB/scFvMEL fusion construct specifically targets human melanoma cells expressing the gp240 cell surface antigen. This construct appears to be cytotoxic at nanomolar levels and has a cytotoxicity profile similar to that of a fusion toxin containing the highly cytotoxic plant toxin gelonin (rGel). Treatment of DOX-resistant tumor cells demonstrated only a modest increase in the IC50 of the GrB/scFvMEL fusion construct. This observation is in line with that of Classen et al. [41], who found that GrB-induced cleavages of PARP and caspases were similar in CEM cells that are sensitive and resistant to various chemotherapeutic agents.
Numerous cellular and molecular events appear to control tumor cell invasion and metastatic spread [3,42,43]. Highly metastatic cells appear to exhibit a higher resistance to apoptosis compared to their poorly disseminated counter parts [2], and we reason that a pharmacological agent acting solely through apoptotic activation would decrease metastatic spread. Our data appear to demonstrate that the GrB/scFvMEL construct is capable of suppressing metastatic spread in an in vitro (Matrigel) assay, and studies are now ongoing to determine whether this agent can suppress metastases in a highly metastatic melanoma xenograft model [44]. Similarly, apoptotic mechanisms also appear to be responsible for mediating the response of tumor cells to ionizing radiation [45–48], and agents that activate caspases and other proapoptotic events appear to sensitize cells to radiotherapy. Our studies indicate that coadministration of the GrB/scFvMEL fusion construct can have a modest cell-specific impact on augmenting the response of tumor cells to X-irradiation.
In addition to its ability to sensitize tumor cells to radiotherapy, we found that this construct also appeared to be highly synergistic with virtually all classes of chemotherapeutic agents tested. This suggests a broad applicability of this agent in combination with conventional chemotherapy. Xenograft model studies are ongoing also to determine whether this agent is capable of augmenting nonspecific toxicity as well as antitumor effects.
Normally, GrB requires PFN, but the fusion construct provides both cell specificity as well as a non-PFN requiring internalization route. The presence of free PFN in disease states could potentially make the construct kill nontarget cells by providing indiscriminant entry into normal cells. Our previous data suggest that the addition of PFN to nontarget cells exposed to GrB/scFvMEL results in significant toxicity [18]. There are several diseases indicating that a significantly higher expression of PFN is present in the peripheral blood circulation [49,50]. This presents the possibility of causing nonspecific toxicity. The targeted delivery of GrB into melanoma cells by scFvMEL makes this fusion construct effective on tumor (not nontarget) cells.
It is surprising that the GrB construct is as active as toxins, which means that GrB is superior to most other toxins as a therapeutic agent for human cancer because GrB is a human protein. Humans are supposed to have a strong immunologic tolerance against endogenous GrB; a humoral or cellular immune response against GrB should not occur. Toxins such as rGel, Pseudomonas exotoxin, or Ricin toxin A chain, which are derived from plants, are highly immunogenic in humans [51] and tend to induce drug resistance after several treatments [52]. Because GrB activates numerous independent cascade events through enzymatic mechanisms of action, the development of resistance to GrB is unlikely.
Other studies targeting GrB [53,54] to other tumor cell types appear to indicate that there is broad applicability for this as a platform technology. Kurschus et al. reported that retargeting of GrB to Lewis Y-positive surface receptors by GrB/dsFv-B3 conjugate could lead to PFN-independent target cell death with efficacy similar to that of dsFv-B3 targeted Pseudomonas exotoxin fragment 38. The data also indicated that the activity of GrB was only weakly inhibited by plasma proteins [54].
The studies demonstrated that the targeted delivery of GrB can be achieved adequately to kill target cells. The fusion construct GrB/scFvMEL specifically kills gp240-bearing cells and sensitizes cells to chemotherapy and radiation therapy. The fusion construct has the potential to reduce the invasion of tumor cells. This is proof-of-core concept that targeted/pharmacological control of apoptotic processes can be achieved. GrB-based immunotoxins should be useful as a new class of immunotoxins with low immunogenicity utilizing programmed cell death for therapeutic purposes.
Abbreviations
- GrB
human granzyme B
- scFv
recombinant single-chain Fv antibody
- rGel
recombinant gelonin
- A375DR
A375 doxorubicin-resistant
Footnotes
This research was supported, in part, by the Clayton Foundation for Research.
References
- 1.Bajetta E, Del Vecchio M, Bernard-Marty C, Vitali M, Buzzoni R, Rixe O, Nova P, Aglione S, Taillibert S, Khayat D. Metastatic melanoma: chemotherapy. Semin Oncol. 2002;29:427–445. doi: 10.1053/sonc.2002.35238. [DOI] [PubMed] [Google Scholar]
- 2.Glinsky GV, Glinsky VV. Apoptosis and metastasis: a superior resistance of metastatic cancer cells to programmed cell death. Cancer Lett. 1996;101:43–51. doi: 10.1016/0304-3835(96)04112-2. [DOI] [PubMed] [Google Scholar]
- 3.Glinsky GV, Glinsky VV, Ivanova AB, Hueser CJ. Apoptosis and metastasis: increased apoptosis resistance of metastatic cancer cells is associated with the profound deficiency of apoptosis execution mechanisms. Cancer Lett. 1997;115:185–193. doi: 10.1016/s0304-3835(97)04738-1. [DOI] [PubMed] [Google Scholar]
- 4.Awada A, Cardoso F, Atalay G, Giuliani R, Mano M, Piccart MJ. The pipeline of new anticancer agents for breast cancer treatment in 2003. Crit Rev Oncol Hematol. 2003;48:45–63. doi: 10.1016/s1040-8428(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 5.Jansen B, Wacheck V, Heere-Ress E, Schlagbauer-Wadl H, Hoeller C, Lucas T, Hoermann M, Hollenstein U, Wolff K, Pehamberger H. Chemosensitisation of malignant melanoma by BCL2 antisense therapy. Lancet. 2000;356:1728–1733. doi: 10.1016/S0140-6736(00)03207-4. [DOI] [PubMed] [Google Scholar]
- 6.Pak BJ, Lee J, Thai BL, Fuchs SY, Shaked Y, Ronai Z, Kerbel RS, Ben David Y. Radiation resistance of human melanoma analyzed by retroviral insertional mutagenesis reveals a possible role for dopachrome tautomerase. Oncogene. 2004;23:30–38. doi: 10.1038/sj.onc.1207007. [DOI] [PubMed] [Google Scholar]
- 7.Rockmann H, Schadendorf D. Drug resistance in human melanoma: mechanisms and therapeutic opportunities. Onkologie. 2003;26:581–587. doi: 10.1159/000074156. [DOI] [PubMed] [Google Scholar]
- 8.Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 9.Kantor RR, Ng AK, Giacomini P, Ferrone S. Analysis of the NIH workshop monoclonal antibodies to human melanoma antigens. Hybridoma. 1982;1:473–482. doi: 10.1089/hyb.1.1982.1.473. [DOI] [PubMed] [Google Scholar]
- 10.Kantor RR, Albino AP, Ng AK, Ferrone S. Biosynthesis and intracellular processing of four human melanoma associated antigens. Cancer Res. 1986;46:5223–5228. [PubMed] [Google Scholar]
- 11.Macey DJ, Denardo SJ, Denardo GL, Goodnight JK, Unger MW. Uptake of indium-111-labeled monoclonal antibody ZME-018 as a function of tumor size in a patient with melanoma. Am J Physiol Imaging. 1988;3:1–6. [PubMed] [Google Scholar]
- 12.Rosenblum MG, Murray JL, Cheung L, Rifkin R, Salmon S, Bartholomew R. A specific and potent immunotoxin composed of antibody ZME-018 and the plant toxin gelonin. Mol Biother. 1991;3:6–13. [PubMed] [Google Scholar]
- 13.Rosenblum MG, Levin B, Roh M, Hohn D, McCabe R, Thompson L, Cheung L, Murray JL. Clinical pharmacology and tissue disposition studies of 131I-labeled anticolorectal carcinoma human monoclonal antibody LiCO 16.88. Cancer Immunol Immunother. 1994;39:397–400. doi: 10.1007/BF01534427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mujoo K, Cheung L, Murray JL, Rosenblum MG. Pharmacokinetics, tissue distribution, and in vivo antitumor effects of the antimelanoma immunotoxin ZME-gelonin. Cancer Immunol Immunother. 1995;40:339–345. doi: 10.1007/BF01519635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenblum MG, Cheung L, Mujoo K, Murray JL. An antimelanoma immunotoxin containing recombinant human tumor necrosis factor: tissue disposition, pharmacokinetic, and therapeutic studies in xenograft models. Cancer Immunol Immunother. 1995;40:322–328. doi: 10.1007/BF01519633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Cheung LH, Marks JW, Rosenblum MG. Recombinant single-chain antibody fusion construct targeting human melanoma cells and containing tumor necrosis factor. Int J Cancer. 2004;108:549–557. doi: 10.1002/ijc.11524. [DOI] [PubMed] [Google Scholar]
- 17.Rosenblum MG, Cheung LH, Liu Y, Marks JW., III Design, expression, purification, and characterization, in vitro and in vivo, of an antimelanoma single-chain Fv antibody fused to the toxin gelonin. Cancer Res. 2003;63:3995–4002. [PubMed] [Google Scholar]
- 18.Liu Y, Cheung LH, Hittelman WN, Rosenblum MG. Targeted delivery of human pro-apoptotic enzymes to tumor cells: in vitro studies describing a novel class of recombinant highly cytotoxic agents. Mol Cancer Ther. 2003;2:1341–1350. [PubMed] [Google Scholar]
- 19.Rosenblum MG, Cheung L, Murray JL, Bartholomew R. Antibody-mediated delivery of tumor necrosis factor (TNF-alpha): improvement of cytotoxicity and reduction of cellular resistance. Cancer Commun. 1991;3:21–27. [PubMed] [Google Scholar]
- 20.Rosenblum MG, Cheung L, Kim SK, Mujoo K, Donato NJ, Murray JL. Cellular resistance to the antimelanoma immunotoxin ZME-gelonin and strategies to target resistant cells. Cancer Immunol Immunother. 1996;42:115–121. doi: 10.1007/s002620050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenblum MG, Marks JW, Cheung LH. Comparative cytotoxicity and pharmacokinetics of antimelanoma immunotoxins containing either natural or recombinant gelonin. Cancer Chemother Pharmacol. 1999;44:343–348. doi: 10.1007/s002800050987. [DOI] [PubMed] [Google Scholar]
- 22.Smyth MJ, Trapani JA. Granzymes: exogenous proteinases that induce target cell apoptosis. Immunol Today. 1995;16:202–206. doi: 10.1016/0167-5699(95)80122-7. [DOI] [PubMed] [Google Scholar]
- 23.Yagita H, Nakata M, Kawasaki A, Shinkai Y, Okumura K. Role of perforin in lymphocyte-mediated cytolysis. Adv Immunol. 1992;51:215–242. doi: 10.1016/s0065-2776(08)60488-5. [DOI] [PubMed] [Google Scholar]
- 24.Shi L, Kraut RP, Aebersold R, Greenberg AH. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J Exp Med. 1992;175:553–566. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orth K, Chinnaiyan AM, Garg M, Froelich CJ, Dixit VM. The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J Biol Chem. 1996;271:16443–16446. [PubMed] [Google Scholar]
- 26.Sutton VR, Davis JE, Cancilla M, Johnstone RW, Ruefli AA, Sedelies K, Browne KA, Trapani JA. Initiation of apoptosis by granzyme B requires direct cleavage of bid, but not direct granzyme B-mediated caspase activation. J Exp Med. 2000;192:1403–1414. doi: 10.1084/jem.192.10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trapani JA, Browne KA, Smyth MJ, Jans DA. Localization of granzyme B in the nucleus. A putative role in the mechanism of cytotoxic lymphocyte-mediated apoptosis. J Biol Chem. 1996;271:4127–4133. doi: 10.1074/jbc.271.8.4127. [DOI] [PubMed] [Google Scholar]
- 28.Osborne CK, Hobbs K, Clark GM. Effect of estrogens and antiestrogens on growth of human breast cancer cells in athymic nude mice. Cancer Res. 1985;45:584–590. [PubMed] [Google Scholar]
- 29.Simstein R, Burow M, Parker A, Weldon C, Beckman B. Apoptosis, chemoresistance, and breast cancer: insights from the MCF-7 cell model system. Exp Biol Med (Maywood) 2003;228:995–1003. doi: 10.1177/153537020322800903. [DOI] [PubMed] [Google Scholar]
- 30.Rubio N, Espana L, Fernandez Y, Blanco J, Sierra A. Metastatic behavior of human breast carcinomas overexpressing the Bcl-x(L) gene: a role in dormancy and organospecificity. Lab Invest. 2001;81:725–734. doi: 10.1038/labinvest.3780281. [DOI] [PubMed] [Google Scholar]
- 31.Kraan MC, Haringman JJ, Weedon H, Barg EC, Smith MD, Ahern MJ, Smeets TJ, Breedveld FC, Tak PP. T cells, fibroblast-like synoviocytes, and granzyme B+ cytotoxic cells are associated with joint damage in patients with recent onset rheumatoid arthritis. Ann Rheum Dis. 2004;63:483–488. doi: 10.1136/ard.2003.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jose MD, Ikezumi Y, van Rooijen N, Atkins RC, Chadban SJ. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation. 2003;76:1015–1022. doi: 10.1097/01.TP.0000083507.67995.13. [DOI] [PubMed] [Google Scholar]
- 33.Humbert M, Devergne O, Cerrina J, Rain B, Simonneau G, Dartevelle P, Duroux P, Galanaud P, Emilie D. Activation of macrophages and cytotoxic cells during cytomegalovirus pneumonia complicating lung transplantations. Am Rev Respir Dis. 1992;145:1178–1184. doi: 10.1164/ajrccm/145.5.1178. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald G, Shi L, Vande VC, Lieberman J, Greenberg AH. Mitochondria-dependent and -independent regulation of Granzyme B-induced apoptosis. J Exp Med. 1999;189:131–144. doi: 10.1084/jem.189.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spaeny-Dekking EH, Hanna WL, Wolbink AM, Wever PC, Kummer AJ, Swaak AJ, Middeldorp JM, Huisman HG, Froelich CJ, Hack CE. Extracellular granzymes A and B in humans: detection of native species during CTL responses in vitro and in vivo. J Immunol. 1998;160:3610–3616. [PubMed] [Google Scholar]
- 36.Smyth MJ, O'Connor MD, Trapani JA. Granzymes: a variety of serine protease specificities encoded by genetically distinct subfamilies. J Leukoc Biol. 1996;60:555–562. doi: 10.1002/jlb.60.5.555. [DOI] [PubMed] [Google Scholar]
- 37.Smyth MJ, Browne KA, Thia KY, Apostolidis VA, Kershaw MH, Trapani JA. Hypothesis: cytotoxic lymphocyte granule serine proteases activate target cell endonucleases to trigger apoptosis. Clin Exp Pharmacol Physiol. 1994;21:67–70. doi: 10.1111/j.1440-1681.1994.tb02438.x. [DOI] [PubMed] [Google Scholar]
- 38.Clement MV, Haddad P, Soulie A, Legros-Maida S, Guillet J, Cesar E, Sasportes M. Involvement of granzyme B and perforin gene expression in the lytic potential of human natural killer cells. Res Immunol. 1990;141:477–489. doi: 10.1016/0923-2494(90)90017-s. [DOI] [PubMed] [Google Scholar]
- 39.Wowk ME, Trapani JA. Cytotoxic activity of the lymphocyte toxin granzyme B. Microbes Infect. 2004;6:752–758. doi: 10.1016/j.micinf.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Cheung LH, Thorpe P, Rosenblum MG. Mechanistic studies of a novel human fusion toxin composed of vascular endothelial growth factor (VEGF)121 and the serine protease granzyme B: directed apoptotic events in vascular endothelial cells. Mol Cancer Ther. 2003;2:949–959. [PubMed] [Google Scholar]
- 41.Classen CF, Falk CS, Friesen C, Fulda S, Herr I, Debatin KM. Natural killer resistance of a drug-resistant leukemia cell line, mediated by up-regulation of HLA class I expression. Haematologica. 2003;88:509–521. [PubMed] [Google Scholar]
- 42.Volm M, Koomagi R. The implications of proliferation and apoptosis for lung cancer metastasis. Oncol Rep. 1999;6:373–376. [PubMed] [Google Scholar]
- 43.Mustika R, Budiyanto A, Nishigori C, Ichihashi M, Ueda M. Decreased expression of Apaf-1 with progression of melanoma. Pigment Cell Res. 2005;18:59–62. doi: 10.1111/j.1600-0749.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- 44.Mujoo K, Cheung L, Murray JL, Rosenblum MG. Pharmacokinetics, tissue distribution, and in vivo antitumor effects of the antimelanoma immunotoxin ZME-gelonin. Cancer Immunol Immunother. 1995;40:339–345. doi: 10.1007/BF01519635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schafer J, Bachtler J, Engling A, Little JB, Weber KJ, Wenz F. Suppression of apoptosis and clonogenic survival in irradiated human lymphoblasts with different TP53 status. Radiat Res. 2002;158:699–706. doi: 10.1667/0033-7587(2002)158[0699:soaacs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Uchida H, Shinoura N, Kitayama J, Watanabe T, Nagawa H, Hamada H. Caspase-8 gene transduction augments radiation-induced apoptosis in DLD-1 cells. Biochem Biophys Res Commun. 2002;292:347–354. doi: 10.1006/bbrc.2002.6643. [DOI] [PubMed] [Google Scholar]
- 47.Rocha S, Soengas MS, Lowe SW, Glanzmann C, Fabbro D, Winterhalter K, Bodis S, Pruschy M. Protein kinase C inhibitor and irradiation-induced apoptosis: relevance of the cytochrome c-mediated caspase-9 death pathway. Cell Growth Differ. 2000;11:491–499. [PubMed] [Google Scholar]
- 48.Belka C, Heinrich V, Marini P, Faltin H, Schulze-Osthoff K, Bamberg M, Budach W. Ionizing radiation and the activation of caspase-8 in highly apoptosis-sensitive lymphoma cells. Int J Radiat Biol. 1999;75:1257–1264. doi: 10.1080/095530099139412. [DOI] [PubMed] [Google Scholar]
- 49.Prpic ML, Kastelan M, Gruber F, Laskarin G, Sotosek V, Strbo N, Zamolo G, Zauhar G, Rukavina D. Perforin expression in peripheral blood lymphocytes and skin-infiltrating cells in patients with lichen planus. Br J Dermatol. 2004;151:433–439. doi: 10.1111/j.1365-2133.2004.06086.x. [DOI] [PubMed] [Google Scholar]
- 50.Rieux-Laucat F, Le Deist F, De Saint BG. Autoimmune lymphoproliferative syndrome and perforin. N Engl J Med. 2005;352:306–307. doi: 10.1056/NEJM200501203520319. [DOI] [PubMed] [Google Scholar]
- 51.Kreitman RJ. Immunotoxins in cancer therapy. Curr Opin Immunol. 1999;11:570–578. doi: 10.1016/s0952-7915(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 52.McGrath MS, Rosenblum MG, Philips MR, Scheinberg DA. Immunotoxin resistance in multidrug resistant cells. Cancer Res. 2003;63:72–79. [PubMed] [Google Scholar]
- 53.Zhao J, Zhang LH, Jia LT, Zhang L, Xu YM, Wang Z, Yu CJ, Peng WD, Wen WH, Wang CJ, et al. Secreted antibody/granzyme B fusion protein stimulates selective killing of HER2-overexpressing tumor cells. J Biol Chem. 2004;279:21343–21348. doi: 10.1074/jbc.M312648200. [DOI] [PubMed] [Google Scholar]
- 54.Kurschus FC, Kleinschmidt M, Fellows E, Dornmair K, Rudolph R, Lilie H, Jenne DE. Killing of target cells by redirected granzyme B in the absence of perforin. FEBS Lett. 2004;562:87–92. doi: 10.1016/S0014-5793(04)00187-5. [DOI] [PubMed] [Google Scholar]