Abstract
B7-H1 molecule increases the apoptosis of tumor-reactive T lymphocytes and reduces their immunogenicity. Breast cancer is the second most common cause of mortality after lung cancer. Direct evidence linking B7-H1 with cancer has been shown in several malignancies; however, its expression in breast cancer has not been investigated. We used immunohistochemistry to investigate the expression of the B7-H1 molecule in 44 breast cancer specimens and to study its correlation with patients' clinicopathological parameters. The expression of B7-H1 was shown in 22 of 44 patients and was not restricted to the tumor epithelium (15 of 44, 34% in tumor cells), but was also expressed by tumor-infiltrating lymphocytes (TIL; 18 of 44, 41%). Interestingly, intratumor expression of B7-H1 was significantly associated with histologic grade III-negative (P = .012), estrogen receptor-negative (P = .036), and progesterone receptor-negative (P = .040) patients. In addition, the expression of B7-H1 in TIL was associated with large tumor size (P = .042), histologic grade III (P = .015), positivity of Her2/neu status (P = .019), and severe tumor lymphocyte infiltration (P = .001). Taken together, these data suggest that B7-H1 may be an important risk factor in breast cancer patients and may represent a potential immunotherapeutic target using monoclonal antibody against the B7-H1 molecule.
Keywords: Breast cancer, B7-H1, PD-L1, tumor-infiltrating lymphocytes, prognostic factors
Introduction
Breast cancer is the leading type of cancer affecting women worldwide and is the second leading cause of mortality after lung cancer [1]. Due to early detection, the majority of breast cancer patients in western countries presents with early-stage disease [2]; locally advanced breast cancer (LABC), a very rare disease, does not exceed 5% of the total diagnosed breast cancer cases. However, LABC is a very common disease in other countries such as Saudi Arabia (about 30%). It affects women at very young ages and is distinctively associated with poor outcome. More than 40% of the cases admitted to the hospital are already at very advanced stages [3,4]. The extensive heterogeneity of breast cancer complicates the precise assessment of tumor aggressiveness; this makes therapeutic decisions difficult and treatment inappropriate in some cases. Therefore, there is a strong need to identify parameters that might predict the effectiveness of new treatments for each patient, as well as to find new therapeutic options besides chemotherapy that can be tailored to each group of patients.
Immunotherapy is currently the major focus, wherein therapeutic cancer vaccines may represent major alternatives and/or adjuvant therapies besides chemotherapy [5]. Cancer cells frequently express tumor antigens that, in principle, can be recognized by the patient's immune system; however, resultant immune responses are ineffective and often do not parallel clinical tumor regression [6]. This raises an essential question in tumor immunology: Why are neoplasms expressing tumor antigens not eliminated by the patient's own immune system? A process named “tumor escape” has been suggested, and several scenarios have been proposed to explain such phenomenon [7,8]. In breast cancer, the presence of a massive lymphocytic infiltrate should constitute immune responses against the tumor; however, immune response seems to be inefficient, and the tumor is able to evade it. It has been shown that such tumor escape has resulted from the induction of apoptosis in Fas-expressing activated lymphocytes by FasL-bearing breast cancer cells [9]. Classic B7-1 and B7-2 costimulatory molecules are known to be expressed in professional antigen-presenting cells and provide positive signals to T lymphocytes through their interactions with CD28 molecule [10]. In addition, other regulatory molecules have been recently discovered and shown to provide a negative inhibitory signal to T lymphocytes. A cell surface glycoprotein B7-H1 (also called PD-L1), which is expressed by antigen-presenting cells, has been shown to induce T-lymphocyte anergy and/or apoptosis after ligation to its T-lymphocyte receptor PD-1 [11–14].
Direct involvement of B7-H1 in the protection of cancer cells from lysis by activated T lymphocytes has been demonstrated [15]. The blockade of the protein or its ligand PD-1 by specific monoclonal antibodies has been shown to reverse this effect and also to potentiate and improve the therapeutic immunity of cancer [16–19]. Moreover, the expression of B7-H1 has been described in several malignancies, including ovary cancer, colon cancer, melanoma, and lung carcinoma [13]; squamous cell carcinoma of the head and neck [16]; glioma [20]; non-small cell lung carcinoma [21]; renal cell carcinoma [22]; and, recently, esophageal cancer [23]. However, its expression in breast cancer has not been well-documented, except in five of six invasive ductal breast cancer patients [24]. Furthermore, a strong link between B7-H1 expression by cancer cells or tumor-infiltrating lymphocytes (TIL) and the patient's clinicopathological status has been shown in non-small cell lung carcinoma [21], renal cell carcinoma [22], and esophageal cancer [23]. To our knowledge, no such study has been carried out in breast cancer. In the present study, we assessed the levels of the B7-H1 protein in 44 breast cancer patients and correlated its expression with the patients' clinicopathological parameters. We found that B7-H1 was expressed in 22 of 44 (50%) breast cancer patients, and that its expression was not restricted to epithelial breast tissues but was also present in TIL. Moreover, we showed great association between B7-H1 expression and bad prognostic factors associated with high-risk patients.
Materials and Methods
Patients and Sample Collection
In this study, we concentrated on breast cancer patients diagnosed with infiltrating ductal carcinoma (IDC), which is the most common subtype of breast cancer, especially among women in the Middle East [25]. Other tumor subtypes, such as infiltrating lobular carcinoma and medullary carcinoma, were excluded from this study because they are very rare in this population [25]. Breast cancer specimens were collected from primary tumors of 44 patients (median age, 45 years) who sought treatment and underwent surgery (breast conservation surgery or total mastectomy) at the King Faisal Specialist Hospital and Research Center from 2003 to 2005. Signed informed consent was obtained from all patients. Twenty-five patients were treated with neoadjuvant chemotherapy prior to surgery, whereas 19 other patients had no chemotherapeutic treatment before surgery. Pathological tumor-node-metastasis staging was as follows: stage I (n = 4), stage II (n = 19), and stage III (n = 20). One patient had an unknown lymph node status (n = 1).
On excision of tissues by a surgeon, an anatomic pathologist obtained a sample of the tumor tissue (denoted T) and an adjacent normal breast tissue from the same breast having the tumor (denoted N). Tissues from either T or N were fixed in formalin and embedded in paraffin for routine histopathological analysis. Other pieces of tissues (N and T) were taken and snap-frozen in liquid nitrogen after being embedded in optimal cutting temperature (OCT) compound (Miles Laboratories, Elkhart, IN) and preserved at -80°C until processed.
All normal (N) breast tissues were confirmed by the pathologist to have normal morphology before the results were analyzed. Six normal samples showed evidence of tumor cell infiltration and were designated as NA (indicative of no true normal tissue). Normal breast tissues were also obtained from two healthy women who underwent plastic surgery and were designated as BP.
Breast Cancer Cell Lines and Fluorescence-Activated Cell Sorter (FACS) Analysis
The MCF-7 and MDA-MB-231 breast cancer cell lines were obtained from ATCC (Manassas, VA) and maintained in culture using a complete DMEM. These cell lines were used as negative and positive controls, respectively, for B7-H1 expression. The MCF-7 and MDA-MB-231 cell lines were stained with an anti-B7-H1-PE-labeled antibody (ebioscience, San Diego, CA). After antibody staining, cells were washed twice with cold phosphate-buffered saline (PBS) containing 2% fetal bovine serum, suspended in 300 µl of PBS, and analyzed using FACS Scan (Immunocytometry Systems; Becton Dickinson, San Jose, CA).
Immunohistochemistry
Routine hospital tests were evaluated by immunohistochemistry on formalin-fixed, paraffin-embedded breast cancer samples for Her2/neu, estrogen receptor, and progesterone receptor status. HercepTest, a commercially available kit (Dako Corp., Carpinteria, CA), was used for Her2/neu receptor staining, according to the manufacturer' instructions. A score between 0 and 3+ was recorded as illustrated in HercepTest kit guidelines. For the purpose of this study, only 3+ samples were considered Her2/neu-positive. The estrogen and progesterone receptors were stained with relevant specific antibodies (Novocastra, Newcastle upon Tyne, UK).
For B7-H1 staining, fresh tissues that have been snap-frozen in the OCT compound were sectioned, using a cryotome (Shandon, Pittsburgh, PA), into 4 to 8 µm and adhered to Superfrost slides (Fisherbrand, Pittsburgh, PA). After overnight air drying, the sections were fixed in 4°C cold acetone for 10 to 20 minutes, covered with aluminum foil, and stored frozen at -80°C until use.
Before immunostaining, the sections were incubated in PBS (Sigma, St. Louis, MO), pH 7.4, for a few minutes. Endogenous peroxidase was blocked for 15 minutes with 0.3% hydrogen peroxide (Fisher Biotech, Fair Lawn, NJ) in PBS containing 0.1% sodium azide. After two washes in PBS, sections were blocked with 10% goat serum (Dako Corp.) for 30 minutes, followed by addition of a primary antibody. Sections were stained using either a single-staining or a double-staining procedure:
Single staining: B7-H1 primary antibody (MIH1 clone; ebioscience) was used at 1:50 dilution and incubated overnight at 4°C in a humidified chamber. After washing thrice in PBS, the sections were stained for 30 minutes at room temperature with Labeled Polymer (EnVision+) horseradish peroxidase (HRP) detection kit (Dako Corp.). After washing thrice with PBS, the sections turned red, using the chromogen 3-amino-9-ethyl carbazole (Sigma), and the sections were counterstained for 1 minute with Harris hematoxylin (Acros Organics, Morris Plains, NJ). Double staining: Two different species antibodies were used at the same time. Mouse anti-B7-H1 antibody (1:10 dilution) was added to one of the following rabbit polyclonal antibodies: pan-Cytokeratin (diluted 1:100; Dako Corp.), CD3 (diluted 1:50; Dako Corp.), or CD8 (diluted 1:50; Abcam, Cambridge, UK) and incubated overnight at 4°C in a humidified chamber. Nonimmunized mouse isotype matching for B7-H1 and normal rabbit IgG were used as negative controls for other rabbit antibodies. On the following day, the sections were washed thrice with PBS and incubated with Labeled Polymer (EnVision+) HRP detection kit (Dako Corp.) mixed with swine anti-rabbit antibody at 1:50 (Dako Corp.) for 30 minutes at room temperature. After washing in PBS, the substrates were added one at a time, starting with Fast Red [for alkaline phosphatase (AP); Dako Corp.] for 15 minutes followed by DAB+ (for HRP; Dako Corp.). The sections were counterstained for 1 minute with Harris hematoxylin (Acros Organics).
For double staining with B7-H1 and Foxp3, rabbit polyclonal anti-Foxp3 antibody (diluted 1:500; Abcam) was added first for 1 hour at room temperature and washed thrice with PBS followed by Labeled Polymer (EnVision+; anti-rabbit/mouse) AP detection kit for 30 minutes at room temperature. After washing thrice in PBS, the mouse anti-B7-H1 antibody (1:10 dilution) was added and incubated for 2 hours at room temperature in a humidified chamber. On the following day, the sections were washed thrice with PBS and incubated with Labeled Polymer (EnVision+; anti-mouse) HRP detection kit (Dako Corp.) for 30 minutes at room temperature. After washing in PBS, the substrates were added one at a time, starting with Fast Red (for AP; Dako Corp.) for 15 minutes followed by DAB+ (for HRP; Dako Corp.). The sections were counterstained for 15 seconds with Instant Hematoxylin (Shandon).
For double staining with B7-H1 and CD4, two different antibodies with different mouse IgG isotypes were used at the same time. The anti-B7-H1 antibody (IgG1 at 1:10 dilution) was added to IgG2a CD4 antibody (Serotec, Raleigh, NC; diluted 1:50) and incubated overnight in a humidified chamber. On the following day, the sections were washed thrice with PBS, and two different isotype-specific secondary antibodies (goat anti-mouse IgG1-HRP and goat anti-mouse IgG2a AP; both from Southern Biotech, Birmingham, AL) were diluted at 1:50 in PBS containing 10% AB serum (Sigma) and incubated for 30 minutes at room temperature. After washing in PBS, the substrates were added one at a time, starting with Fast Red (for AP; Dako Corp.) for 15 minutes followed by DAB+ (for HRP; Dako Corp.). The sections were counterstained for 1 minute with Harris hematoxylin (Acros Organics). Slides were washed with distilled water, dried, and cover-slipped.
The percentages of tumor cells and TIL that stained positive for B7-H1 were quantified at 5 to 10 increments by two independent pathologists who had no prior knowledge of patient details. Diagnosis (type of breast cancer) was also confirmed at the time of reading. The extent of TIL was assessed and recorded as 0 (absent), 1 (focal), 2 (moderate), and 3 (severe).
Histologic Grade Evaluation
Histologic grades of breast cancer sections were evaluated according to Scarff-Bloom-Richardson (SBR) classification [26].
Statistical Analysis
Statistical analyses were used to determine the association between B7-H1 expression and the patients' clinicopathological parameters. Relationships were assessed using Fisher exact test. The significance level was set at .05, and all P values were two-sided. The software package SAS 9.1 (SAS Institute, Cary, NC) was used for these analyses.
Results
B7-H1 Is Expressed Specifically in Tumor Tissues
To standardize the B7-H1 staining procedure, two breast cancer cell lines (MCF-7 and MDA-MB-231) were stained with the B7-H1-PE-labeled antibody and analyzed by flow cytometry. The MDA-MB-231 cell line was positive for the membrane expression of B7-H1 (95%), whereas the MCF-7cell line was completely negative (data not shown). We then adhered cells to microscopic slides using cytospin and stained them with an unlabeled B7-H1 antibody. MDA-MB-231 cells stained positive, whereas a negative staining was obtained in the MCF-7 cell line (Figure 1, A and B). Cytospin slides prepared for the MCF-7 and MDA-MB-231 cell lines were used as negative and positive controls, respectively, in the subsequent immunohistochemical staining of patients' tissue sections.
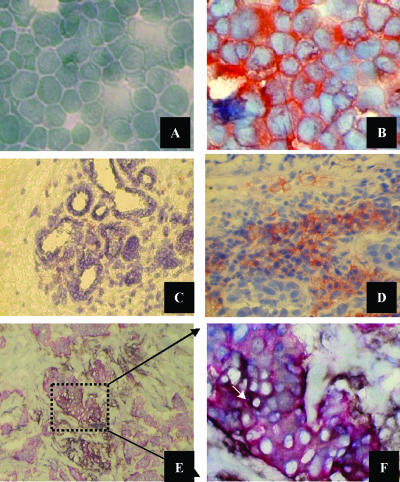
Figure 1.
Immunocytochemical staining of B7-H1-negative MCF-7 (A) and B7-H1-positive MDA-MB-231 (B) breast cancer cell lines confirm their phenotype, as shown by FACS analysis. Photomicrographs, x400 magnification. Representative immunohistochemical staining in a B7-H1-positive breast cancer patient is also presented. (C) A section from adjacent normal breast lobules (N) showing a negative reaction to B7-H1 antibody. (D) A section from the tumor tissue side (T) showing a positive reaction to B7-H1 antibody. Note that the red-orange single stain represents B7-H1 expression. The localization of B7-H1 to epithelial tissues in the tumor section (T) is presented by the double staining (arrow in F; blackish dark color) of pan-Cytokeratin (single red color) and B7-H1 (single brown color). (E) Low magnification, x100. (F) High magnification, x540.
Of the 44 patients stained for the expression of B7-H1, 22 (50%) were found to be positive [15 of 44 (34%) in tumor cells]. The B7-H1 protein was expressed in 37% of patients receiving non-neoadjuvant chemotherapy, whereas its expression has been detected in 60% of the patients treated with neoadjuvant chemotherapy (Table 1). More important, B7-H1-positive sections were restricted only to tumor tissues (T), and B7-H1-negative sections were expressed in all adjacent normal tissues (N). The expression was both membranous and/or cytoplasmic. The intensity of B7-H1 staining ranged between + (low) and +++ (high) subjective scores, whereas the percentage of tumor cells expressing B7-H1 ranged from 1% to 50% of tumor cells in a given section (Table 1). Figure 1, C and D, shows representative stained sections with the B7-H1 antibody in normal tissues (N) and tumor tissues (T), respectively. Two normal breast tissues (BP) obtained during plastic surgery were found to be negative for B7-H1 expression (data not shown). Double staining with the B7-H1 antibody and pan-Cytokeratin antibody confirmed the expression of B7-H1 by tumor cells (Figure 1, E and F). All isotype antibody-stained slides were negative in both tumor and normal tissues (data not shown).
Table 1.
Expression of B7-H1 in Breast Cancer Cells and TIL.
| B7-H1 Expression | |||||||||||||
| Non-Neoadjuvant Chemotherapy Group | Neoadjuvant Chemotherapy Group | ||||||||||||
| Patient number | N | T | % Tumor | % TIL | TIL Score | Adjusted Score | Patient number | N | T | % Tumor | % TIL | TIL Score | Adjusted Score |
| 1 | NA | - | 0 | 20 | - | - | 1 | ||||||
| 2 | - | - | 1 | 21 | - | ++ | 0 | 60 | 3 | 180 | |||
| 3 | - | - | 3 | 22 | - | ++ | 30 | 0 | 1 | 0 | |||
| 4 | NA | - | 2 | 23 | - | +++ | 30 | 0 | 1 | 0 | |||
| 5 | - | - | 1 | 24 | - | - | 1 | ||||||
| 6 | - | + | 5 | 1* | 1 | 1 | 25 | - | ++ | 0 | 30 | 3 | 90 |
| 7 | NA | ++ | 0 | 40 | 3 | 120 | 26 | - | - | 1 | |||
| 8 | - | - | 1 | 27 | - | + | 1 | 0 | 2 | 0 | |||
| 9 | - | +++ | 20 | 40 | 3 | 120 | 28 | NA | ++ | 0 | 40 | 2 | 80 |
| 10 | - | +++ | 5 | 50 | 3 | 150 | 29 | - | ++ | 10 | 20 | 3 | 60 |
| 11 | - | - | 2 | 30 | - | - | 2 | ||||||
| 12 | - | + | 50 | 70 | 3 | 210 | 31 | NA | + | 5 | 0 | 1 | 0 |
| 13 | - | - | 1 | 32 | - | + | 1 | 1 | 2 | 2 | |||
| 14 | - | +++ | 30 | 50 | 2 | 100 | 33 | - | - | 2 | |||
| 15 | - | - | 3 | 34 | - | ++ | 20 | 70 | 3 | 210 | |||
| 16 | - | - | 0 | 35 | - | +++ | 50 | 2 | 2 | 4 | |||
| 17 | - | - | 0 | 36 | - | - | 0 | ||||||
| 18 | - | - | 1 | 37 | - | +++ | 5 | 50 | 2 | 100 | |||
| 19 | - | ++ | 0 | 30 | 1 | 30 | 38 | - | +++ | 0 | 70 | 2 | 140 |
| 39 | - | - | 1 | ||||||||||
| 40 | - | - | 1 | ||||||||||
| 41 | - | - | 1 | ||||||||||
| 42 | NA | +++ | 0 | 10 | 2 | 20 | |||||||
| 43 | - | +++ | 10 | 5 | 1 | 5 | |||||||
| 44 | - | - | 1 | ||||||||||
| B7-H1-positive | 7/19 | 37% | B7-H1-positive | 15/25 | 60% | ||||||||
N, adjacent normal tissues; T, tumor tissues; NA, not available; -, negative staining intensity; +, low staining intensity; ++, medium staining intensity; +++, high staining intensity.
Adjusted score is calculated by multiplying the percentage of B7-H1 -expressing TIL by the TIL score.
The expression was checked in tumor cells and TIL, and the percentage of cells expressing B7-H1 was quantified in 5% to 10% increments by an anatomic pathologist.
B7-H1 Is Expressed in TIL
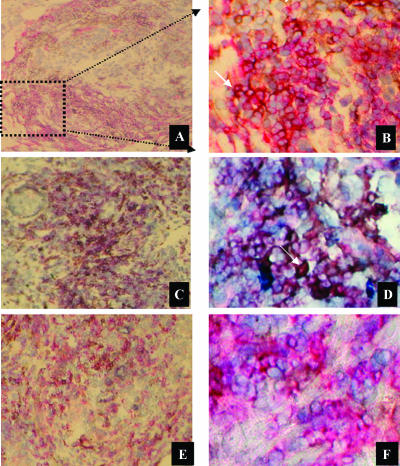
B7-H1 was found to be present in TIL of 18 patients (41 %), in addition to its expression in tumor cells. The intensity of B7-H1 staining ranged between + (low) and +++ (high), and the percentage of TIL expressing B7-H1 ranged from 1% to 70% of TIL in a given section (Table 1). B7-H1 expression by TIL in the sections was evaluated using double staining of B7-H1 in CD3+ T lymphocytes (Figure 2, A and B). The expression of B7-H1 by TIL was not necessarily associated with its presence in the tumor epithelium (only 11 of 18 patients had expression in both). Moreover, the majority of TIL presented in the patient sections was generally of the CD4+ T-lymphocyte phenotype, with only a minority of CD8+ T lymphocytes (data not shown). B7-H1 expression in TIL was highly associated with a CD4+ phenotype (Figure 2, C and D), whereas its expression in CD8+ T lymphocytes was minimal (Figure 2, E and F). We have investigated whether CD4+ T lymphocytes expressing the B7-H1 molecule are of the T-regulatory (T-reg) type. We used anti-Foxp3 antibody, together with the anti-B7-H1 antibody, to double-stain known B7-H1+ TIL sections. We have found that B7-H1+ CD4 T lymphocytes are negative for Foxp3 expression (Foxp3+ TIL were negative for B7-H1 expression).
Figure 2.
Representative immunohistochemical staining shows the expression of B7-H1 by TIL. The expression of B7-H1 by TIL is shown using double staining (arrow in B; blackish dark color) for B7-H1 (single brown color) and CD3 (single red color), and using a nuclear (blue color) counterstain. (A) Low magnification, x130. (B) High magnification, x540. The subsets of TIL expressing B7-H1 were identified using double staining (arrow in D) for B7-H1 (single brown color) and CD4 (single red color). (C) Low magnification, x130. (D) High magnification, x540. The double staining of B7-H1 and CD8 molecules is shown. (E) Low magnification, x130. (F) High magnification, x540.
B7-H1 Expression Correlates with Clinicopathological Parameters of Patients
Table 2 shows the correlation between the clinicopathological data of patients and B7-H1 expression in tumor cells or in TIL. In tumor cells, the expression of B7-H1 was significantly associated with histologicgrade III-negative (P= .012), estrogen receptor-negative (P = .036), and progesterone receptor-negative (P = .040) patients. However, the expression of B7-H1 in TIL was highly associated with large tumor size (P= .042), histologic grade III (P= .015), positivity of Her2/neu status (P = .019), and severe tumor lymphocyte infiltration (P = .001). There was no significant correlation with age, lymph node metastasis, and treatment with neoadjuvant chemotherapy.
Table 2.
Correlation between B7-H1 Expression in Tumor Cells and TIL and the Clinicopathological Parameters of 44 Breast Cancer Patients.*
| B7-H1 in Tumors | P† | B7-H1 in TIL | P | |||
| + | - | + | - | |||
| Age (years) | ||||||
| <40 | 5 (33)‡ | 10 (67) | .692 | 6 (40) | 9 (60) | .552 |
| ≥40 | 8 (28) | 21 (72) | 9 (31) | 20 (69) | ||
| Tumor size (cm) | ||||||
| <4 | 6 (25) | 18 (75) | .469 | 5 (21) | 19 (79) | .042 |
| ≥4 | 7 (35) | 13 (65) | 10 (50) | 10 (50) | ||
| Lymph node metastasis§ | ||||||
| Negative | 6 (40) | 9 (60) | .384 | 5 (33) | 10 (67) | .631 |
| 1–3 | 3 (30) | 7 (70) | 2 (20) | 8 (80) | ||
| 4–9 | 1 (9) | 10 (91) | 5 (45) | 6 (55) | ||
| ≥10 | 2 (29) | 5 (71) | 3 (43) | 4 (57) | ||
| Histologic grade (SBR) | ||||||
| II | 3 (13) | 20 (87) | .012 | 4 (17) | 19 (83) | .015 |
| III | 10 (48) | 11 (52) | 11 (52) | 10 (48) | ||
| Her2/neu status | ||||||
| Positive | 7 (44) | 9 (56) | .119 | 9 (56) | 7 (44) | .019 |
| Negative | 6 (21) | 22 (79) | 6 (21) | 22 (79) | ||
| Estrogen receptor status | ||||||
| Positive | 7 (21) | 26 (79) | .036 | 10 (30) | 23 (70) | .356 |
| Negative | 6 (55) | 5 (46) | 5 (45) | 6 (55) | ||
| Progesterone receptor status | ||||||
| Positive | 4 (17) | 20 (83) | .040 | 7 (29) | 17 (71) | .450 |
| Negative | 9 (45) | 11 (55) | 8 (40) | 12 (60) | ||
| Neoadjuvant chemotherapy | ||||||
| Without | 5 (26) | 14 (74) | .682 | 6 (32) | 13 (68) | .759 |
| With | 8 (32) | 17 (68) | 9 (36) | 16 (64) | ||
| Lymphocyte infiltration | ||||||
| None | 0 (0) | 4 (100) | .280 | 0 (0) | 4 (100) | .001 |
| Focal | 5 (28) | 13 (72) | 2 (11) | 16 (89) | ||
| Moderate | 3 (25) | 9 (75) | 5 (42) | 7 (58) | ||
| Severe | 5 (50) | 5 (50) | 8 (80) | 2 (20) | ||
In interpreting data and correlating them with clinicopathological parameters, the 5% expression of cells was the cutoff point below which the number of patients was considered as negative and above which the number of patients was considered as positive.
P values in italics represent significant data.
Numbers inside parentheses are percentages of patients.
One sample has unknown LN+ status.
Discussion
The balance between positive and negative signals is of great importance in maximizing the ability of immune response to defend the host while maintaining immunologic tolerance and preventing autoimmunity [27]. In this respect, overexpression of one of the costimulatory molecules by T lymphocytes or antigen-presenting cells can have a deleterious effect on the host immune system. This can result in either inhibition of the immune system and permission of invasion by cancer cells, or stimulation of the immune system to generate autoimmunity. For example, although the expression of B7-H1 by microglial cells is an important immune-inhibitory molecule capable of downregulating T-lymphocyte activation in the central nervous system to prevent immunopathological damage [28], expression of this molecule by malignant cells constitutes an important immune escape mechanism in cancer [13,15,29].
In spite of remarkable advances in breast cancer (traditional, targeted, and neoadjuvant) therapies, approximately 40% of women still ultimately die of this disease [30]. Manipulating the immune system to specifically recognize and destroy tumor cells may represent a major alternative approach for the management of cancer [5,6]. For example, passive immunotherapeutic strategies, including the infusion of an anti-Her2/neu monoclonal antibody (Herceptin), have been shown to be effective in a subset of breast cancer patients overexpressing the Her2/neu gene [31]. Nevertheless, active immunotherapeutic strategies with cancer vaccines, wherein the patient's immune system is manipulated to specifically eradicate breast tumor cells, may offer further theoretical advantages over other therapeutic strategies. However, the presence of an existing active immune tolerance and the antigenic variability that arises from the genetic instability of breast cancer limit the clinical efficacy of such therapeutic vaccination in patients with metastatic and advanced diseases [32]. Moreover, there is an extensive evidence for immune defects in breast cancer patients, including a lower absolute number of peripheral blood lymphocytes [33] and elevated numbers of functionally immunosuppressive CD4+CD25+ T-reg lymphocytes in both peripheral blood and tumor microenvironment [34]. In addition, dendritic cells obtained from the peripheral blood and lymph nodes of patients with operable breast cancer had a substantial decrease in major histocompatibility complex class II and B7.2 expression, and IL-12 production [35], which are essential elements for an effective immune response.
In addition, it has been shown that breast tumor cells are able to evade the host immune system by induction of apoptosis in Fas-expressing activated lymphocytes by FasL-bearing breast cancer cells, highlighting the importance of FasL as an immunoprotective molecule [9]. Another immunoregulatory molecule named B7-H1 has been shown in recent years to be directly involved in the protection of cancer cells from destruction by activated T lymphocytes [15]. A factor such as lipopolysaccharide in the cancer microenvironment has been shown to activate Toll-like receptor-4 on tumor cells and to induce the synthesis of various proteins, including B7-H1 [36]. The expression of this molecule has been described in several malignancies [13,16,20–23] where a strong link between its expression by cancer cells and the patient's clinicopathological status has been demonstrated [21–23]. However, its expression in breast cancer has not been well-investigated, except in six patients with IDC [24]. IDC is the most common subtype of breast cancer [30], especially among women in the Middle East [25].
In the present investigation, we examine the expression patterns of the B7-H1 protein in a larger number of subjects, including 44 breast cancer patients with IDC, to correlate its expression with patients' clinicopathological data. Twenty-two of 44 (50%) patients expressed the B7-H1 protein, in which the expression was based on tumor cells, TIL, or both. The percentage of cells expressing the B7-H1 was quantified in 5% to 10% increments by an anatomic pathologist. B7-H1 was expressed in 60% of patients treated with neoadjuvant chemotherapy, whereas its expression was 37% in chemotherapy-naive patients. Although its expression seems to be higher in the neoadjuvant-treated patients, there was no significant difference between the two groups. The relatively low number of patients in each group makes it difficult to draw a definitive conclusion. Nevertheless, the higher expression of B7-H1 in patients with neoadjuvant chemotherapy could simply be due to the fact that the patients receiving neoadjuvant chemotherapy usually have LABC (tumor size ≥4 cm) and therefore have worse prognosis. In a relatively large number of patients, Tringler et al. [37] reported a significant correlation between breast cancer patients treated with neoadjuvant chemotherapy and expression of another T lymphocyte-inhibitory molecule named B7-H4. An important implication of a high expression of B7-H1 in patients treated with chemotherapy is the possibility that B7-H1-expressing tumor cells are resistant to chemotherapy, leading to tumor dormancy. It has been shown recently that long-term persistent leukemic cells have increased B7-H1 expression and resist cytotoxic T lymphocyte-mediated lysis [38].
Interestingly, the expression of the B7-H1 protein was restricted to tumor tissues (T), as evidenced by its complete absence in adjacent normal tissues (N) isolated from the same patient's breast, as well as in normal breast tissues taken from patients undergoing plastic surgery. This observation is very important especially when considering the B7-H1 molecule as a potential therapeutic target in breast cancer. Anti-B7-H1-specific monoclonal antibodies that can potentiate and improve therapeutic immunity have already been investigated in other malignancies [16–19]. The expression of B7-H1 in breast cancer was both membranous and/or cytoplasmic, and this is consistent with what has been found in previous studies where other types of tumors were examined.
Another important finding in this study is the expression of B7-H1 by TIL. A similar finding has also been reported in renal cell carcinoma [22]. B7-H1 inhibits anti-cancer immune response. The mechanism by which the tumor-associated B7-H1 protein inhibits anti-cancer immune response has been shown to be interaction with its T-lymphocyte ligand PD-1, leading to impairment in both cytokine production and T-lymphocyte apoptosis [12,15,20,39]. However, the expression of B7-H1 by activated TIL can inhibit T-lymphocyte clonal expansion either by reverse signaling process [40] or by inhibition of other T lymphocytes by binding to PD-1 receptors in other T lymphocytes (T-T interaction), as has been demonstrated very recently by Seo et al. [41]. The expression of B7-H1 by CD4+CD25+ T-reg lymphocytes has been previously described by Greenwald et al. [42]. Schreiner et al. [43] have recently shown that B7-H1-dependent immune inhibition is mediated, in part, by CD4+CD25+ T-reg lymphocytes. In addition, it has been demonstrated that both peripheral blood and the tumor microenvironment of breast cancer patients had elevated numbers of functionally immunosuppressive T-reg [34]. We have shown in the present work that B7-H1 is mainly expressed by CD4+ T lymphocytes rather than by CD8+ T lymphocytes and is not of the T-reg subtype. A possible interaction between T lymphocytes expressing B7-H1 and T-reg may exist, as has been shown recently by Krupnick et al. [44].
We have also examined the relationship between the expression of B7-H1 in tumor cells or in TIL and the clinicopathological parameters of patients. Generally accepted prognostic factors in breast cancer include the following: patient age, tumor size, histologic grade, lymph node involvement, and hormonal receptors status [45]. Our findings indicate that B7-H1 correlates with important prognostic factors, which are associated with high-risk patients. The expression of B7-H1 was significantly associated with large tumor size (≥4 cm; for TIL, P = .042), histologic grade III (for tumor, P = .012; for TIL, P = .015), Her2/neu-negative status (for TIL, P = .019), negative estrogen receptor (for tumor, P = .036), and negative progesterone receptor (for tumor, P = .040). There was no significant correlation with age and lymph node metastasis. Similarly, a significant correlation between high histologic grade and B7-H1 expression was reported in renal cell carcinoma, whereas large tumor size was marginal (P = .051) [22]. Concerning progesterone status, Tringler et al. [37] have found a significant correlation between B7-H4 and progesterone-negative patients, similar to our findings. Both B7-H1 and B7-H4 molecules belong to the T lymphocyte-inhibitory members of the B7 family [42]. Further studies are needed to reveal if this converse relationship is due to molecular and/or clinicopathological parameters, which may reflect a direct linkage between progesterone status and expression of these molecules.
Thompson et al. [22] have found a correlation between B7-H1 expression and lower survival rate among renal cell carcinoma patients. B7-H1 has been shown to provide a negative inhibitory signal to T lymphocytes when expressed by antigen-presenting cells by inducing T-lymphocyte anergy and/or apoptosis [11–14]. Therefore, it is possible that, by inhibiting the immune system, B7-H1 provides more advantages for the tumor to grow. Alternatively, a lower survival rate may be due to the association of tumors with higher growth rate (large tumor size and high histologic grade) or lower differentiation (high histologic grade, estrogen receptor-negative status, and progesterone receptor-negative status), which is known to lower the survival of cancer patients [45].
We recognize one drawback that relates to the relatively low number of patients included in this current study. In particular, the available antibody we used to stain breast cancer sections is reliable only for immunohistochemical staining of cryogenic sections and is not functional for staining of embedded tissues [13]. This will, therefore, not allow for a retrospective study of archival paraffin-embedded materials. The collection of fresh frozen samples from breast cancer patients at the King Faisal Specialist Hospital and Research Center for this study was initiated in 2003.
Finally, the expression of the B7-H1 molecule in breast cancer patients may represent an important additional risk factor and may be considered as a potential immunotherapeutic target using monoclonal antibodies against this molecule. Further studies are, however, warranted to dissect the potential role of this B7-H1 molecule as a therapeutic, prognostic, and/or diagnostic factor.
Acknowledgements
We are very grateful to the administration of the research center and the Research Advisory Council for their support. We would like to thank Valorie Balde for excellent technical advice, Ayodele Alaiya for critical review, and Zuha Al-Mukhlafi for analysis of FACS data.
Abbreviations
- TIL
tumor-infiltrating lymphocytes
- LABC
locally advanced breast cancer
- IDC
infiltrating ductal carcinoma
Footnotes
This work was sponsored by the Research Advisory Council (proposal grant 2030 034).
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9:606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- 3.Ezzat AA, Ibrahim EM, Raja MA, Al-Sobhi S, Rostom A, Stuart RK. Locally advanced breast cancer in Saudi Arabia: high frequency of stage III in a young population. Med Oncol. 1999;16:95–103. doi: 10.1007/BF02785842. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim EM, al-Mulhim FA, al-Amri A, al-Muhanna FA, Ezzat AA, Stuart RK, Ajarim D. Breast cancer in the eastern province of Saudi Arabia. Med Oncol. 1998;15:241–247. doi: 10.1007/BF02787207. [DOI] [PubMed] [Google Scholar]
- 5.Dermime S, Armstrong A, Hawkins RE, Stern PL. Cancer vaccines and immunotherapy. Br Med Bull. 2002:149–162. doi: 10.1093/bmb/62.1.149. [DOI] [PubMed] [Google Scholar]
- 6.Dermime S, Gilham DE, Shaw DM, Davidson EJ, Meziane el K, Armstrong A, Hawkins RE, Stern PL. Vaccine and antibody-directed T cell tumour immunotherapy. Biochim Biophys Acta. 2004;1704:11–35. doi: 10.1016/j.bbcan.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Rivoltini L, Carrabba M, Huber V, Castelli C, Novellino L, Dalerba P, Mortarini R, Arancia G, Anichini A, Fais S, et al. Immunity to cancer: attack and escape in T lymphocyte-tumor cell interaction. Immunol Rev. 2002;188:97–113. doi: 10.1034/j.1600-065x.2002.18809.x. [DOI] [PubMed] [Google Scholar]
- 8.Dermime S, Mavroudis D, Jiang YZ, Hensel N, Molldrem J, Barrett AJ. Immune escape from a graft-versus-leukemia effect may play a role in the relapse of myeloid leukemias following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997;19:989–999. doi: 10.1038/sj.bmt.1700778. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez LS, Eliza M, Niven-Fairchild T, Naftolin F, Mor G. The Fas/Fas-ligand system: a mechanism for immune evasion in human breast carcinomas. Breast Cancer Res Treat. 1999;54:245–253. doi: 10.1023/a:1006102601215. [DOI] [PubMed] [Google Scholar]
- 10.Abbas AK, Sharpe AH. T-cell stimulation: an abundance of B7s. Nat Med. 1999;5:1345–1346. doi: 10.1038/70905. [DOI] [PubMed] [Google Scholar]
- 11.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 13.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 14.Selenko-Gebauer N, Majdic O, Szekeres A, Hofler G, Guthann E, Korthauer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, et al. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003;170:3637–3644. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- 15.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 17.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 18.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 19.Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol. 2005;17:133–144. doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
- 20.Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7467. [PubMed] [Google Scholar]
- 21.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 22.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 ex-pression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 24.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 25.Al-Kuraya K, Schraml P, Sheikh S, Amr S, Torhorst J, Tapia C, Novotny H, Spichtin H, Maurer R, Mirlacher M, et al. Predominance of high-grade pathway in breast cancer development of Middle East women. Mod Pathol. 2005;18:891–897. doi: 10.1038/modpathol.3800408. [DOI] [PubMed] [Google Scholar]
- 26.Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong A, Dermime S. Developing effective cancer vaccines: design and monitoring are critical. Br J Cancer. 2001;84:1433–1436. doi: 10.1054/bjoc.2001.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magnus T, Schreiner B, Korn T, Jack C, Guo H, Antel J, Ifergan I, Chen L, Bischof F, Bar-Or A, et al. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J Neurosci. 2005;25:2537–2546. doi: 10.1523/JNEUROSCI.4794-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong H, Chen L. B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med. 2003;81:281–287. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- 30.American Cancer Society, author. Breast Cancer Facts and Figures 2003–2004. Atlanta, GA, USA: American Cancer Society; 2004. [Google Scholar]
- 31.Emens LA, Davidson NE. Trastuzumab in breast cancer. Oncology (Huntington) 2004;18:1117–1128. (discussion 1112–1131, 1118–1137) [PubMed] [Google Scholar]
- 32.Emens LA, Reilly RT, Jaffee EM. Breast cancer vaccines: maximizing cancer treatment by tapping into host immunity. Endocr-Relat Cancer. 2005;12:1–17. doi: 10.1677/erc.1.00671. [DOI] [PubMed] [Google Scholar]
- 33.Caras I, Grigorescu A, Stavaru C, Radu DL, Mogos I, Szegli G, Salageanu A. Evidence for immune defects in breast and lung cancer patients. Cancer Immunol Immunother. 2004;53:1146–1152. doi: 10.1007/s00262-004-0556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 35.Pockaj BA, Basu GD, Pathangey LB, Gray RJ, Hernandez JL, Gendler SJ, Mukherjee P. Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Ann Surg Oncol. 2004;11:328–339. doi: 10.1245/aso.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 36.Huang B, Zhao J, Li H, He KL, Chen Y, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 37.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7-H4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11:1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 38.Saudemont A, Quesnel B. In a model of tumor dormancy, long-term persistent leukemic cells have increased B7-H1 and B7.1 expression and resist CTL-mediated lysis. Blood. 2004;104:2124–2133. doi: 10.1182/blood-2004-01-0064. [DOI] [PubMed] [Google Scholar]
- 39.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 40.Dong H, Strome SE, Matteson EL, Moder KG, Flies DB, Zhu G, Tamura H, Driscoll CL, Chen L. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J Clin Invest. 2003;111:363–370. doi: 10.1172/JCI16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seo SK, Seo HM, Jeong HY, Choi IW, Park YM, Yagita H, Chen L, Choi IH. Co-inhibitory role of T-cell-associated B7-H1 and B7-DC in the T-cell immune response. Immunol Lett. 2005;102:222–228. doi: 10.1016/j.imlet.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 43.Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung HP, Weller M, Wiendl H. Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol. 2004;155:172–182. doi: 10.1016/j.jneuroim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Krupnick AS, Gelman AE, Barchet W, Richardson S, Kreisel FH, Turka LA, Colonna M, Patterson GA, Kreisel D. Cutting edge: murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J Immunol. 2005;175:6265–6270. doi: 10.4049/jimmunol.175.10.6265. [DOI] [PubMed] [Google Scholar]
- 45.Chang J, Clark GM, Allred DC, Mohsin S, Chamness G, Elledge RM. Survival of patients with metastatic breast carcinoma: importance of prognostic markers of the primary tumor. Cancer. 2003;97:545–553. doi: 10.1002/cncr.11083. [DOI] [PubMed] [Google Scholar]