Abstract
Epstein-Barr virus (EBV) latent infection is a critical event in nasopharyngeal carcinoma (NPC) tumorigenesis. EBV-encoded genes have been shown to be involved in immune evasion and in the regulation of various cellular signaling cascades. To elucidate the roles of EBV in NPC development, stable infection of EBV in nasopharyngeal epithelial cell lines was established. Similar to primary tumors of NPC, these infected cells exhibited a type II EBV latency expression pattern. In this study, multiple cellular signaling pathways in EBV-infected cells were investigated. We first demonstrated that in vitro EBV infection resulted in the activation of STAT3 and NFκB signal cascades in nasopharyngeal epithelial cells. Increased expression of their downstream targets (c-Myc, Bcl-xL, IL-6, LIF, SOCS-1, SOCS-3, VEGF, and COX-2) was also observed. Moreover, EBV latent infection induced the suppression of p38-MAPK activities, but did not activate PKR cascade. Our findings suggest that EBV latent infection is able to manipulate multiple cellular signal cascades to protect infected cells from immunologic attack and to facilitate cancer development.
Keywords: Nasopharyngeal carcinoma, Epstein-Barr virus, NFκB, STAT3, cell signaling
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most prevalent types of cancers in southern China and Southeast Asia. More than 50,000 new NPC cases are reported each year in these regions [1]. In contrast to other head and neck cancers, the unique feature of NPC is its close association with Epstein-Barr virus (EBV). Serologic and molecular studies have suggested that EBV may play a contributory role in NPC tumorigenesis. Notably, in high-grade precancerous lesions and in invasive carcinoma of the nasopharynx in the abovementioned regions, a clonal EBV genome is consistently detected [1]. All EBV-infected NPC cells exhibit type II latency, with expression of EBV-encoded small RNA (EBER), EBV-associated nuclear antigen-1 (EBNA1), latent membrane protein 1/2 (LMP1 and LMP2), BamHI A RNA transcripts (BART), and BARF1 protein [1,2]. EBV is considered to be an oncogenic virus, owing to its ability to immortalize and transform primary cells in vitro [2]. Among EBV latent proteins, LMP1 and BARF1 have been reported to be able to transform B lymphocytes, fibroblasts, or epithelial cells in vitro [2,3]. LMP2 has been shown to mediate B-lymphocyte survival, to transform epithelial cells, and to inhibit cell differentiation [4,5]. EBNA1 and EBER, which are expressed in all types of EBV latency, have been proposed to play roles in immune evasion [3,6]. Furthermore, almost all of the EBV latent gene-encoded products are able to interact with different cellular signal proteins, suggesting the involvement of EBV latent infection in manipulating parts of a host's cellular programs [1,3,6].
In vitro, EBV can infect and efficiently transform B lymphocytes, providing a system to study the interaction of EBV with B cells [2]. In contrast, most epithelial cells are not susceptible to EBV infection in cell culture systems [7]. In addition, deriving stable EBV-positive cell lines from carcinoma tissues has been largely disappointing, owing to the loss of EBV genomes during cell line establishment [8]. Lack of suitable EBV-infected epithelial cell lines leads to poor understanding of the impact of EBV latent infection on cellular signaling cascades in human nasopharyngeal epithelial cells.
In this study, persistent EBV latent infection in malignant or immortalized nasopharyngeal epithelial cell lines was established with a recombinant EBV (rEBV) carrying a neomycin resistance (neor) gene and a green fluorescent protein (EGFP) gene. We also demonstrated that cellular signaling was altered by EBV in nasopharyngeal epithelial cells.
Materials and Methods
Cell Lines
NPC cell lines HK1 and C666-1 were routinely maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) [9,10]. The NP69, NP69-pLNSX, and NP69-LMP1 nasopharyngeal epithelial cell lines were grown in keratinocyte-SFM medium (Invitrogen, Carlsbad, CA) [11,12].
EBV Infection
Before infection, the EGFP-neorEBV-infected Akatacells were induced for the production of infectious rEBV by surface IgG cross-linking [13]. First, dialyzed anti-human IgG goat serum was added to a cell suspension (5 x 106 ml-1) to give a final concentration of 0.8 % (vol/vol). The cells were then incubated in RPMI medium with 3% FBS at 37°C for 4 hours with shaking. After sIgG cross-linking, 1 ml of EGFP-neorEBV-infected Akata cell suspension was added to the target cells, which were maintained in a T25 flask with 3 ml of fresh medium. Both cultures were then incubated for 2 to 6 days at 37°C in 5% CO2, with replacement of half of the medium after every 2 days of incubation. After completion of the infection step, cocultivated cultures were thoroughly washed four times with phosphate-buffered saline to remove residual viable Akata cells, and 3 ml of fresh medium was added. One day after infection, the cells were fed medium containing 50 to 500 µg/ml G418 for EBV-positive cell selection. After 2 to 3 weeks of selection, EBV-positive cells were observed to be green under a fluorescent microscope. The EBV-positive cells were then maintained in drug-free medium, and G418 treatment was performed only after every three to four subcultures to prevent the generation of drug-resistant EBV-negative cells. After subculture for 20 passages, the majority of the cells was still green, indicating the presence of EBV in the cells. All the experiments were then performed between passages 21 and 25. The cells were fed serum-free medium for 12 hours before cell signal pathway analyses to eliminate the influence of serum on cellular signaling.
In Situ Hybridization (ISH)
Detection of EBER (EBER-1 and EBER-2) was carried out with an EBV Probe ISH Kit (Novocastra, Newcastle, UK), as previously described [14].
Semiquantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
First-strand cDNA was synthesized from 5 µg of total RNA with oligo-dT primer and SuperScript II reverse transcriptase (Invitrogen) followed by PCR amplification using gene-specific primers listed in Table 1. To collect PCR products at the linear range, the number of PCR cycles was optimized for each primer set. For the detection of each specific gene, independent RT-PCR reactions were performed in duplicate using different sets of RNA samples as templates.
Table 1.
Oligonucleotide Primers Used in RT-PCR Analysis.
| Transcript | Primer | Oligonucleotide Sequence |
| EBER1 | 5′ | AGG ACC TAC GCT GCC CTA GA |
| 3′ | AAA ACA TGC GGA CCA CCA GC | |
| Q-EBNA1 | 5′ | GTG CGC TAC CGG ATG GCG |
| Y3-EBNA1 | 5′ | TGG CGT GTG ACG TGG TGT AA |
| K-EBNA1 | 3′ | GCT CCT GTT CCA CCG TGG GT |
| EBNA2 | 5′ | GCT GCT ACG CAT TAG AGA CC |
| 3′ | TCC TGG TAG GGA TTC GAG GG | |
| LMP1 | 5′ | AGC GAC TCT GCT GGA AAT GAT |
| 3′ | TGA TTA GCT AAG GCA TTC CCA | |
| LMP2a | 5′ | ATG ACT CAT CTC AAC ACA TA |
| LMP2b | 5′ | CAG TGT AAT CTG CAC AAA GA |
| LMP2 | 3′ | CAT GTT AGG CAA ATT GCA AA |
| BARF1 | 5′ | CAG GTT CAT CGC TCA GCT CC |
| 3′ | CAT GGG AGA TGT TGG CAG C | |
| BamHIA | 5′ | AGA GAC CAG GCT GCT AAA CA |
| 3′ | AAC CAG CTT TCC TTT CCG AG | |
| BZLF1 | 5′ | TTC CAC AGC CTG CAC CAG TG |
| 3′ | GGC AGC AGC CAC CTC ACG GT | |
| BRLF1 | 5′ | GAC CAA GCT ACC AGA GTC TAT |
| 3′ | CAG AAT CGC ATT CCT CCA GCG A | |
| CD19 | 5′ | CTG AGG ATG AGC CCC TGG GT |
| 3′ | GCC CAT CGG GAT TAT CCA TGT | |
| IL-6 | 5′ | TGG TGT TGC CTG CTG CCT TC |
| 3′ | CTC ATC TGC ACA GCT CTG GC | |
| LIF | 5′ | GGC CCG GAC ACC CAT AGA CG |
| 3′ | CCA CGC GCC ATC CAG GTA AA | |
| SOCS-1 | 5′ | CAC GCA CTT CCG CAC ATT CC |
| 3′ | TCC AGC AGC TCG AAG AGG CA | |
| SOCS-3 | 5′ | CTC GCC ACC TAC TGA ACC CTC |
| 3′ | AAG CGG GGC ATC GTA CTG GT | |
| GAPDH | 5′ | CTC AGA CAC CAT GGG GAA |
| 3′ | ATG ATC TTG AGG CTG TTG |
Indirect Immunofluorescence Assay
Cells for immunofluorescence assay were cultured in a slide chamber (Coster, Cambridge, MA) for 2 days and then were fixed with 100% methanol, in which the EGFP fluorescent signal of EBV-infected cells is destroyed. Immunostaining was performed by standard procedures. Antibodies against STAT3 were obtained from Cell Signaling, Danvers, MA.
Western Blot Analysis and Immunohistochemistry Analysis
A detailed procedure of Western blot analysis has been previously described [15]. Briefly, the cells were lysed in cell lysis buffer (Cell Signaling). The cell lysates (25–100 µg of protein) were separated in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then electrophoretically transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) for blotting. To investigate the expression of activated STAT3 in primary undifferentiated NPCs, immunohistochemistry analysis of 24 formalin-fixed paraffin-embedded sections of NPC cases was conducted by using antiphospho-STAT3 polyclonal antibody (Cell Signaling).
Electrophoretic Mobility Shift Assay (EMSA)
EMSA was performed according to the protocol of Gel Shift Assay Systems (Promega, Madison, WI). Briefly, 10 µg of nuclear proteins extracted by NE-PER Nuclear and Cytoplasmic Extraction reagents (Pierce, Rockford, IL) was incubated with 32P-labeled NFκB consensus oligonucleotide (Santa Cruz Biotechnology, Sta. Cruz, CA) for 20 minutes at room temperature. After incubation, mixtures were subjected to autoradiography and electrophoresis in a 5% polyacrylamide gel. Competition assay was performed with a 50-fold excess unlabeled NFκB consensus probe or a mutant probe (Santa Cruz Biotechnology). Super gel shift assay was performed with antibodies against NFκB-p65 and NFκB-p50 (Santa Cruz Biotechnology). Antibody against STAT3 (Santa Cruz Biotechnology) was used as nonspecific antibody.
Results
Infection of Nasopharyngeal Epithelial Cell Lines by EGFP-neorEBV
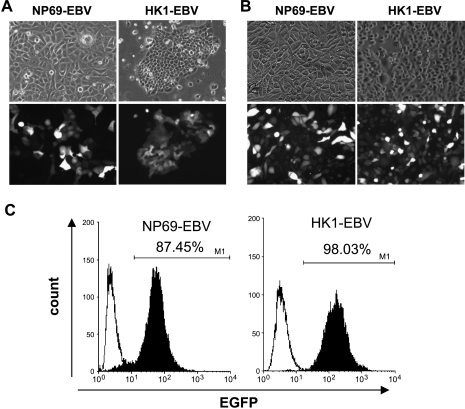
Direct cell-to-cell contact between EBV-producing B cells (Akata) and target cells was recently shown to be highly efficient in the transmission of EBV into a variety of human epithelial cells [7]. EBV transmission by cell-to-cell contact occurs independently of the CR2 receptor that mediates EBV entry into B cells [16]. In the present study, the SV40-immortalized nasopharyngeal epithelial NP69 cell line and the EBV-negative HK1 NPC cell line were subjected to EBV infection by cocultivation with Akata B cells. The latter carries an rEBV (EGFP-neorEBV) containing a neor gene and an EGFP gene. The introduction of G418-resistant (neor) and EGFP genes into the EBV genome allows selective growth of EBV-infected nasopharyngeal epithelial cells. After neomycin selection, drug-resistant cells were obtained. Although infection efficiencies were different between NP69 and HK1 cells, the cells with green fluorescence under fluorescent microscopy increased from passage 5 (Figure 1A) to passage 20 (Figure 1B) postinfection, indicating enrichment of the EGFP-neorEBV population. A high percentage of the EBV-positive cells was stably maintained in both NP69-EBV and HK1-EBV lines after passage 20. Fluorescence-activated cell sorter analysis at passage 23 postinfection demonstrated that 87.45% of EBV-infected NP69 (NP69-EBV) cells and 98.03% of HK-1 (HK1-EBV) cells were EBV-positive (Figure 1C). ISH also confirmed the presence of EBER in the majority of cells in these two EBV-infected cell lines (Figure 2A). More than 85% of the cells showed expression of EBER transcripts. The findings showed that direct cell-to-cell contact was an efficient method for the establishment of EBV-infected nasopharyngeal epithelial cells.
Figure 1.
Detection of EGFP-neorEBV in nasopharyngeal epithelial cells. Neomycin-resistant cells at (A) passage 5 and at (B) passage 20 postinfection exhibited a fluorescent signal under fluorescence microscopy, indicating EGFP-neorEBV infection (original magnification, x40). (C) Fluorescence-activated cell sorter analysis of the EGFP expression of epithelial cells at passage 23 postinfection. Filled curves represent the fluorescence intensity of EBV-infected cells, whereas black lines denote the fluorescence intensity of uninfected cells.
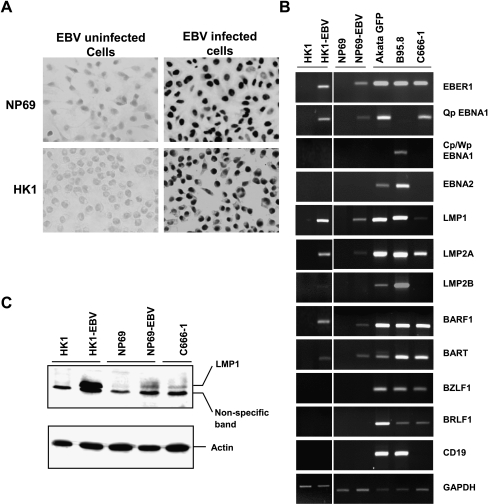
Figure 2.
EBV latency expression pattern in nasopharyngeal epithelial cells. (A) In situ RNA hybridization of EBER in EGFP-neorEBV-infected cells. (B) Semiquantitative RT-PCR analysis of EBV-associated transcripts and CD19 in EBV-infected nasopharyngeal epithelial cells. GAPDH was used as loading control. (C) Western blot analysis of LMP1 protein expression in EBV-infected cells.
EBV Infection of Nasopharyngeal Epithelial Cells Results in Type II Latency
In addition to detecting EBER transcripts by ISH, we have examined a latent gene expression pattern in two EBV-infected nasopharyngeal epithelial cell lines (NP69-EBV and HK1-EBV) by RT-PCR analysis (Figure 2B). B95.8 LCL cells, Akata B cells carrying recombinant EGFP-neorEBV, and C666-1 NPC cells were included as controls. The EBV-infected nasopharyngeal epithelial cell lines (NP69-EBV and HK1-EBV) expressed Qp-EBNA1, EBER-1, LMP1, LMP2A, BART, and BARF1 genes, but not EBNA2 and Cp/Wp-EBNA1. LMP2B was detected only in HK1-EBV cells, but not in NP69-EBV cells. Expression of lytic BZLF1 and BRLF1 was negligible. These EBV expression patterns, especially the characteristic Qp promoter usage, correspond to type II latency infection and are similar to those of the EBV-positive NPC cell line C666-1 and primary biopsies [1,10]. Lack of expression of the B-cell-specific maker CD19 in all G418-resistant cell lines confirmed the absence of contaminating Akata B cells (Figure 2B). In addition, LMP1 protein expression was examined in EBV-infected cells by Western blot analysis. The EBV-positive NPC cell line C666-1 was included as control. Similar to RT-PCR results in Figure 2B, HK1-EBV cells exhibited strong levels of LMP1, whereas NP69-EBV cells expressed a relatively low level (which was still higher than that of C666-1 cells) (Figure 2C). As controls, B95.8 cells were shown to exhibit a type III latency expression pattern. They expressed BZLF1, BRLF1, and almost all EBV latent genes. For EBNA1, only Cp/Wp promoter activity was detected. In Akata GFP cells, we detected the activity of the Qp promoter, but not of the Cp/Wp promoter. However, expression of genes for type III latency (e.g., EBNA2) and of lytic genes (BZLF1 and BRLF1) was also seen. It is suspected that RT-PCR analysis may capture a few cells that are in the lytic cycle.
Activation of STAT3 Pathway in Nasopharyngeal Epithelial Cells by EBV Latent Infection
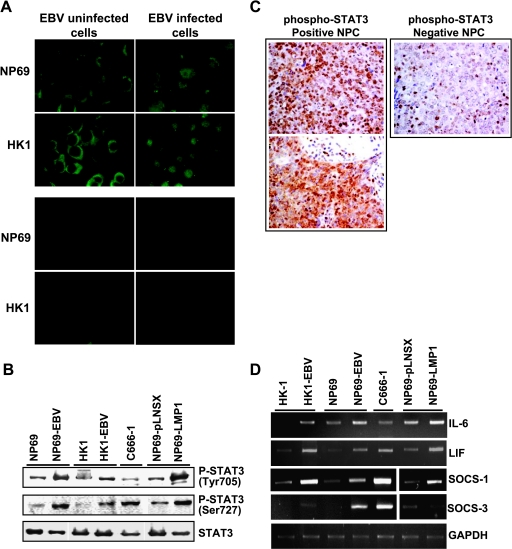
By immunofluorescent staining with STAT3-specific antibody in NP69 and HK1 cells with and without infection (Figure 3A, upper panel), the majority of STAT3 was detected in the nucleus of EBV-infected cells, indicating the activation of STAT3. In uninfected cells, STAT3 was localized in the cytoplasm, showing that STAT3 was inactive. No signal was seen in the negative control lacking primary antibody (Figure 3A, lower panel), indicating the specificity of STAT3 immunofluorescent staining. For further confirmation, Western blot analysis with antibodies specific to phospho-STAT3 (Tyr 705) and STAT3 (Ser 727) was performed. In addition to NP69-EBV, HK1-EBV, and C666-1, the LMP1 expressing NP69 cells (NP69-LMP1) and the empty vector pLNSX expressing NP69 cells (NP69-pLNSX) were also included for analysis. Both HK1-EBV and NP69-EBV demonstrated a strong expression of phospho-STAT3 when compared with their EBV-negative counterparts (Figure 3B). The C666-1 and NP69-LMP1 cells also showed strong STAT3 activity. The detection of STAT3 activation in NP69-LMP1 cells indicated that LMP1 can activate STAT3 cascade. As STAT3 activation was highly increased in EBV latent infection in nasopharyngeal epithelial cell lines, the finding suggests that activation of STAT3 by EBV may be associated with NPC pathogenesis. By using immunohistochemistry analysis with antibody specific to phospho-STAT3, STAT3 activation was investigated in primary NPCs. Phospho-STAT3 was detectable in 17 of 24 (70.8%) tumors examined (Figure 3C). All of the 24 primary tumors were positive for EBER ISH. No LMP1 protein was detected in seven primary tumors that showed no phospho-STAT3 staining. In the present study, we also determined the expression of STAT3-positive activators (including IL-6 and LIF) and STAT3-negative activators (including SOCS-1 and SOCS-3) by RT-PCR (Figure 3D). Increased expression of IL-6, LIF, and SOCS-1 was observed in all EBV-infected cells, as well as in NP69-LMP1 cells. Obvious induction of SOCS-3 was also detected in all EBV-positive cell lines, whereas the NP69-LMP1 cells expressed lower levels of SOCS-3 relative to the NP69-pLNSX cells.
Figure 3.
Activation of STAT pathways in EBV-infected nasopharyngeal epithelial cells. (A) Immunofluorescent staining with STAT3-specific antibody (original magnification, x100; upper panel). Lower panel shows negative controls in which no primary antibody was added. (B) Western blot analysis of STAT3 activation using phosphorylation-specific antibodies. Actin was included as loading control. (C) Immunohistochemical staining for phospo-STAT3. Left: Positive staining of phospho-STAT3 was exhibited in two primary NPC samples (original magnification, x400). Right: A case of NPC showed negative staining of phospho-STAT3. Positive staining of lymphocytes as internal positive control (original magnification, x400). (D) Expression of STAT3-associated genes in EBV-infected nasopharyngeal epithelial cells. Semiquantitative RT-PCR analysis of IL-6, LIF, SOCS-1, and SOCS-3.
Activation of NFκB Pathway in Nasopharyngeal Epithelial Cells by EBV Latent Infection
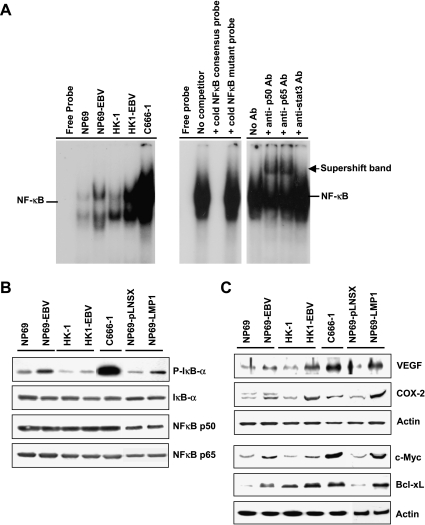
In addition to the STAT pathway, we also examined the effects of EBV latent infection on the proinflammatory NFκB pathway by EMSA. Increased activation of NFκB was observed in all cell lines with EBV infection (Figure 4A). Western blot analysis also detected increased IκB phosphorylation in EBV-infected cell lines (Figure 4B) and in NP69-LMP1 cells, demonstrating that EBV could increase the activity of NFκB signaling, possibly as a result of the effects of the LMP1 protein. Otherwise, there was no significant change in the total amounts of IκB, p50-NFκB, and p65-NFκB proteins after EBV infection, suggesting that EBV infection has no effect on the expression of NFκB signaling proteins but affects only NFκB activity (Figure 4B).
Figure 4.
Effects of EBV latent infection on the NFκB pathway in nasopharyngeal epithelial cells. (A) EMSA showing the kinetics of NFκB binding activity in nuclear extracts isolated from EBV-infected and uninfected cells (left panel). The specificity of binding was determined by competition experiments in which a nuclear extract isolated from C666-1 cells was analyzed in the presence of a 50-fold excess of an unlabelled NFκB consensus probe or an NFκB mutant probe (right panel, lanes 1–4). The activation of p50-NFκB and p65-NFκB complexes was examined by supershift analysis using antibodies against p50 and p65. Antibody against STAT3 was used as negative control (right panel, lanes 5–8). (B) Western blot analysis of NFκB activity using antibody specific for the phosphorylated form of IκB. The total levels of IκB, p50-NFκB, and p60-NFκB were also examined. (C) Western blot analysis of NFκB downstream targets (VEGF and COX-2) associated with cell invasion, and of c-Myc and Bcl-xL in EBV-infected nasopharyngeal epithelial cells.
By Western blot analysis, we showed that the NFκB downstream targets (COX-2 and VEGF) associated with invasion and angiogenesis were upregulated in protein levels in the NP69-LMP1 cell line and in all EBV-infected cell lines (Figure 4C). Moreover, c-Myc and Bcl-xL, which have been reported to be induced by STAT3 and NFκB, were also increased in the NP69-EBV, HK1-EBV, C666-1, and NP69-LMP1 cell lines (Figure 4C).
Effect of EBV Latent Infection on p38-MAPK and PKR Pathways
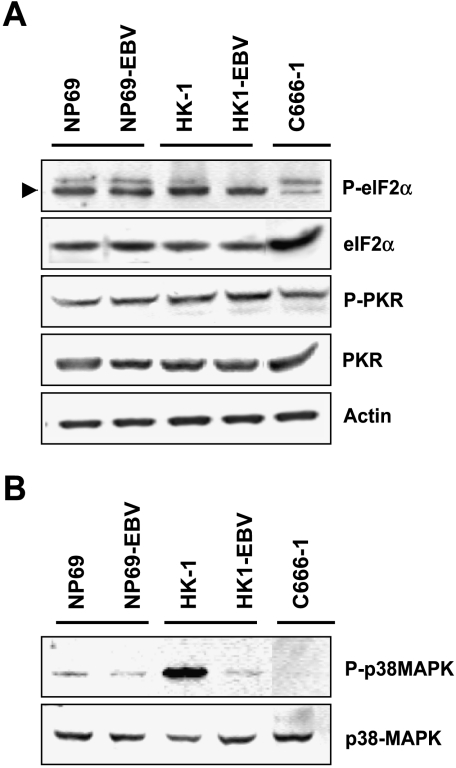
To investigate the effects of EBV on signaling cascades associated with immune response, we then examined PKR signaling, which is one of the major pathways induced after viral infection. However, levels of phospho-eIF-2α and phospho-PKR kinases had no significant change in EBV-infected nasopharyngeal epithelial cells (Figure 5A). This finding suggests that the PKR pathway failed to activate on EBV latent infection. By investigating the p38-MAPK pathway that is responsible for cytokine expression, we found that the level of phospho-p38 MAPK was dramatically reduced in HK1 cells on EBV latent infection and that the signal was undetectable in C666-1 EBV-positive NPC cells, although their basal level of p38-MAPK was clearly indicated. We detected low levels of phospho-p38 MAPK in NP69 cells. No significant change of p38 MAPK phosphorylation was found in EBV-infected NP69 cells (Figure 5B).
Figure 5.
Western blot analysis of the activity of (A) PKR and (B) p38-MAPK pathways in EBV-infected nasopharyngeal epithelial cells with antibodies specific for the phosphorylated forms of eIF-2α, PKR, and p38-MAPK.
Discussion
The roles of EBV latent infection in the tumorigenesis of NPC are of interest because of the close association of this virus with NPC. However, owing to the limitations of the EBV-positive nasopharyngeal epithelial cell model, the critical functions of EBV in NPC development remain poorly understood. By cocultivation with Akata B cells harboring rEBV, we established a stable infection of EBV in both NP69 and HK-1 nasopharyngeal epithelial cell lines. The infected cells exhibited a typical type II latent gene expression pattern. This is also reminiscent of a specific EBV latency program in NPC primary tumors [1].
The current study is the first report of multiple cellular signaling pathways being investigated in EBV-infected nasopharyngeal epithelial cells. In particular, activation of the STAT3 and NFκB pathways is predominant. Upregulation of their downstream targets was also demonstrated. Moreover, suppression of p38-MAPK signaling was demonstrated in EBV latently infected nasopharyngeal cancer cells, whereas PKR signal activity had no significant change.
STAT3 is a transcription factor that is activated by a number of ligands, including IL-6 and LIF. In response to the binding of ligands to cytokine receptors, tyrosine residues on STAT proteins are phosphorylated by Janus activated kinase family kinases. The expression of IL-6 and LIF can also be mediated by STAT3 activity [17]. Previous studies have shown that LMP1 can increase the activity of STAT3 and the expression of IL-6 [18]. Here, we showed that EBV infection and expression of LMP1 could activate STAT3 signaling and increase the expression IL-6 and LIF. These findings suggest that the expression of LMP1 in EBV latently infected epithelial cells is able to activate STAT3 signal cascade. Moreover, the expression of IL-6 and LIF mediated by LMP1 may generate a positive feedback loop to persistently activate STAT3. Constitutive activation of STAT3 has been found in a variety of malignancies, including NPC [19,20]. In the present study, we also demonstrated that constitutive activation of STAT3 was common in primary tumors of NPC. Because clonal EBV genomes and LMP1 expression are detected in high-grade preinvasive lesions and invasive carcinomas, EBV may play a critical role in transforming nasopharyngeal epithelial cells through activation of STAT3 signaling [1]. Aside from the STAT3 pathway, we found that NFκB signaling was activated in EBV latently infected nasopharyngeal epithelial cells. Therefore, increased expression of VEGF, COX-2, c-Myc, and Bcl-xL in EBV-infected cells is possibly related to the activation of NFκB and STAT3 signaling [21,22]. By upregulating the apoptosis suppressor Bcl-xL and the cell cycle activator c-Myc, EBV latent infection may mediate cell growth and prevent the apoptosis of nasopharyngeal epithelial cells. Moreover, activation of STAT3 was found to be required for de novo epithelial carcinogenesis, by maintaining the survival of DNA-damaged stem cells and mediating the proliferation necessary for clonal expansion of initiated cells during tumor promotion [23]. Furthermore, both VEGF and COX-2 are potent angiogenic factors; an association of VEGF with metastatic NPC has been reported [24]. Induction of COX-2 and VEGF by EBV may therefore play a role in angiogenesis of NPC.
It has been noted that the EBV genome is commonly lost during the establishment of NPC cell lines from biopsies or xenografts, implying that, unlike in vivo growth, EBV is not necessary for maintaining the growth of carcinoma cells in vitro [8]. This observation suggests that EBV may have other important roles in vivo, which may include, but are not limited to, conferring protection to the carcinoma cells from immune surveillance. Previous studies from other workers have shown that STAT3 can inhibit inflammation in various cell types [25,26]. Ablation of STAT3 gene in bone marrow cells results in overexpression of inflammatory cytokines and dramatic expansion of myeloid cells [25]. Wang et al. [26] have also indicated that blocking of STAT3 signaling in tumor cells leads to secretion of inflammatory cytokines, which can activate innate immune cells against tumor cells. In contrast, constitutive STAT3 signaling in tumor cells inhibits the production of multiple proinflammatory mediators and induces the production of pleiotropic factors that inhibit the maturation of dendritic cells [26]. Additionally, STAT3 signaling can induce the expression of SOCS (suppressor of cytokine signaling) proteins, which are negative regulators of cytokine-induced STAT activity [27]. It seems, however, that the increased expression of SOCS1 and SOCS3 observed in this study did not result in the suppression of STAT3 activity in EBV-infected cells. A study of T-cell lymphoma has shown that constitutive SOCS3 expression blocks IFN-α-mediated growth inhibition without affecting STAT3 activation. SOCS3 was also suggested to be a protector of tumor cells [28]. Thus, the increased activation of STAT3 as well as the expression of SOCS1 and SOCS3 by EBV in NPC cells may protect tumor cells from innate and adaptive immune responses.
p38-MAPK is a stress-dependent kinase that is also associated with the induction of inflammatory cytokines. Furthermore, PKR is a key mediator of the antiviral effect of IFN-α. It is also responsible for IFN synthesis and signaling. Our recent study of EBER expression in NP69 cells has shown that EBER is able to suppress the activation of PKR and p38-MAPK [29]. Here, in EBV latently infected nasopharyngeal epithelial cells in which EBER was expressed, we observed suppression of p38-MAPK activity and failure to activate the PKR/eIF-2α pathway, both possibly associated with the expression of EBER. Accordingly, EBV may confer protection to latently infected nasopharyngeal epithelial cells (especially the infected neoplastic cells) from immune attack, thus facilitating tumor development. This possibility is still under investigation.
Overall, this study reports our observations on important cellular signaling cascades that are affected by EBV latent infection in nasopharyngeal epithelial cells. The current findings suggest that the activation of STAT3 and NFαB, as well as the suppression of p38-MAPK, is crucial for EBV to protect infected cells from immunologic attack and to facilitate cancer development.
Abbreviations
- NPC
nasopharyngeal carcinoma
- EBV
Epstein-Barr virus
- IFN
interferon
Footnotes
This work was completed under the supervision and support of Dolly P. Huang, who passed on recently. This work was supported by the Kadoorie Charitable Foundations and the Hong Kong Research Grant Council, (central allocation: HKUST 2/03C). A. K. F. Lo is a postdoctoral fellow supported by a Croucher Foundation Fellowship, (Hong Kong).
This manuscript is dedicated to Dolly P. Huang in memory of her invaluable guidance in the design and conduct of experiments described herein.
References
- 1.Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell. 2004;5:423–428. doi: 10.1016/s1535-6108(04)00119-9. [DOI] [PubMed] [Google Scholar]
- 2.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 3.Young LS, Murray PG. Epstein-Barr virus and oncogenesis: from latent genes to tumours. Oncogene. 2003;22:5108–5121. doi: 10.1038/sj.onc.1206556. [DOI] [PubMed] [Google Scholar]
- 4.Portis T, Longnecker R. Epstein-Barr virus, (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway. Oncogene. 2004;23:8619–8628. doi: 10.1038/sj.onc.1207905. [DOI] [PubMed] [Google Scholar]
- 5.Scholle F, Bendt KM, Raab-Traub N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol. 2000;74:10681–10689. doi: 10.1128/jvi.74.22.10681-10689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanbo A, Inoue K, Adachi-Takasawa K, Takada K. Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt's lymphoma. EMBO J. 2002;21:954–965. doi: 10.1093/emboj/21.5.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imai S, Nishikawa J, Takada K. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J Virol. 1998;72:4371–4378. doi: 10.1128/jvi.72.5.4371-4378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin CT, Chan WY, Chen W, Huang HM, Wu HC, Hsu MM, Chuang SM, Wang CC. Characterization of seven newly established nasopharyngeal carcinoma cell lines. Lab Invest. 1993;68:716–727. [PubMed] [Google Scholar]
- 9.Huang DP, Ho JH, Poon YF, Chew EC, Saw D, Lui M, Li CL, Mak S, Lai SH, Lau WH. Establishment of a cell line (NPC/HK1) from a differentiated squamous carcinoma of the nasopharynx. Int J Cancer. 1980;26:127–132. doi: 10.1002/ijc.2910260202. [DOI] [PubMed] [Google Scholar]
- 10.Cheung ST, Huang DP, Hui AB, Lo KW, Ko CW, Tsang YS, Wong N, Whitney BM, Lee JC. Nasopharyngeal carcinoma cell line (C666-1) consistently harbouring Epstein-Barr virus. Int J Cancer. 1999;83:121–126. doi: 10.1002/(sici)1097-0215(19990924)83:1<121::aid-ijc21>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Tsao SW, Wang X, Liu Y, Cheung YC, Feng H, Zheng Z, Wong N, Yuen PW, Lo AK, Wong YC, et al. Establishment of two immortalized nasopharyngeal epithelial cell lines using SV40 large Tand HPV16E6/E7 viral oncogenes. Biochim Biophys Acta. 2002;1590:150–158. doi: 10.1016/s0167-4889(02)00208-2. [DOI] [PubMed] [Google Scholar]
- 12.Lo AK, Liu Y, Wang XH, Huang DP, Yuen PW, Wong YC, Tsao GS. Alterations of biologic properties and gene expression in nasopharyngeal epithelial cells by the Epstein-Barr virus-encoded latent membrane protein 1. Lab Invest. 2003;83:697–709. doi: 10.1097/01.lab.0000067480.44925.10. [DOI] [PubMed] [Google Scholar]
- 13.Maruo S, Yang L, Takada K. Roles of Epstein-Barr virus glycoproteins gp350 and gp25 in the infection of human epithelial cells. J Gen Virol. 2001;82:2373–2383. doi: 10.1099/0022-1317-82-10-2373. [DOI] [PubMed] [Google Scholar]
- 14.Chan AS, To KF, Lo KW, Mak KF, Pak W, Chiu B, Tse GM, Ding M, Li X, Lee JC., Jr High frequency of chromosome 3p deletion in histologically normal nasopharyngeal epithelia from southern Chinese. Cancer Res. 2000;60:5365–5370. [PubMed] [Google Scholar]
- 15.Lo AK, Huang DP, Lo KW, Chui YL, Li HM, Pang JC, Tsao SW. Phenotypic alterations induced by the Hong Kong-prevalent Epstein-Barr virus-encoded LMP1 variant (2117-LMP1) in nasopharyngeal epithelial cells. Int J Cancer. 2004;109:919–925. doi: 10.1002/ijc.20051. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y, Tung CH, Huang YT, Lu J, Chen JY, Tsai CH. Requirement for cell-to-cell contact in Epstein-Barr virus infection of nasopharyngeal carcinoma cells and keratinocytes. J Virol. 1999;73:8857–8866. doi: 10.1128/jvi.73.10.8857-8866.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Hutt-Fletcher L, Cao L, Hayward SD. A positive autoregulatory loop of LMP1 expression and STAT activation in epithelial cells latently infected with Epstein-Barr virus. J Virol. 2003;77:4139–4148. doi: 10.1128/JVI.77.7.4139-4148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 20.Hsiao JR, Jin YT, Tsai ST, Shiau AL, Wu CL, Su WC. Constitutive activation of STAT3 and STAT5 is present in the majority of nasopharyngeal carcinoma and correlates with better prognosis. Br J Cancer. 2003;89:344–349. doi: 10.1038/sj.bjc.6601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murono S, Inoue H, Tanabe T, Joab I, Yoshizaki T, Furukawa M, Pagano JS. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc Natl Acad Sci USA. 2001;98:6905–6910. doi: 10.1073/pnas.121016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 23.Chan KS, Sano S, Kiguchi K, Anders J, Komazawa N, Takeda J, DiGiovanni J. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest. 2004;114:720–728. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guang-Wu H, Sunagawa M, Jie-En L, Shimada S, Gang Z, Tokeshi Y, Kosugi T. The relationship between microvessel density, the expression of vascular endothelial growth factor (VEGF), and the extension of nasopharyngeal carcinoma. Laryngoscope. 2000;110:2066–2069. doi: 10.1097/00005537-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, Kano A, Iwamoto Y, Li E, Craft JE, et al. STAT3 deletion during hematopoiesis causes Crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci USA. 2003;100:1879–1884. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 27.Paukku K, Silvennoinen O. STATs as critical mediators of signal transduction and transcription: lessons learned from STAT5. Cytokine Growth Factor Rev. 2004;15:435–455. doi: 10.1016/j.cytogfr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Brender C, Lovato P, Sommer VH, Woetmann A, Mathiesen AM, Geisler C, Wasik M, Odum N. Constitutive SOCS-3 expression protects T-cell lymphoma against growth inhibition by IFNalpha. Leukemia. 2005;19:209–213. doi: 10.1038/sj.leu.2403610. [DOI] [PubMed] [Google Scholar]
- 29.Wong HL, Wang X, Chang RC, Jin DY, Feng H, Wang Q, Lo KW, Huang DP, Yuen PW, Takada K, et al. Stable expression of EBERs in immortalized nasopharyngeal epithelial cells confers resistance to apoptotic stress. Mol Carcinog. 2005;44:92–101. doi: 10.1002/mc.20133. [DOI] [PubMed] [Google Scholar]