Abstract
Human leukocyte antigen G (HLA-G) molecules corresponding to nonclassic class I genes of the major histocompatibility complex exhibit immunomodulatory properties. They are either membrane-bound or solubly expressed during certain tumoral malignancies. Soluble human leukocyte antigen G (sHLA-G) molecules seem more frequently expressed than membrane-bound isoforms during hematologic malignancies, such as lymphoproliferative disorders. Assay of these molecules by enzyme-linked immunosorbent assay in patients suffering from another hematologic disorder (acute leukemia) highlights increased sHLA-G secretion. This increased secretion seems more marked in acute leukemia subtypes affecting monocytic and lymphoid lineages such as FABM4 and FABM5, as well as both B and T acute lymphoblastic leukemia (ALL). Moreover, this study uses in vitro cytokine stimulations and reveals the respective potential roles of granulocyte-macrophage colony-stimulating factor and interferon-γ in increasing this secretion in FABM4 and ALL. Correlations between sHLA-G plasma level and clinical biologic features suggest a link between elevated sHLA-G level and 1) the absence of anterior myelodysplasia and 2) high-level leukocytosis. All these findings suggest that sHLA-G molecules could be a factor in tumoral escape from immune survey during acute leukemia.
Keywords: Soluble human leukocyte antigen G (sHLA-G), acute myeloblastic leukemia (AML), acute lymphoblastic leukemia (ALL), myelodysplasia, immunomodulation
Introduction
Human leukocyte antigen G (HLA-G) is a nonclassic major histocompatibility complex class I gene encoding for a protein with a cytoplasmic tail that is shorter than those of its classic counterparts [1], showing restrictive distribution [2–4] and lower polymorphism [5]. By alternative splicing, the HLA-G gene generates seven transcriptional isoforms encoding four membrane-bound protein isoforms (HLA-G1, HLA-G2, HLA-G3, and HLA-G4) [6,7] and three soluble isoforms (HLA-G5, HLA-G6, and HLA-G7) [8,9]. Membrane-bound HLA-G isoforms are expressed by extravillous cytotrophoblast cells [4], fetal capillary endothelial cells [2], endovascular cells [10], and thymic epithelium cells [3]. They can also be detected in tumoral pathologies such as melanomas [11]; breast [12], renal [13], and lung carcinomas [14]; gliomas [15]; and cutaneous lymphomas [16].
HLA-G is the only class I gene that allows the generation of a spliced transcript with the persistence of intron 4 or intron 2 containing a stop codon, thus preventing translation upstream of the intracytoplasmic domain. It has also been shown that a shedding form of HLA-G1 protein (sHLA-G1) [17,18], which is cleaved by metalloproteinases, coexists with HLA-G5 soluble isoforms generated by the translation of spliced mRNA [17]. HLA-G5 is composed of α1, α2, and α3 domains, whereas HLA-G6 is composed of α1 and α3 domains, allowing an association with light-chain β2-microglobulin and CD8 molecules. A new variant, HLA-G7, has been recently described. This variant is composed of α1 domains only, but little is known about its functions [9]. HLA-G5 is the full-length soluble isoform, in the same way that HLA-G1 is for membrane-bound isoforms, and it has the same properties as those of other soluble isoforms. Interest in soluble human leukocyte antigen G (sHLA-G) molecules is constantly growing. Indeed, sHLA-G molecules are expressed during pregnancy by cytotrophoblast cells invading the maternal decidua and are secreted by villous trophoblasts [19]. Basal plasma level has been detected in amniotic fluid and in healthy subjects, without any difference between the sexes [20]. Secretion of sHLA-G isoforms could be detected in the sera of heart-transplanted patients and during tumoral pathologies. Secretion has been observed in ascites of patients suffering from ovarian and breast carcinomas [21] and melanomas [22]. However, the cell type responsible for the secretion has not yet been defined, although monocytes are considered as good candidates under physiological conditions. An increase in sHLA-G secretion has also been detected in the plasma of patients who have developed B or T non-Hodgkin lymphoma (NHL) [23].
The immunomodulatory role of HLA-G is well-known in the context of pregnancy, as well as during immune reactions through interactions with inhibitory receptors. HLA-G1 has been demonstrated to inhibit cytotoxic lysis by NK cells, as well as by lymphocyte T CD8+ cells through direct interactions with ILT-2 and p49KIR2DL4 receptors [24–27] or through indirect interactions with CD94/NKG2A after induction of HLA-E expression [28]. Moreover, sHLA-G isoforms appear to be able to induce the apoptosis of CD8+ T cells through ligation with CD8 [29], a characteristic shared with classic soluble HLA class I molecules, according to some authors [30,31]. HLA-G1 and HLA-G5 are also capable of: 1) inhibiting the allogeneic proliferation of T cells [32,33]; 2) interfering with the priming of naive CD8+ T cells; and 3) inhibiting allogeneic lysis in gliomas and myoblast models [15,34,45].
Soluble proteins seem to be more frequently expressed than membrane-bound isoforms in hematologic malignancies, as demonstrated for lymphoproliferative disorders [23]. This study aims to investigate the expression of sHLA-G in another hematologic tumoral process affecting less mature cells—acute leukemia. This tumoral process comprises the proliferation of immature and undifferentiated hematopoietic cells called blasts, which induces the blockage of normal hematopoietic differentiation. Some subtypes of these pathologies, such as clonal hematopoietic disorders characterized by cytopenia and bone marrow dysplasia, can also be associated with myelodysplasia. This results from the proliferation, differentiation, and apoptotic processes of hematopoietic precursors, often evolving into acute myeloid leukemia. Acute leukemia subtypes have been classified according to the French-American-British (FAB) classification and more recently according to the World Health Organization classification. This study focuses on six FAB subtypes of acute leukemia, using a classification routinely adopted on diagnosis for the period considered: 1) four acute myeloblastic leukemia (AML) subtypes: FABM1 (myeloblastic leukemia without maturation), FABM2 (myeloblastic leukemia with maturation), FABM4 (myelomonocytic leukemia), and FABM5 (monocytic leukemia); and 2) Tor B acute lymphoblastic leukemias (ALLs) (B-ALL and T-ALL). In one of our previous studies, we showed that HLA-G is transcribed in 2 of 13 cases of acute leukemia characterized by a monocytic contingent [35]. In a subsequent study, no HLA-G surface or cytoplasm expression was detected in normal or malignant cells, including 17 AML and 15 ALL cases [36]. Moreover, some authors have failed to find surface HLA-G expression in leukemic cells without incubation using interferon-γ (IFN-γ) [37], whereas others have not detected any HLA-G expression at all [38]. Because we previously found an increase of sHLA-G molecules in mature lymphoid malignancies [39], contrasting with the absence of cell surface expression, this study focuses on the expression of sHLA-G molecules in immature hematopoietic malignancies (i.e., acute leukemia). We also investigate here the impact of cytokine microenvironment on sHLA-G secretion, based on previous studies performed on the U937 cell line [36], T-NHL cells [23], pulmonary tumors [14], and cutaneous lymphomas [16]. In this study, the sera of patients suffering from acute leukemia were assayed by the enzyme-linked immunosorbent assay (ELISA) sandwich method, yielding increased sHLA-G levels for both AML and ALL pathologies and being even more marked for FABM4 and FABM5 as well as for B-ALL and T-ALL. The study of cytokine impact revealed a potential role of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IFN-γ in AML and ALL pathologies, respectively, in controlling the increase in sHLA-G levels. We also established correlations between elevated levels of sHLA-G and two clinical biologic criteria (myelodysplasia and leukocytosis).
Methods
Patients
Seventy-five patients suffering from acute leukemia who were treated at the Department of Hematology, Rennes Hospital, were included in this retrospective study (between February 2001 and April 2004). The pathologies were classified according to the system used at that time (i.e., FAB classification): 47 patients suffered from AML (7 with FABM1, 27 with FABM2, 7 with FABM4, and 6 with FABM5) and 28 patients suffered from ALL (11 with B-ALL and 17 with T-ALL). Plasma samples were obtained from EDTA-anticoagulated blood after centrifugation (1000g) at 4°C and then cryopreserved at -80°C. Plasma from healthy subjects (n = 37) was purchased from the Etablissement Français du Sang Bretagne (Rennes, France) and cryopreserved at -80°C before being used as controls.
Cytokines
Interleukin (IL) 2 (107 U/mg), IFN-γ (107 U/mg), and IL-10 (>5 x 105 U/mg) were supplied by Tebu-Peprotech (Le Perrayen-Yvelines, France). Human GM-CSF (specific activity > 107 U/mg) was provided by Gentaur (Brussels, Belgium).
Cell Cultures
Mononuclear cell samples were obtained from 21 patients suffering from AML and exhibiting high sHLA-G levels (FABM1, n = 5; FABM2, n = 5; FABM4, n = 5; FABM5, n = 6) and from 12 patients suffering from ALL (B-ALL, n = 6; T-ALL, n = 6). Mononuclear cells from leukemic blood were obtained by Ficoll density gradient centrifugation and cryopreserved at -196°C. Cell samples were made up of >80% tumoral cells. Constant numbers of cells (1 x 106ml-1) were cultured in six-well plates for 48 hours in a culture medium (RPMI; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 2 mM glutamine, 1% sodium pyruvate, and penicillin-streptomycin. The cultures were prepared in the presence or absence of different combinations of cytokines. The cytokines were used at the following concentrations under different conditions: IFN-γ alone (500 U/ml), IFN-γ (500 U/ml) with GM-CSF (100 U/ml), IFN-γ (100 U/ml) with GM-CSF (100 U/ml) and IL-2 (220 U/ml), and IL-10 alone (50 ng/ml).
RNA Isolation and Reverse Transcription Polymerase Chain Reaction (PCR)
Total RNA was extracted from each culture condition using TRI reagent (Euromedex, Mundolsheim, France), according to the manufacturer's recommendations. RNA was then quantified by a spectrophotometer. cDNA synthesis was performed using Superscript II Reverse Transcriptase (Invitrogen), according to the manufacturer's recommendations.
Real-Time Quantitative PCR
cDNA of leukemic cells from all HLA-G isoform transcripts were quantified by duplex PCR during 40 cycles in the presence of TaqMan Universal PCR Master Mix on an ABI prism 7000 instrument (Applied Biosystems, Foster City, CA), as previously described [40]. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as endogenous control (Applied Biosystems). All samples were analyzed in duplicate. Quantification is given relative to transcripts levels of JEG3, a cell line expressing HLA-G transcripts. The transcription levels were calculated as follows: ΔCt sample = Ct HLA-G - Ct GAPDH and ΔΔCt = ΔCt sample - ΔCt JEG3. The quantity of HLA-G transcripts is defined by 2ΔΔCt.
Specific sHLA-G ELISA
sHLA-G isoforms (HLA-G5 and sHLA-G1) were quantified using a specific sandwich ELISA, with MEM-G/9 and anti-β2-microglubulin horseradish peroxidase as capture and revelation antibodies, respectively, which were validated at the “Wet Workshop for Quantification of Soluble HLA-G in Essen, 2004” [41]. Standard curves were obtained by the quantification of serial dilutions of calibrated supernatants of the LCL 721.221-G5 cell line and by validation by an internal control consisting of another HLA-G5 sample of known concentration that was purified as previously described [33]. HLA-G concentrations of the samples were determined from their optical densities in relation to standard experimental curves.
Determination of Cytokine Profile in Plasma Samples
Levels of IL-10, IL-4, IL-6, IL-2, IFN-γ, and tumor necrosis factor α (TNF-α) in the plasma of patients suffering from acute leukemia were determined using Cytometric Bead Array (BD Biosciences, Mountain View, CA) on a FACSCalibur cytometer (BD Biosciences).
Statistical Analysis and Clinical Biologic Correlations
The statistical tests used here (i.e., Student's t test, Wilcoxon matched-pair rank test, and Mann-Whitney U test) are discussed in detail in Results. Correlations of clinical biologic criteria with HLA-G levels were evaluated using one-way analysis of variance (ANOVA). Tests were considered significant when P < .05.
Results
sHLA-G Molecules Are Increased in the Plasma of Patients Suffering from Acute Leukemias
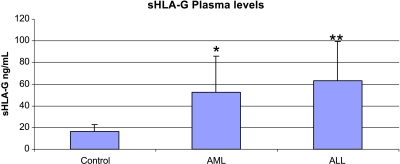
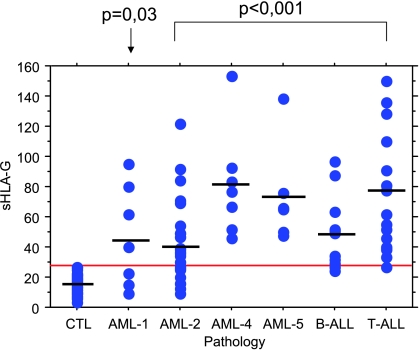
The mean sHLA-G plasma level of healthy subjects (n = 37) is 16.23 ± 6.17 ng/ml. This level is considered as the physiological level closest to that found in other ELISA studies [22,42]. The threshold above which sHLA-G level is considered pathologic in patients is defined as follows: mean of control plasma level +2 SD (>28.6 ng/ml). Quantitative variables are expressed as mean ± SEM. Differences between healthy subjects and patients suffering from AML and ALL are analyzed here using Student's t test and are considered as significant when P < .01. sHLA-G level means are significantly increased for AML patients (n = 47; mean = 52.8 ± 32.7 ng/ml, P < .01) as well as for ALL patients (n = 28; mean = 63.4 ±35.8 ng/ml, P < .001) (Figure 1). Thirty-five of 47 (74%) patients with AML are considered as pathologic, according to their sHLA-G plasma level. Interestingly, FABM4 and FABM5 yield the highest means, with values of 81.4 ± 35.7 and 73.7 ± 33.5 ng/ml, respectively. In contrast, FABM1 and FABM2 levels (46.4 ± 33.3 and 42.6 ± 26.6 ng/ml, respectively) are lower. All patients suffering from FABM4 and FABM5 show crossing of their sHLA-G pathologic threshold (Figure 2). In addition, 25 ALL patients (89%) display an increased sHLA-G plasma level. T-ALL cases exhibit more elevated means (i.e., 72.9 ± 39.6 ng/ml), and 16 of 17 patients show an increase in their sHLA-G plasma level (Figure 2). Seven of 11 patients suffering from B-ALL show an increased sHLA-G level, but to a lesser extent with respect to the sHLA-G mean level (49.6 ± 24.6 ng/ml). Each of the sHLA-G plasma levels for the different pathology subtypes is significantly increased compared to those of controls (P < .001), except for FABM1 (P = .03), according to the nonparametric Mann-Whitney U test that is suitable for small sample sizes (significant when P < .001).
Figure 1.
sHLA-G plasma levels of leukemic patients measured by specific ELISA compared with those of healthy subjects. Mean plasma levels ± SD (ng/ml) are represented by histograms for healthy subjects (n = 37), AML (n = 47), and ALL (n = 28). Both AML (*P < .01) and ALL (**P < .001) show a significant increase in sHLA-G level compared to that in controls, according to Student's t test. AML = acute myeloblastic leukemia; ALL = acute lymphoblastic leukemia.
Figure 2.
Plasma levels of sHLA-G as a function of acute leukemia subtype. Each dot represents a plasma level of one patient measured by specific ELISA. The horizontal line represents the sHLA-G positivity threshold defined by the mean of the sHLA-G level of healthy subjects (n = 37) +2 SD (28.6 ng/ml). Horizontal bars represent mean plasma levels for each pathology. sHLA-G plasma levels considered as pathologic are distributed as follows: 4 of 7 for FABM1, 18 of 27 for FABM2, 7 of 7 for FABM4, 6 of 6 for FABM5, 7 of 11 for B-ALL, and 16 of 17 for T-ALL. All subtypes show significantly increased sHLA-G levels, according to the Mann-Whitney U test (P < .001), except for FABM1 (P = .03).
sHLA-G Secretion Is Induced In Vitro after Cytokine Stimulation of FABM4, T-ALL, and B-ALL Cells
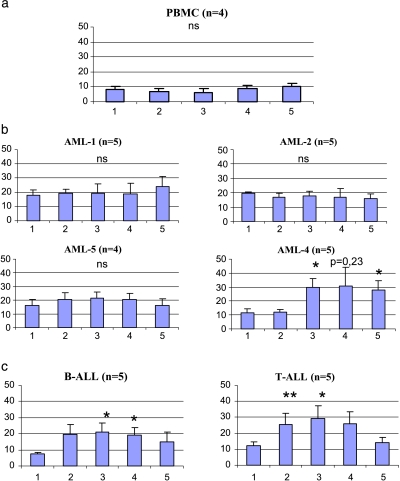
An in vitro study was performed on cells provided by patients selected for their high sHLA-G plasma levels. Different results are observed according to the subtype of AML (Figure 3). We compare variations of sHLA-G secretion between untreated and cytokine-stimulated cells using the Wilcoxon matched-pair signed rank test, which is suitable for the analysis of paired variations in small sample sizes (n < 20). P < .05 is considered significant.
Figure 3.
sHLA-G levels of culture supernatants measured by specific ELISA with or without 48 hours of cytokine stimulation of acute leukemia cells. Histograms represent sHLA-G levels (ng/ml) ± SE for the following conditions: (1) control (without stimulation); (2) IFN-γ (500 U/ml); (3) IFN-γ (500 U/ml) + GM-CSF (100 U/ml); (4) IFN-γ (100 U/ml) + GM-CSF (100 U/ml) + IL-2 (220 U/ml); (5) IL-10 (50 ng/ml). (a) Stimulation ofPBMC from healthy donors. No sHLA-G secretion increase is observed whatever the condition. (b) Stimulation of AML cells. Only FABM4 shows a significant increase in sHLA-G secretion (*P < .05) for conditions (3) and (5), according to the Wilcoxon paired test. FABM1, FABM2, and FABM5 cell stimulations do not yield any increase in sHLA-G level compared to that in controls. (c) Stimulation of ALL cells. B-ALL and T-ALL show a significant increase in sHLA-G secretion (*P < .05) for conditions (3) and (4) compared to that in controls. In addition, T-ALL cells stimulated under condition (2) show a significant increase in sHLA-G secretion (P < .05).
Stimulation of peripheral blood mononuclear cells (PBMC) provided by nonselected healthy controls (n = 5) exhibiting basal sHLA-G levels did not yield any significant increase in sHLA-G levels compared with that in control conditions, without any cytokine stimulation (Figure 3a). Cytokine stimulation of FABM1 (n = 5) and FABM2 (n = 5) cells failed to produce any increase in sHLA-G secretion compared to that in controls corresponding to nonstimulated leukemic cells, under any of the tested conditions (Figure 3b). By contrast, stimulation of FABM4 cells (n = 5) led to a significant increase in sHLA-G secretion compared to that in controls (11.8 ng/ml), in the presence of IFN-γ and GM-CSF (29.6 ng/ml) or with IL-10 alone (28.1 ng/ml). Although we observed an increase in sHLA-G level in the presence of IFN-γ, GM-CSF, and IL-2 (31 ng/ml), it remains nonsignificant. However, FABM5 cells (n = 4) do not seem to react in the presence of any of the tested cytokines. We also observed that leukemic cells from the lymphoid lineage are sensitive to in vitro cytokine stimulation. Both B-ALL and T-ALL show significant responses in terms of their sHLA-G secretion compared to that in controls, as illustrated in Figure 3c (three- to four-fold increase), except for conditions with IL-10 or IFN-γ stimulation on B-ALL (increase from 7.45 to 19.4 ng/ml, although nonsignificant).
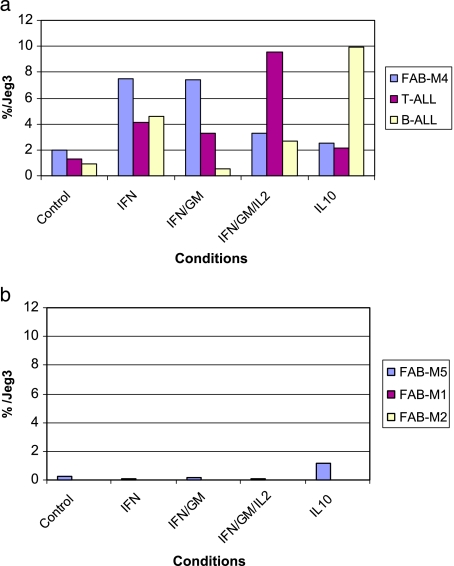
The results obtained from transcript level studies also show an increase in the HLA-G mRNA level compared to that in controls. The stimulation was performed using reactive cells tested for sHLA-G secretion (FABM4, B-ALL, and T-ALL), as illustrated in Figure 4a. In contrast, nonresponding pathologies tested for secretion (FABM1, FABM2, and FABM5) failed to show any increase in the HLA-G mRNA level, whatever the situation (Figure 4b).
Figure 4.
mRNA levels of the HLA-G transcripts of pathologic cell samples stimulated by cytokines for 48 hours, as evaluated by real-time quantitative PCR, quoted relative to mRNA levels of JEG3 (%), with the choriocarcinoma cell line constitutively expressing HLA-G transcripts. The transcription level of JEG3 is defined arbitrarily as 100%. (a) Three representative cases (FABM4, B-ALL, and T-ALL) of pathologies responding to cytokine stimulations as well as (b) three representative cases of nonresponding pathologies (FABM5, FABM1, and FABM2) are illustrated by histograms representing HLA-G mRNA levels according to each condition: (1) control (without stimulation); (2) IFN-γ (500 U/ml); (3) IFN-γ (500 U/ml) - GM-CSF(100 U/ml); (4) IFN-γ (100 U/ml) - GM-CSF (100 U/ml) - IL-2 (220 U/ml); (5) IL-10 (50 ng/ml).
Cytokine Profile in the Studied Pathologies
The specific cytokine profile Th1/Th2 (levels of IL-2, IFN-γ, TNF-α, IL-4, IL-6, and IL-10) was evaluated for all patients included in the sHLA-G level study. Except for a few patients with detectable levels of plasma IL-6, IL-10, and, sometimes, IFN-γ, the analysis failed to show any correlation between these levels and the pathology subtype or the HLA-G plasma level. However, TNF-α and IL-2 were detected in none of the patients.
Clinical Biologic Correlations
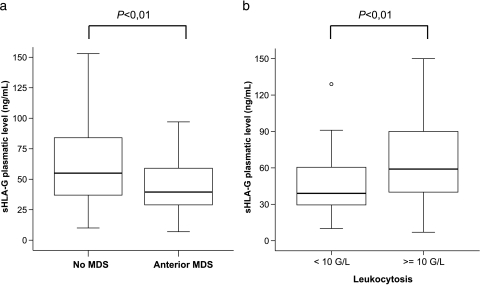
We performed one-way ANOVA between the HLA-G plasma levels of patients and relevant clinical biologic criteria for leukemias. No correlation was found with remission delay, occurrence of relapse, or death. However, ANOVA statistics reveal a highly significant relationship between elevated sHLA-G plasma levels and the absence of anterior myelodysplasia (P < .01) (Figure 5a), along with high-level leukocytosis (P < .01) (Figure 5b).
Figure 5.
Correlation of sHLA-G plasma levels with clinical biologic criteria. Upper and lower limits represent the 75th and 25th percentiles, respectively. The middle line indicates medians. (a) Correlation with anterior myelodysplasia. The two boxes represent the following groups: patients without anterior myelodysplasia and patients with anterior myelodysplasia. ANOVA reveals a significant difference in sHLA-G levels between patients with and patients without anterior myelodysplasia (P < .01). (b) Correlation with leukocytosis. The two boxes represent the following groups: low-level leukocytosis (<10 g/l) and hyperleukocytosis (>10 g/l). ANOVA reveals a significant difference in sHLA-G levels between patients with high and patients with low leukocytosis (P < .01).
Discussion
In this retrospective study, we report that sHLA-G molecules increase in patients suffering from different acute leukemia subtypes. We also show that the cytokine environment is able to stimulate sHLA-G secretion by pathologic cells in an in vitro model, but in a differential way according to cell lineage. Previous studies on HLA-G expression in leukemic pathologies have provided discordant conclusions. We confirm the results of other authors who have not found membrane-bound or cytoplasmic HLA-G expression [36,38]. sHLA-G isoforms seem to be more frequent than membrane-bound isoforms in solid tumors (ovarian and breast cancer ascites), as well as in hematopoietic malignancies such as lymphoproliferative disorders [23,39].
Higher HLA-G levels were found in ALL and are compatible with previous data obtained on lymphoproliferative disorders. Moreover, T CD4+ lymphocytes were demonstrated as potential sHLA-G secretors during mixed allogeneic reactions [32], as well as in one case of Sezary syndrome (T cutaneous lymphoma) [23]. High sHLA-G plasma level in T lineage (72.9 ng/ml) corresponds to five times the physiologic level. FABM4 and FABM5, which correspond to myelomonocytic or monocytic proliferation, equally exhibit high sHLA-G levels (81.4 and 73.7 ng/ml, respectively). These findings are also consistent with speculations on the type of secreting cells. Under physiological conditions, monocytes are suspected to be the main sHLA-G secretors [43]. In addition, other cells from monocytic lineage, such as myeloid dendritic cells, are able to secrete HLA-G molecules [33]. Previous transcriptional studies on leukemia support these two statements because only lymphoid and monocytic tumoral cells are able to express HLA-G transcripts [35].
In vitro cytokine stimulations of leukemic cells show an effect of the previously described cytokines, alone or in combination (IFN-γ, GM-CSF, IL-2, or IL-10), on sHLA-G secretion. This ability to induce HLA-G expression has been demonstrated in the myelomonocytic cell line U937 for membrane-bound expression [36]. Moreover, the same phenomenon has been observed in T-NHL cells [23]. Other teams have induced HLA-G expression on the surface of myeloblastic leukemia cells by IFN-γ stimulation [37]. This latter result is subject to debate due to lack of incubation with AB serum, as suggested by Polakovaetal. [38]. It seems that IFN-γ preferentially activates HLA-G secretion in ALL cells. Sensitivity to IFN-γ regarding HLA-G expression has been previously reported in human gliomas [15], ovarian carcinoma cells [44], and myoblast models [45], as well as in monohistiocytic cell lines [36]. The effect of IFN-γ on HLA-G transcription might appear surprising because the common classic HLA class I interferon-stimulating response element (ISRE) is truncated in the HLA-G gene. Nevertheless, a functional ISRE upstream of the promoter region of HLA-G gene could explain the action of IFN-γ [46,47]. However, no exact correlation can be established under each condition at the level of soluble proteins and transcripts, suggesting that posttranscriptional mechanisms are involved, as previously described [14].
GM-CSF and IL-10 exert an influence on HLA-G secretion for FABM4 cells, in contrast to FABM5. This latter difference in susceptibility could be related to the presence of a mature monocytic component in FABM4, contrary to FABM5, which is defined by immature monocytic proliferation. Indeed, IL-10 has been detected and seems associated with sHLA-G expression at protein and transcript levels in pulmonary tumors and cutaneous lymphomas [14,16]. Thus, IL-10 appears able to induce sHLA-G expression in trophoblasts and monocytes [48].
The effect observed with cytokine stimulation in sHLA-G in some pathology subtypes seems to be partly related to a more elevated transcript level.
The results of cytokine stimulations led us to investigate if a particular plasma cytokine profile was characteristic of leukemic patients, knowing that data on this subject are scarce. Although only IL-10, IL-6, and IFN-γ could be detected in some cases, no conclusion could be drawn. However, we suggest that the tested cytokines can reach elevated levels near the site of initial proliferation (i.e., the bone marrow), assuming that a large number of cytokines can be synthesized by the medullar microenvironment.
In acute leukemias, several factors are relevant to prognosis, including cytogenetic abnormalities and myelodysplasia, which are now included in the World Health Organization classification [49]. The present statistical study failed to reveal any correlation with prognosis. However, two biologic features could be correlated with a high sHLA-G level: absence of anterior myelodysplasia and high-level leukocytosis (leukocyte number per unit volume of blood). The inverse correlation between sHLA-G level and anterior myelodysplasia may appear surprising, considering the poor prognosis impact of this background. However, this finding can be explained by a potential link between sHLA-G secretion and a de novo acute leukemic process unrelated to chronic pathology, such as dysplasia. A correlation between hyperleukocytosis and sHLA-G merely reflects the frequent hypercellular pattern of pathologies expressing higher levels of sHLA-G.
Because membrane-bound and sHLA-G molecules are well-known for their immunoregulatory actions [50], their expression could favor tumor escape, as has been shown in melanomas [11] and gliomas [15]. In this study, we did not find any correlation with survival or relapse delay. These findings could be related to the relatively low number of patients in the present cohort, in particular in some FAB subtypes. Because the studied pathologies are strongly acute, the immune response might not be efficient in time, explaining why no effect of sHLA-G could be observed over a short delay. It would be appropriate to carry out a kinetic study to determine the evolution of sHLA-G level and its potential association with remission or relapse episodes. The part devoted to HLA-G in tumor escape remains to be more precisely determined in these acute pathologies. The mechanisms responsible for this abnormal secretion should be further examined in a therapeutic perspective, so that we can modulate the expression of sHLA-G molecules in association with other factors.
Acknowledgement
We thank M. S. N. Carpenter for postediting of English style.
Footnotes
This work was supported by grants from the Ligue Nationale contre le Cancer (Comités des Côtes d'Armor et d'Ille-et-Vilaine).
F.G. and Y.S. are recipients of fellowships from the Ligue Nationale contre le Cancer (Comité des Côtes d'Armor) and Conseil Général des Côtes d'Armor.
References
- 1.Geraghty DE, Koller BH, Orr HT. A human major histo-compatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci USA. 1987;84:9145–9149. doi: 10.1073/pnas.84.24.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaschitz A, Lenfant F, Mallet V, Hartmann M, Bensussan A, Geraghty DE, Le Bouteiller P, Dohr G. Endothelial cells in chorionic fetal vessels of first trimester placenta express HLA-G. Eur J Immunol. 1997;27:3380–3388. doi: 10.1002/eji.1830271237. [DOI] [PubMed] [Google Scholar]
- 3.Crisa L, McMaster MT, Ishii JK, Fisher SJ, Salomon DR. Identification of a thymic epithelial cell subset sharing expression of the class Ib HLA-G molecule with fetal trophoblasts. J Exp Med. 1997;186:289–298. doi: 10.1084/jem.186.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 5.Kirszenbaum M, Djoulah S, Hors J, Prost S, Dausset J, Carosella ED. Polymorphism of HLA-G gene and protein. J Reprod Immunol. 1999;43:105–109. doi: 10.1016/s0165-0378(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 6.Ishitani A, Geraghty DE. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc Natl Acad Sci USA. 1992;89:3947–3951. doi: 10.1073/pnas.89.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirszenbaum M, Moreau P, Gluckman E, Dausset J, Carosella E. An alternatively spliced form of HLA-G mRNA in human trophoblasts and evidence for the presence of HLA-G transcript in adult lymphocytes. Proc Natl Acad Sci USA. 1994;91:4209–4213. doi: 10.1073/pnas.91.10.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii T, Ishitani A, Geraghty DE. A soluble form of the HLA-G antigen is encoded by a messenger ribonucleic acid containing intron 4. J Immunol. 1994;153:5516–5524. [PubMed] [Google Scholar]
- 9.Paul P, Cabestre FA, Ibrahim EC, Lefebvre S, Khalil-Daher I, Vazeux G, Quiles RM, Bermond F, Dausset J, Carosella ED. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum Immunol. 2000;61:1138–1149. doi: 10.1016/s0198-8859(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 10.Proll J, Blaschitz A, Hutter H, Dohr G. First trimester human endovascular trophoblast cells express both HLA-C and HLA-G. Am J Reprod Immunol. 1999;42:30–36. doi: 10.1111/j.1600-0897.1999.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 11.Paul P, Rouas-Freiss N, Khalil-Daher I, Moreau P, Riteau B, Le Gal FA, Avril MF, Dausset J, Guillet JG, Carosella ED. HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc Natl Acad Sci USA. 1998;95:4510–4515. doi: 10.1073/pnas.95.8.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefebvre S, Antoine M, Uzan S, McMaster M, Dausset J, Carosella ED, Paul P. Specific activation of the non-classical class I histocompatibility HLA-G antigen and expression of the ILT2 inhibitory receptor in human breast cancer. J Pathol. 2002;196:266–274. doi: 10.1002/path.1039. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim EC, Guerra N, Lacombe MJ, Angevin E, Chouaib S, Carosella ED, Caignard A, Paul P. Tumor-specific up-regulation of the nonclassical class I HLA-G antigen expression in renal carcinoma. Cancer Res. 2001;61:6838–6845. [PubMed] [Google Scholar]
- 14.Urosevic M, Kurrer MO, Kamarashev J, Mueller B, Weder W, Burg G, Stahel RA, Dummer R, Trojan A. Human leukocyte antigen G up-regulation in lung cancer associates with high-grade histology, human leukocyte antigen class I loss and interleukin-10 production. Am J Pathol. 2001;159:817–824. doi: 10.1016/S0002-9440(10)61756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Bornemann A, Meyermann R, Weiss EH, Melms A, Weller M. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol. 2002;168:4772–4780. doi: 10.4049/jimmunol.168.9.4772. [DOI] [PubMed] [Google Scholar]
- 16.Urosevic M, Willers J, Mueller B, Kempf W, Burg G, Dummer R. HLA-G protein up-regulation in primary cutaneous lymphomas is associated with interleukin-10 expression in large cell T-cell lymphomas and indolent B-cell lymphomas. Blood. 2002;99:609–617. doi: 10.1182/blood.v99.2.609. [DOI] [PubMed] [Google Scholar]
- 17.Park GM, Lee S, Park B, Kim E, Shin J, Cho K, Ahn K. Soluble HLA-G generated by proteolytic shedding inhibits NK-mediated cell lysis. Biochem Biophys Res Commun. 2004;313:606–611. doi: 10.1016/j.bbrc.2003.11.153. [DOI] [PubMed] [Google Scholar]
- 18.Dong Y, Lieskovska J, Kedrin D, Porcelli S, Mandelboim O, Bushkin Y. Soluble nonclassical HLA generated by the metalloproteinase pathway. Hum Immunol. 2003;64:802–810. doi: 10.1016/s0198-8859(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 19.Solier C, Aguerre-Girr M, Lenfant F, Campan A, Berrebi A, Rebmann V, Grosse-Wilde H, Le Bouteiller P. Secretion of pro-apoptotic intron 4-retaining soluble HLA-G1 by human villous trophoblast. Eur J Immunol. 2002;32:3576–3586. doi: 10.1002/1521-4141(200212)32:12<3576::AID-IMMU3576>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 20.Rebmann V, Pfeiffer K, Passler M, Ferrone S, Maier S, Weiss E, Grosse-Wilde H. Detection of soluble HLA-G molecules in plasma and amniotic fluid. Tissue Antigens. 1999;53:14–22. doi: 10.1034/j.1399-0039.1999.530102.x. [DOI] [PubMed] [Google Scholar]
- 21.Singer G, Rebmann V, Chen YC, Liu HT, Ali SZ, Reinsberg J, McMaster MT, Pfeiffer K, Chan DW, Wardelmann E, et al. HLA-G is a potential tumor marker in malignant ascites. Clin Cancer Res. 2003;9:4460–4464. [PubMed] [Google Scholar]
- 22.Ugurel S, Rebmann V, Ferrone S, Tilgen W, Grosse-Wilde H, Reinhold U. Soluble human leukocyte antigen-G serum level is elevated in melanoma patients and is further increased by interferon-alpha immunotherapy. Cancer. 2001;92:369–376. doi: 10.1002/1097-0142(20010715)92:2<369::aid-cncr1332>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 23.Sebti Y, Le Friec G, Pangault C, Gros F, Drenou B, Guilloux V, Bernard M, Lamy T, Fauchet R, Amiot L. Soluble HLA-G molecules are increased in lymphoproliferative disorders. Hum Immunol. 2003;64:1093–1101. doi: 10.1016/j.humimm.2003.08.345. [DOI] [PubMed] [Google Scholar]
- 24.Cantoni C, Verdiani S, Falco M, Pessino A, Cilli M, Conte R, Pende D, Ponte M, Mikaelsson MS, Moretta L, et al. p49, a putative HLA class I-specific inhibitory NK receptor belonging to the immunoglobulin superfamily. Eur J Immunol. 1998;28:1980–1990. doi: 10.1002/(SICI)1521-4141(199806)28:06<1980::AID-IMMU1980>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 25.Chumbley G, King A, Robertson K, Holmes N, Loke YW. Resistance of HLA-G and HLA-A2 transfectants to lysis by decidual NK cells. Cell Immunol. 1994;155:312–322. doi: 10.1006/cimm.1994.1125. [DOI] [PubMed] [Google Scholar]
- 26.Rouas-Freiss N, Goncalves RM, Menier C, Dausset J, Carosella ED. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci USA. 1997;94:11520–11525. doi: 10.1073/pnas.94.21.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouas-Freiss N, Marchal RE, Kirszenbaum M, Dausset J, Carosella ED. The alpha1 domain of HLA-G1 and HLA-G2 inhibits cytotoxicity induced by natural killer cells: is HLA-G the public ligand for natural killer cell inhibitory receptors. Proc Natl Acad Sci USA. 1997;94:5249–5254. doi: 10.1073/pnas.94.10.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarro F, Llano M, Bellon T, Colonna M, Geraghty DE, Lopez-Botet M. The ILT2(LIR1) and CD94/NKG2A NK cell receptors respectively recognize HLA-G1 and HLA-E molecules co-expressed on target cells. Eur J Immunol. 1999;29:277–283. doi: 10.1002/(SICI)1521-4141(199901)29:01<277::AID-IMMU277>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Fournel S, Aguerre-Girr M, Huc X, Lenfant F, Alam A, Toubert A, Bensussan A, Le Bouteiller P. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol. 2000;164:6100–6104. doi: 10.4049/jimmunol.164.12.6100. [DOI] [PubMed] [Google Scholar]
- 30.Contini P, Ghio M, Merlo A, Brenci S, Filaci G, Indiveri F, Puppo F. Soluble HLA class I/CD8 ligation triggers apoptosis in EBV-specific CD8+ cytotoxic T lymphocytes by Fas/Fas-ligand interaction. Hum Immunol. 2000;61:1347–1351. doi: 10.1016/s0198-8859(00)00212-3. [DOI] [PubMed] [Google Scholar]
- 31.Contini P, Ghio M, Poggi A, Filaci G, Indiveri F, Ferrone S, Puppo F. Soluble HLA-A, -B, -C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol. 2003;33:125–134. doi: 10.1002/immu.200390015. [DOI] [PubMed] [Google Scholar]
- 32.Lila N, Rouas-Freiss N, Dausset J, Carpentier A, Carosella ED. Soluble HLA-G protein secreted by allo-specific CD4+ T cells suppresses the allo-proliferative response: a CD4+ T cell regulatory mechanism. Proc Natl Acad Sci USA. 2001;98:12150–12155. doi: 10.1073/pnas.201407398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Friec G, Laupeze B, Fardel O, Sebti Y, Pangault C, Guilloux V, Beauplet A, Fauchet R, Amiot L. Soluble HLA-G inhibits human dendritic cell-triggered allogeneic T-cell proliferation without altering dendritic differentiation and maturation processes. Hum Immunol. 2003;64:752–761. doi: 10.1016/s0198-8859(03)00091-0. [DOI] [PubMed] [Google Scholar]
- 34.Wiendl H, Mitsdoerffer M, Weller M. Hide-and-seek in the brain: a role for HLA-G mediating immune privilege for glioma cells. Semin Cancer Biol. 2003;13:343–351. doi: 10.1016/s1044-579x(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 35.Amiot L, Onno M, Renard I, Drenou B, Guillaudeux T, Le Bouteiller P, Fauchet R. HLA-G transcription studies during the different stages of normal and malignant hematopoiesis. Tissue Antigens. 1996;48:609–614. doi: 10.1111/j.1399-0039.1996.tb02682.x. [DOI] [PubMed] [Google Scholar]
- 36.Amiot L, Onno M, Drenou B, Monvoisin C, Fauchet R. HLA-G class I gene expression in normal and malignant hematopoietic cells. Hum Immunol. 1998;59:524–528. doi: 10.1016/s0198-8859(98)00041-x. [DOI] [PubMed] [Google Scholar]
- 37.Mizuno S, Emi N, Kasai M, Ishitani A, Saito H. Aberrant expression of HLA-G antigen in interferon gamma-stimulated acute myelogenous leukaemia. Br J Haematol. 2000;111:280–282. doi: 10.1046/j.1365-2141.2000.02345.x. [DOI] [PubMed] [Google Scholar]
- 38.Polakova K, Krcova M, Kuba D, Russ G. Analysis of HLA-G expression in malignant hematopoetic cells from leukemia patients. Leuk Res. 2003;27:643–648. doi: 10.1016/s0145-2126(02)00228-x. [DOI] [PubMed] [Google Scholar]
- 39.Amiot L, Le Friec G, Sebti Y, Drenou B, Pangault C, Guilloux V, Leleu X, Bernard M, Facon T, Fauchet R. HLA-G and lymphoproliferative disorders. Semin Cancer Biol. 2003;13:379–385. doi: 10.1016/s1044-579x(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 40.Moreau P, Mouillot G, Rousseau P, Marcou C, Dausset J, Carosella ED. HLA-G gene repression is reversed by demethylation. Proc Natl Acad Sci USA. 2003;100:1191–1196. doi: 10.1073/pnas.0337539100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rebmann V, Lemaoult J, Rouas-Freiss N, Carosella ED, Grosse-Wilde H. Wet Workshop for Quantification of Soluble HLA-G in Essen, 2004 [report] Hum Immunol. 2005;66:853–863. doi: 10.1016/j.humimm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Creput C, Durrbach A, Charpentier B, Carosella ED, Rouas-Freiss N. HLA-G: immunoregulatory molecule involved in allograft acceptance. Nephrologie. 2003;24:451–456. [PubMed] [Google Scholar]
- 43.Rebmann V, Busemann A, Lindemann M, Grosse-Wilde H. Detection of HLA-G5 secreting cells. Hum Immunol. 2003;64:1017–1024. doi: 10.1016/j.humimm.2003.08.354. [DOI] [PubMed] [Google Scholar]
- 44.Malmberg KJ, Levitsky V, Norell H, de Matos CT, Carlsten M, Schedvins K, Rabbani H, Moretta A, Soderstrom K, Levitskaya J, et al. IFN-gamma protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism. J Clin Invest. 2002;110:1515–1523. doi: 10.1172/JCI15564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiendl H, Behrens L, Maier S, Johnson MA, Weiss EH, Hohlfeld R. Muscle fibers in inflammatory myopathies and cultured myoblasts express the nonclassical major histocompatibility antigen HLA-G. Ann Neurol. 2000;48:679–684. [PubMed] [Google Scholar]
- 46.Lefebvre S, Berrih-Aknin S, Adrian F, Moreau P, Poea S, Gourand L, Dausset J, Carosella ED, Paul P. A specific interferon (IFN)-stimulated response element of the distal HLA-G promoter binds IFN-regulatory factor 1 and mediates enhancement of this nonclassical class I gene by IFN-beta. J Biol Chem. 2001;276:6133–6139. doi: 10.1074/jbc.M008496200. [DOI] [PubMed] [Google Scholar]
- 47.Gobin SJ, van den Elsen PJ. Transcriptional regulation of the MHC class Ib genes HLA-E, HLA-F, and HLA-G. Hum Immunol. 2000;61:1102–1107. doi: 10.1016/s0198-8859(00)00198-1. [DOI] [PubMed] [Google Scholar]
- 48.Moreau P, Adrian-Cabestre F, Menier C, Guiard V, Gourand L, Dausset J, Carosella ED, Paul P. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol. 1999;11:803–811. doi: 10.1093/intimm/11.5.803. [DOI] [PubMed] [Google Scholar]
- 49.Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 50.LeMaoult J, Rouas-Freiss N, Carosella ED. Immunotolerogenic functions of HLA-G: relevance in transplantation and oncology. Autoimmun Rev. 2005;4:503–509. doi: 10.1016/j.autrev.2005.04.006. [DOI] [PubMed] [Google Scholar]