Abstract
Resistance to chemotherapy is a common problem encountered in the treatment of head and neck squamous cell carcinoma (HNSCC). Chemoresistant HNSCC tumors frequently overexpress antiapoptotic proteins, such as Bcl-xL. (-)-Gossypol, the negative enantiomer of a cottonseed polyphenol, binds to Bcl-xL and was recently been shown to inhibit HNSCC proliferation in vitro. In this study, we assessed the in vivo efficacy of (-)-gossypol in an orthotopic xenograft model of HNSCC, using two human HNSCC cell lines with high Bcl-xL expression levels. Both produced tumors in a murine floor-of-mouth model that mimics human HNSCC, exhibiting growth and invasion into adjacent tissues. Mice were randomized into three groups: vehicle control and two daily intraperitoneal (-)-gossypol treatment groups (5 and 15 mg/kg). Tumors were measured twice weekly. In the control group, tumors grew progressively, whereas in (-)-gossypol treatment groups, tumor growth was significantly suppressed. The mitotic rate in tumors from (-)-gossypol-treated animals was significantly lower than that in controls, and an increase in the percentage of apoptotic cells was observed in treated tumors versus controls. Residual tumors remained growth-suppressed for 2 weeks after cessation of (-)-gossypol treatment. Our results demonstrate that (-)-gossypol can inhibit tumor growth in an orthotopic model of aggressive HNSCC.
Keywords: Chemoresistance, (-)-gossypol, head and neck cancer, oral cancer, Bcl-xL
Introduction
In the United States, head and neck squamous cell carcinoma (HNSCC) accounts for more deaths annually than cervical cancer, melanoma, or lymphoma [1]. Aggressive surgical treatment of HNSCC is physically and emotionally debilitating. Chemotherapy (cisplatin), combined with radiation therapy, has enabled some patients to avoid radical surgery without altering disease prognosis [2]. However, the development of resistance to chemotherapy limits the effectiveness of such organ-sparing treatment approaches. Chemotherapeutic agents, particularly DNA-damaging agents such as cisplatin, function by inducing apoptosis in cancer cells [3,4]. Thus, dysregulation of apoptosis can lead to decreased tumor cell death in response to chemotherapy and/or radiation. Overexpression of the antiapoptotic protein Bcl-xL is common in both HNSCC tumor samples [5] and HNSCC cell lines [6]. In laryngeal cancer, high expression levels of Bcl-xL are associated with poor response to chemotherapy and radiation, requiring subsequent laryngectomy [5]. Recently, we have shown that high Bcl-xL expression directly correlates with resistance to cisplatin treatment in HNSCC cells in vitro [7]. Hence, the use of adjuvant agents that target antiapoptotic proteins in HNSCC may overcome cisplatin resistance, and thus has the potential to decrease patient morbidity and enhance survival.
Recently, a number of potential therapeutics targeting Bcl-2 family members have been described (reviewed in Fesik [8]). (-)-Gossypol, a small-molecule Bcl-2 and Bcl-xL inhibitor, is a polyphenol derived from cottonseed. Racemic gossypol, composed of both (-)-gossypol and (+)-gossypol, is used in herbal medicines in China. Studies on melanoma, breast cancer, and colon cancers have shown that racemic gossypol is well-tolerated and is moderately effective in reducing tumor volume [9–12]. We and others [6,13] have shown that (-)-gossypol, but not (+)-gossypol, binds to the BH3 pocket of Bcl-xL. Furthermore, the negative, but not the positive, enantiomer of gossypol effectively inhibits HNSCC cancer cell growth in vitro [6,9,10,13].
In the current study, we investigated the potential of (-)-gossypol to suppress HNSCC tumor growth in an in vivo orthotopic xenograft mouse model of aggressive human head and neck cancer. Two cell lines representing laryngeal cancer and oral cancer were used, and the ability of (-)-gossypol to suppress tumor cell growth as a single agent was determined. Effects on tumor growth, mitotic activity, and apoptosis were assessed.
Materials and Methods
Cell Culture
UM-SCC-17B and UM-SCC-1 HNSCC cell lines [14] were cultured as described previously in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY) containing 10% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin, and 50 µg/ml L-glutamine [15]. After appropriate Institutional Review Board approval for Human Subject Research had been obtained, primary oral keratinocytes were cultured from human gingival tissues in keratinocyte growth medium (KGM; Cambrex Corp., East Rutherford, NJ), as described previously [16].
(-)-Gossypol Preparation
(-)-Gossypol was purified using the method described previously [6]. It was determined by high-pressure liquid chromatography analysis to have a chemical purity of >97% and a chiral purity of >95%.
Cell Proliferation/Viability Assay
To study cell proliferation, cells were plated at 100,000 cells/well in six-well cell culture plates. After 36 to 48 hours of growth in conditions mentioned above, the medium was replaced with a 1:1 mixture of DMEM and KGM, supplemented with (-)-gossypol at various concentrations. After 5 days, the number of viable cells was determined by trypan blue enumeration assay. Cells were trypsinized, washed, and stained with trypan blue, and then viable cells were counted using a hemocytometer. Experiments were performed in triplicate.
Additionally, the MTT assay, which measures cell viability based on the mitochondrial conversion of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) from a soluble tetrazolium salt into an insoluble colored formazan precipitate, followed by spectrophotometric quantification, was used to verify trypan blue data. Cells were plated at 5000 cells/well in 96-well plates, incubated overnight, and then treated with a range of (-)-gossypol concentrations. Sample plates were incubated for 6 days in 300 µl of a 1:1 mixture of DMEM and KGM containing (-)-gossypol or solvent controls. MTT assays were then performed according to the manufacturer's protocol (Roche Diagnostics, Mannheim, Germany). Each experimental condition was repeated in 10 wells, and the results were averaged. Percent absorbance relative to control was plotted as a linear function of drug concentration. Fifty percent inhibitory concentration (IC50) was identified as the drug concentration required to achieve 50% growth inhibition relative to untreated controls.
Immunodeficient Mouse Tumor Model
Six-week-old athymic nude mice (NCr-nu/nu strain; National Cancer Institute, Frederick, MD) weighing 18 to 25 g were anesthesized with 100 mg/kg ketamine and 10 mg/kg xylazine, administered intraperitoneally. HNSCC cell lines, grown to 50% to 70% confluence, were suspended in DMEM at 1 to 3 million cells per 0.2 ml and injected submucosally in the floor of the mouth. Using digital calipers, tumors were measured biweekly in two dimensions, and tumor volume was calculated using the formula: a x a x b/2, where a is the smaller dimension. Tumor cell dose was optimized for each cell line to produce 4- to 5-mm tumors, corresponding to tumors 35 to 60 mm3 in diameter, within ∼2 to 3 weeks. Prior to initiating treatment, the mice were randomly assigned to three groups. (-)-Gossypol was dissolved in ethanol, and water was added to make a stock solution with a final ethanol content of 10%. Daily intraperitoneal administration of 5 mg/kg (-)-gossypol, 15 mg/kg (-)-gossypol, or 10% ethanol (vehicle control) was then initiated, with daily injection volumes of approximately 200 µl per animal. The two (-)-gossypol dosage levels were chosen based on previous studies, which suggested that these levels should be non-toxic and potentially effective [17]; indeed, both dosages were well tolerated by the mice in our study.
Tissue Microscopy
After euthanizing the mice, tumors were removed from the animals and fixed in 10% formalin solution. Tissues were paraffin-embedded, and 5-µm-thick sections were stained with hematoxylin and eosin. To analyze mitotic rate in tumor specimens, slides were scored by two independent investigators. Mitoses were counted in 20 high-power fields for each specimen. Subsequently, the mitotic index (the average number of mitoses per high-power field) was determined for each tumor. For immunohistochemistry, paraffin-embedded sections were deparaffinized and rehydrated. To examine apoptosis in the specimens, sections were stained by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining using ApopTag Plus Assay system (Chemicon International, Temecula, CA), according to the manufacturer's instructions. Ten high-power fields were examined for each sample, and the number of cells staining positive in each field was determined by a pathologist. Values were then averaged to provide an apoptotic rate for each tumor specimen, and percentage (versus control) was determined for each tumor group.
Statistical Analysis
Tumor growth rates were analyzed using a mixed-effects model. The mixed-effects model combines fixed effects (treatment group) with random effects (intercept at start of treatment, growth rate per tumor) into a general linear model framework. The size of the tumor within an animal is allowed to have random deviation around a group mean, as well as random deviation in the growth rate about a group mean. The tumor growth rate was modeled using both a linear time effect (per day) and a quadratic time effect (per day squared). However, only the linear time effect was significant in the modeling process.
The values for the mitotic index and apoptotic scoring were compared using Student's t test for univariate analysis. P < .05 was considered statistically significant.
Results
Cancer Cell Proliferation In Vitro Inhibited By (-)-Gossypol Treatment
Prior to in vivo testing, we investigated the effects of (-)-gossypol on the growth of two HNSCC cell lines, UM-SCC-1 and UM-SCC-17B, as well as on primary normal human oral keratinocytes in vitro. Both HNSCC cell lines expressed high levels of Bcl-xL [6]. In the current study, we employed both a direct measurement of viable cells by dye exclusion and an indirect metabolic assay to assess cell growth inhibition by (-)-gossypol. Both tumor lines and normal keratinocytes were grown in the presence or absence of varying concentrations of (-)-gossypol (Figure 1) and showed dose-dependent inhibition of cell growth over the range tested (0–25 µM). Through the dye exclusion method, the IC50 for both HNSCC cell lines was found to be approximately 4 µM (-)-gossypol (Figure 1A). In contrast, normal keratinocytes were less sensitive to (-)-gossypol, with an IC50 of approximately 12.5 µM. Through the metabolic assay method, the IC50 values for (-)-gossypol were found to be 3, 6, and 17 µM for UM-SCC-1, UM-SCC-17B, and human oral keratinocytes, respectively (Figure 1B). These results are consistent with the selective growth inhibition of HNSCC cells by (-)-gossypol.
Figure 1.
Growth inhibition of HNSCC cells by (-)-gossypol. (A) UM-SCC-17B and UM-SCC-1 cell lines and primary human oral keratinocytes were continuously exposed to various concentrations of (-)-gossypol over 5 days in a medium composed of a 1:1 mixture of KGM and DMEM. Try pan blue viable cell enumeration assays were performed in triplicate for each data point. Data are expressed as percentages of untreated control values. Error bars indicate standard deviation. (B) UM-SCC-17B and UM-SCC-1 cell lines and primary human oral keratinocytes were continuously exposed to varying concentrations of (-)-gossypol over 6 days for MTT cell survival assays, performed in a medium composed of a 1:1 mixture of KGM and DMEM (n= 10 for each point on the graph). Data are expressed as percentages of untreated control values.
Optimization of the Xenograft Mouse Model of Human HNSCC
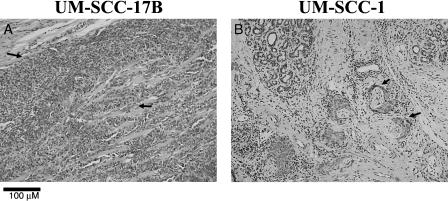
The mouse floor-of-the-mouth model for human oral cancer was optimized for xenografts using the UM-SCC-17B and UM-SCC-1 cell lines. We did not observe tumor encapsulation—a difficulty that can be encountered at other injection sites. Tumor doses of 1 x 106 and 2.5 x 106 cells for UM-SCC-17B and UM-SCC-1, respectively, yielded 35- to 60-mm3-diameter tumors in the floor-of-the-mouth region at 2 to 4 weeks after injection. Histopathology was confirmed by hematoxylin and eosin-stained sections of murine xenografts. UM-SCC-17B (Figure 2A) and UM-SCC-1 (Figure 2B) xenografts exhibited morphologic and cytologic features consistent with aggressive squamous cell carcinoma. UM-SCC-17B (Figure 2A) exhibited infiltrating cords of tumor cells invading skeletal muscles. Histopathological findings included invasive sheets of neoplastic epithelial cells exhibiting nuclear pleomorphism, nuclear hyperchromatism, and increased number of mitoses (Figure 2A, arrows). Of note, UM-SCC-1 exhibited areas of perineural invasion that are apparent in a photomicrograph presented here (Figure 2B, arrows).
Figure 2.
UM-SCC-17B and UM-SCC-1 murine xenografts exhibit morphologic and cytologic features consistent with invasive squamous cell carcinoma. Representative sections of hematoxylin and eosin-stained murine xenografts are shown. (A) UM-SCC-17B tumor exhibits neoplastic epithelial islands, some of which invade skeletal muscle. Cytologic changes, including nuclear pleomorphism, nuclear hyperchromatism, and increased number of mitoses (arrows), are observed. (B) UM-SCC-1 tumor exhibits areas of perineural invasion (arrows).
(-)-Gossypol Suppresses the Growth of UM-SCC-17B and UM-SCC-1 Xenograft Tumors
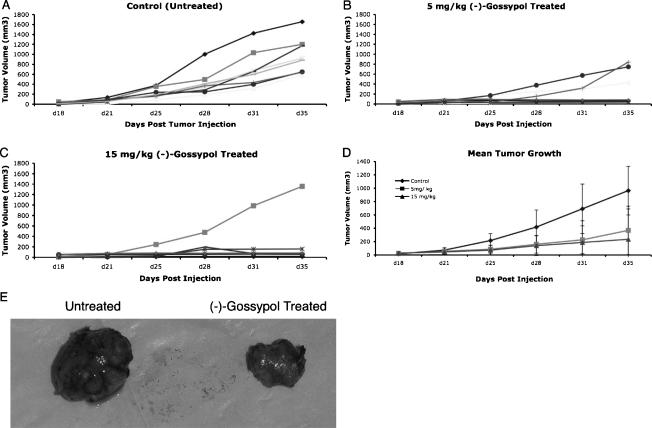
Mice with UM-SCC-17B and UM-SCC-1 xenograft tumors were treated with (-)-gossypol to investigate its effects on tumor growth in vivo (Figures 3 and 4, respectively). Thirty mice were injected in the floor of the mouth with 1 x 106 UM-SCC-17B cells. Eighteen days after tumor injection, mice were randomized to three groups, and intraperitoneal injections of 5 mg/kg (-)-gossypol, 15 mg/kg (-)-gossypol, or drug vehicle solution were initiated (n = 10 in each group). Tumor growth curves (tumor volume) for the mice up to day 35 are shown in Figure 3. For the control mice that received the drug vehicle solution, there was progressive growth of tumors (Figure 3A). In both 5 and 15 mg/kg (-)-gossypol treatment groups, a significant suppressive effect on tumor growth was observed (Figure 3, B, C, and E); however, four tumors in the treatment groups escaped: three at the lower dose (Figure 3B) and one at the higher dose (Figure 3C). The mean tumor growth for the three groups of mice is shown in Figure 3D. There was a significantly lower growth rate for both the 5- and 15-mg/kg (-)-gossypol-treated mice compared to the control mice. By day 28, control animals had, on average, tumors that are 253 mm3 (SE = 95, P = .008) and 268 mm3 (SE = 95, P = .006) larger than those in animals treated with 5 and 15 mg/kg (-)-gossypol, respectively, as estimated by our regression model. On day 35, the average tumor of the control animal was estimated to be 620 mm3 (SE = 164, P < .001) and 712 mm3 (SE = 162, P < .001) larger than the average tumor for animals treated with 5 and 15 mg/kg (-)-gossypol. Despite the greater number of “escapees” in the 5-mg/kg (-)-gossypol group compared with that in the 15-mg/kg (-)-gossypol group, the difference in tumor growth between these two treatment groups was not significant.
Figure 3.
(-)-Gossypol suppresses the growth of UM-SCC-17B xenograft mouse tumors. Thirty mice were injected with 1 x 106 UM-SCC-17B cells in the floor of the mouth. Eighteen days after tumor injection, the mice were randomized to three groups (n = 10 in each group), and intraperitoneal injections of the drug vehicle solution (A), 5 mg/kg (-)-gossypol (B), or 15 mg/kg (-)-gossypol (C) were initiated. Tumor growth curves (tumor volume) for the mice up to day 35 are shown. (D) The mean tumor growth for the three groups of mice is shown. Error bars indicate standard deviation. There was a significantly lower growth rate for (-)-gossypol-treated mice (both 5 and 15 mg/kg) compared to that of control mice on day 28 (P ≤ . 008 and P ≤ . 006, respectively) and day 35 (P ≤ .001 for both). The difference in tumor growth between the 5- and the 15-mg/kg (-)-gossypol groups was not significant. (E) Gross appearance of representative tumors from the control group and the 15-mg/kg (-)-gossypol treatment group, on the left and right, respectively, demonstrating the difference in tumor size between the untreated and the (-)-gossypol-treated animals.
Figure 4.
(-)-Gossypol suppresses the growth of UM-SCC-1 xenograft mouse tumors. Thirty mice were injected with 2.5 x 106 UM-SCC-1 cells in the floor of the mouth. Twenty-six days after tumor injection, mice were randomized to three groups (n = 10 in each group), and intraperitoneal injections of the drug vehicle solution (A), 5 mg/kg (-)-gossypol (B), or 15 mg/kg (-)-gossypol (C) were initiated. Tumor growth curves (tumor volume) for the mice up to day 43 are shown. (D) The mean tumor growth for the three groups of mice is shown. Error bars indicate standard deviation. There was a significantly lower growth rate for (-)-gossypol-treated mice (both 5 and 15 mg/kg) compared to that in control mice on day 29 (P ≤ .009 and P ≤ .016, respectively) and day 43 (P ≤ .0001 and P ≤ .001, respectively). The difference in tumor growth between the 5- and the 15-mg/kg (-)-gossypol groups was not significant.
In similar studies, 30 mice were injected with 2.5 x 106 UM-SCC-1 cells. Twenty-six days after tumor injection, once the tumors had reached a diameter of 4 to 5 mm, the mice were randomized to three groups, and daily intraperitoneal injections of 5 mg/kg (-)-gossypol, 15 mg/kg (-)-gossypol, or drug vehicle solution were initiated (n = 10 in each group). Tumor growth curves for the mice up to day 43 are shown in Figure 4. For the control mice that received the drug vehicle solution, there was progressive growth of tumors (Figure 4A). In both the 5- and the 15-mg/kg (-)-gossypol treatment groups, a suppressive effect on tumor growth was observed (Figure 4, B and C, respectively). The mean tumor growth for the three groups of mice is shown in Figure 4D. (-)-Gossypol significantly suppressed tumor growth in both the 5- and 15-mg/kg (-)-gossypol-treated mice compared to that in the control mice. By day 29, the average tumor for animals treated with 5 or 15 mg/kg (-)-gossypol was estimated to be 57 mm3 (SE = 21, P = .009) and 53 mm3 (SE = 22, P = .016) smaller than that of the control animal. On day 43, the difference was estimated to be 331 mm3 (SE = 48, P ≤ .0001) and 315 mm3 (SE = 49, P ≤ .001), for the 5- and the 15-mg/kg (-)-gossypol groups, respectively, with the average tumor of the treated animal being significantly smaller than the average tumor of the control animal.
(-)-Gossypol Tumor Growth Suppression Is Sustained Following Drug Withdrawal
Although tumor growth-suppressive effects in the (-)-gossypol groups were striking and highly significant when compared to that in untreated controls, we noted that there were small residual tumor masses in the mice. To determine the effect of stopping (-)-gossypol treatment, all treatments were stopped on day 43 for three control and three (-)-gossypol-treated UM-SCC-1 tumors, and tumor measurements were taken biweekly. The other mice in each of the treatment groups and the vehicle control were euthanized at this time point, and the tumors were harvested for histology. The results of treatment cessation are illustrated in Figure 5. For the (-)-gossypol-treated mice, there was continued arrest of tumor growth for at least 2 weeks following cessation of drug use, suggesting that the drug's growth-suppressive effects persist for a prolonged period. However, after 2 weeks, the tumors grew progressively, and the mice were euthanized on day 91. All the control mice had to be euthanized by day 57 due to progressive tumor growth.
Figure 5.
(-)-Gossypol treatment suppresses the growth of UM-SCC-1 xenograft mouse tumors after the cessation of (-)-gossypol administration. Mice were injected with 2.5 x 106 UM-SCC-1 cells in the floor of the mouth. Forty-three days after tumor injection, drug administration was stopped in three animals from the control group (A) and in three animals from the 15-mg/kg (-)-gossypol treatment group (B). (The remaining animals were euthanized at this time.) Animals were followed, and tumors were measured until euthanization on day 57 for the control group (A) and on day 91 for the previously treated group (B). (-)-Gossypol suppression of tumor growth lasted several weeks after administration was stopped.
Mitotic Index Is Lower in (-)-Gossypol-Treated Tumors
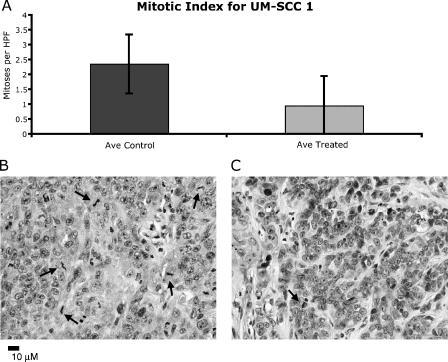
To investigate differences in proliferation between the control and the (-)-gossypol-treated tumors, tumor sections from each group were stained with hematoxylin and eosin and examined in a blinded fashion for the number of mitotic figures per high-power field (mitotic index). For each tumor cell line, three control and six (-)-gossypol-treated animals were scored. In Figure 6, the mitotic indices for the control and the treated UM-SCC-17B tumors are graphically represented. There was a statistically significant difference (P < .01) between the mitotic indices of tumors in the control mice (3.88 ± 1.27) and in the treated mice (1.48 ± 0.85). As shown in the top panel of Figure 7, the mitotic indices for the control and the (-)-gossypol-treated UM-SCC-1 tumors are 2.35 ± 0.99 and 0.95 ± 0.82, respectively. Although not statistically significant, the trend for UM-SCC-1 was the same as that for UM-SCC-17B, with treated cells displaying a lower mitotic index. The lower panels of Figures 6 and 7 show a representative histology of treated and untreated tumors, demonstrating disparity in mitoses (arrows) in representative high-power fields.
Figure 6.
Treatment of UM-SCC-17B tumors with (-)-gossypol causes a significant decrease in the number of mitotic figures. (A) Graphic representation ofmitotic index in 20 high-power fields in control and (-)-gossypol-treated tumors (P ≤ .01). Error bars indicate standard deviation. Representative high-power fields from control (B) and 15-mg/kg (-)-gossypol (C)-treated UM-SCC-17B tumors, stained with hematoxylin and eosin. Arrows indicate mitotic figures.
Figure 7.
Treatment of UM-SCC-1 tumors with (-)-gossypol causes a decrease in the number of mitotic figures. (A) Graphic representation ofmitotic index in 20 high-power fields in control and (-)-gossypol-treated tumors. The decrease in (-)-gossypol-treated tumors was not statistically significant. Error bars indicate standard deviation. Representative high-power fields from control (B) and 15-mg/kg (-)-gossypol (C)-treated UM-SCC-1 tumors, stained with hematoxylin and eosin. Arrows indicate mitotic figures.
Increase in Apoptosis in (-)-Gossypol-Treated Tumors
To investigate the level of apoptosis occurring in the tumor samples, tumor sections from each cell line (three control and six treated) were TUNEL-stained. (Figure 8). The average number of apoptotic cells per field was 67% greater in the treatment group that that in the untreated group for UM-SCC-17B cell line tumors, and it was 28% greater in the treatment group that that in the control group for UM-SCC-1 cell line tumors. The difference was statistically significant for UM-SCC-17B (P < .005), but not for UM-SCC-1.
Figure 8.
Treatment of UM-SCC-17B tumors with (-)-gossypol leads to an increased rate ofapoptosis, as indicated by TUNEL staining. (A) Control UM-SCC-17B tumor from an animal that did not receive (-)-gossypol. Note that there is positive staining in some cells (brown). (B) UM-SCC-17B tumor from an animal that received 5 mg/kg (-)-gossypol. Note the increase in the proportion of staining relative to (A).
Discussion
The treatment of advanced HNSCC remains a daunting clinical problem. Despite an armamentarium that includes surgery, radiation, and chemotherapy, survival in HNSCC has not improved over the past four decades. A high Bcl-xL level has been identified as a marker for the poor response of HNSCC to chemotherapy. Previous work in breast, lung, and prostate cancers has demonstrated that Bcl-xL overexpression facilitates tumor growth and mediates chemotherapy resistance, presumably by inhibiting chemotherapy-induced apoptosis [18–21]. This has led us to investigate the use of Bcl-xL inhibitors as possible adjuvants to standard chemotherapy for the treatment of HNSCC.
Various potential strategies exist for modulating Bcl-xL activity, including antisense nucleotides, antibodies, peptide inhibitors, and small molecules [22]. However, antisense nucleotides, antibodies, and peptide approaches are limited by poor in vivo stability, difficulties in tissue delivery, and/or high costs. Small-molecule inhibitors are typically organic compounds that bind to specific domains of a target protein. Potentially, these compounds are advantageous due to superior in vivo stability, good cell permeability, and various possible administrative routes [22]. In recent years, a number of different small-molecule compounds, both naturally occurring and synthetic, have been described as inhibitors of Bcl-xL [23–28]. Using a structure-based database screening approach, (-)-gossypol was identified as a small molecule with a particularly high affinity for Bcl-xL. Gossypol is derived from a cottonseed extract used in herbal medicines, and the racemic compound has been studied for anticancer activity on a variety of human tumors [9,11,12,15]. Prior studies from our laboratory have demonstrated that (-)-gossypol has an in vitro growth-inhibitory effect against multiple human HNSCC cell lines, with relatively little impact on normal fibroblasts or keratinocytes [6]. Furthermore, we have shown that (-)-gossypol induces apoptosis in a high fraction of cells in cisplatin-resistant HNSCC cell lines that overexpress Bcl-xL [6,7].
Cell culture studies allow rapid investigation of the effects of novel agents on a variety of different cancers. However, there are limitations to in vitro studies of anticancer agents. These studies cannot evaluate the potential systemic toxicity of the drug, or the role of the surrounding tissues in either the growth of solid tumors or their responsiveness to the drug. Clearly, in vivo experiments are important for the study of new anticancer drugs prior to human clinical trials. Although a variety of mouse models of HNSCC have been described, studies have demonstrated advantages to orthotopic tumor placement [29]. These include smaller tumor inocula, better local tumor modeling, and patterns of regional and distant metastasis analogous to human HNSCC. The floor-of-the-mouth model is advantageous to the study of HNSCC, as the tumors behave like human HNSCC, growing progressively and invading surrounding tissues. We used an immunodeficient mouse model for our in vivo studies, in which cells from two HNSCC tumor lines expressing high levels of Bcl-xL were injected intraorally into submucosal tissues of the floor of the mouth. Under optimized conditions, this tumor model consistently produced tumors 35 to 60 mm3 in diameter within 2 to 3 weeks of HNSCC cell inoculation. Accurate measurement of tumors at the orthotopic site is further facilitated by the hairless nature of the nude mouse. Tumors in the floor of the mouth are well-tolerated by the mice. The orthotopic xenograft system is thus a useful, reproducible model for the in vivo evaluation of novel therapeutic agents for head and neck cancer.
In this study, we show that (-)-gossypol potently suppresses tumor growth in an immunodeficient mouse model of human HNSCC. This growth inhibition was noted in tumors arising from both HNSCC cell lines. The drug was well-tolerated by the mice, with the only observed side effect being moderate weight loss (<5%), which was reversible on drug withdrawal. We used two (-)-gossypol dosages for both cell lines tested, but we observed no significant differences in tumor inhibition between the two dosing levels. Both treatment levels were below the dosage established as the maximum tolerated dose for mice [17]. Interestingly, following drug withdrawal, there was arrest of tumor growth for 3 weeks. However, a small number of the treated mice had persistent tumors, which, although derived from the same HNSCC cell line, demonstrated resistance to the drug. The tumors from these animals, referred to as “escapees” above, are currently being analyzed to better elucidate potential mechanisms of (-)-gossypol resistance.
The fact that tumors in animals treated with (-)-gossypol demonstrate a decreased mitotic rate is intriguing. Other studies have demonstrated that a number of chemotherapeutic agents, both well-established and experimental, block cell cycle progression and alter the activity of Bcl-xL and related proteins [30–32]. Likewise, antisense oligonucleotides to Bcl-xL induce the growth arrest of tumor cells in vitro [33]. Our data suggest that the tumor cells are inhibited by (-)-gossypol from dividing. Although TUNEL staining indicates that a modest increase in the apoptotic rate occurs in the treated HNSCC tumors, the targeting of Bcl-xL by (-)-gossypol is unlikely to fully explain the dramatic inhibition of tumor growth seen in the treatment groups. A number of additional mechanisms have been described for gossypol-mediated growth inhibition, including protein kinase C inhibition [34], cellular metabolism blockade [35], modulation of cyclin D1 and Rb [36], and generation of reactive oxygen species [37]. It is likely that one or several of these mechanisms contribute to the inhibition of HNSCC tumor growth described here.
In this study, we demonstrate that (-)-gossypol is an effective inhibitor of HNSCC growth at physiologically tolerable doses. In this study, the antitumor growth effect of (-)-gossypol against HNSCC tumors, although significant, was incomplete when it was used as a single agent. The combination of (-)-gossypol with standard chemotherapeutic agents such as cisplatin may lead to a more dramatic reduction in tumors with reduced drug concentrations. A better understanding of the mechanism of action of (-)-gossypol will facilitate the development of effective treatment protocols when administered in conjunction with other chemotherapeutic agents. Indeed, (-)-gossypol's greatest utility may lie in overcoming pathways of tumor drug resistance and, therefore, may serve as an adjuvant to standard chemotherapy regimens that have a different mechanism of action. We have recently demonstrated that cisplatin treatment of HNSCC cells in vitro selects for wild-type p53 and high Bcl-xL expression, supporting the concept that Bcl-xL protects tumor cells from cisplatin-induced apoptosis. Additionally, cisplatin-resistant tumor cells are very sensitive to induction of apoptosis in response to treatment with (-)-gossypol [7].
Tumor resistance limits the effectiveness of conventional therapy and underscores the need to target resistance pathways. (-)-Gossypol, which works in part as a small-molecule inhibitor of Bcl-xL, suppresses the growth of human HNSCC cells in culture. Using a floor-of-the-mouth xenograft mouse model of head and neck cancer, we demonstrate that (-)-gossypol also inhibits the growth of tumors in vivo. This novel therapeutic agent has potential as an adjuvant to traditional chemotherapy in the treatment of HNSCC.
Acknowledgement
We thank Joshua A. Bauer for discussions and experimental insights.
Footnotes
This research was supported by National Institute of Dental and Craniofacial Research (NIDCR) grants DE00452-01 (N.J.D.), DE014620-01A1 (B.S.H.), and R01 DE13346 (T.E.C.); National Cancer Institute (NCI) grant R01 CA83087 (C.R.B.); Department of Defense idea grant BC000914; the Susan G. Komen Breast Cancer Foundation; the CapCure Foundation (S.W.); the University of Michigan's NCI Cancer Center support grant 5 P30 CA46592; Head and Neck Cancer SPORE grant P50 CA97248; and NIDCD Research Center Core grant P30 DC05188 (multiple authors). S.J.W. and K.G.W. were funded by National Institutes of Health training grant T32 DC05356. S.W. has financial interests in Ascenta Pharmaceuticals, which is pursuing the production of (-)-gossypol and other compounds for clinical use.
Keith G. Wolter and Steven J. Wang contributed equally to this manuscript.
References
- 1.Todd R, Donoff RB, Wong DT. The molecular biology of oral carcinogenesis: toward a tumor progression model. J Oral Maxillofac Surg. 1997;55:613–623. doi: 10.1016/s0278-2391(97)90495-x. (discussion, 615–623) [DOI] [PubMed] [Google Scholar]
- 2.The Department of Veterans Affairs Laryngeal Cancer Study Group, author. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 3.Eastman A. Activation of programmed cell death by anticancer agents: cisplatin as a model system. Cancer Cells. 1990;2:275–280. [PubMed] [Google Scholar]
- 4.Hickman JA. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992;11:121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- 5.Trask DK, Wolf GT, Bradford CR, Fisher SG, Devaney K, Johnson M, Singleton T, Wicha M. Expression of Bcl-2 family proteins in advanced laryngeal squamous cell carcinoma: correlation with response to chemotherapy and organ preservation. Laryngoscope. 2002;112:638–644. doi: 10.1097/00005537-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Oliver CL, Bauer JA, Wolter KG, Ubell ML, Narayan A, O'Connell KM, Fisher SG, Wang S, Wu X, Ji M, et al. In vitro effects of the BH3 mimetic, (-)-gossypol, on head and neck squamous cell carcinoma cells. Clin Cancer Res. 2004;10:7757–7763. doi: 10.1158/1078-0432.CCR-04-0551. [DOI] [PubMed] [Google Scholar]
- 7.Bauer JA, Trask DK, Kumar B, Los G, Castro J, Lee JS, Chen J, Wang S, Bradford CR, Carey TE. Reversal of cisplatin resistance with a BH3 mimetic, (-)-gossypol, in head and neck cancer cells: role of wild-type p53 and Bcl-xL. Mol Cancer Ther. 2005;4:1096–1104. doi: 10.1158/1535-7163.MCT-05-0081. [DOI] [PubMed] [Google Scholar]
- 8.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Wang J, Wong SC, Chow LS, Nicholls JM, Wong YC, Liu Y, Kwong DL, Sham JS, Tsa SW. Cytotoxic effect of gossypol on colon carcinoma cells. Life Sci. 2000;67:2663–2671. doi: 10.1016/s0024-3205(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Liu H, Guo R, Ling Y, Wu X, Li B, Roller PP, Wang S, Yang D. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem Pharmacol. 2003;66:93–103. doi: 10.1016/s0006-2952(03)00248-x. [DOI] [PubMed] [Google Scholar]
- 11.Van Poznak C, Seidman AD, Reidenberg MM, Moasser MM, Sklarin N, Van Zee K, Borgen P, Gollub M, Bacotti D, Yao TJ, et al. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res Treat. 2001;66:239–248. doi: 10.1023/a:1010686204736. [DOI] [PubMed] [Google Scholar]
- 12.Blackstaffe L, Shelley MD, Fish RG. Cytotoxicity of gossypol enantiomers and its quinone metabolite gossypolone in melanoma cell lines. Melanoma Res. 1997;7:364–372. doi: 10.1097/00008390-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46:4259–4264. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 14.Carey TE. Head and neck tumor cell lines. In: Hay AGR, Park J-G, editors. Atlas of Human Tumor Cell Lines. Academic Press Inc./Harcourt Brace Jovanovich Publishers: New York City, NY; 1994. pp. 79–120. [Google Scholar]
- 15.Mitra RS, Zhang Z, Henson BS, Kurnit DM, Carey TE, D'Silva NJ. Rap1A and rap1B ras-family proteins are prominently expressed in the nucleus of squamous carcinomas: nuclear translocation of GTP-bound active form. Oncogene. 2003;22:6243–6256. doi: 10.1038/sj.onc.1206534. [DOI] [PubMed] [Google Scholar]
- 16.D'Silva NJ, Mitra RS, Zhang Z, Kurnit DM, Babcock CR, Polverini PJ, Carey TE. Rap1, a small GTP-binding protein, is upregulated during arrest of proliferation in human keratinocytes. J Cell Physiol. 2003;196:532–540. doi: 10.1002/jcp.10331. [DOI] [PubMed] [Google Scholar]
- 17.Mohammad RM, Wang S, Aboukameel A, Chen B, Wu X, Chen J, Al-Katib A. Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-X(L) [(-)-gossypol] against diffuse large cell lymphoma. Mol Cancer Ther. 2005;4:13–21. [PubMed] [Google Scholar]
- 18.Hopkins-Donaldson S, Cathomas R, Simoes-Wust AP, Kurtz S, Belyanskaya L, Stahel RA, Zangemeister-Wittke U. Induction of apoptosis and chemosensitization of mesothelioma cells by Bcl-2 and Bcl-xL antisense treatment. Int J Cancer. 2003;106:160–166. doi: 10.1002/ijc.11209. [DOI] [PubMed] [Google Scholar]
- 19.Liu QY, Stein CA. Taxol and estramustine-induced modulation of human prostate cancer cell apoptosis via alteration in Bcl-xL and bak expression. Clin Cancer Res. 1997;3:2039–2046. [PubMed] [Google Scholar]
- 20.Liu JR, Fletcher B, Page C, Hu C, Nunez G, Baker V. Bcl-xL is expressed in ovarian carcinoma and modulates chemotherapy-induced apoptosis. Gynecol Oncol. 1998;70:398–403. doi: 10.1006/gyno.1998.5125. [DOI] [PubMed] [Google Scholar]
- 21.Reed JC. Mechanisms of Bcl-2 family protein function and dysfunction in health and disease. Behring-Inst-Mitt. 1996:72–100. [PubMed] [Google Scholar]
- 22.Huang Z. Bcl-2 family proteins as targets for anticancer drug design. Oncogene. 2000;19:6627–6631. doi: 10.1038/sj.onc.1204087. [DOI] [PubMed] [Google Scholar]
- 23.Klasa RJ, Gillum AM, Klem RE, Frankel SR. Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev. 2002;12:193–213. doi: 10.1089/108729002760220798. [DOI] [PubMed] [Google Scholar]
- 24.Kutzki O, Park HS, Ernst JT, Orner BP, Yin H, Hamilton AD. Development of a potent Bcl-x(L) antagonist based on alpha-helix mimicry. J Am Chem Soc. 2002;124:11838–11839. doi: 10.1021/ja026861k. [DOI] [PubMed] [Google Scholar]
- 25.Tzung SP, Kim KM, Basanez G, Giedt CD, Simon J, Zimmerberg J, Zhang KY, Hockenbery DM. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat Cell Biol. 2001;3:183–191. doi: 10.1038/35055095. [DOI] [PubMed] [Google Scholar]
- 26.Becattini B, Kitada S, Leone M, Monosov E, Chandler S, Zhai D, Kipps TJ, Reed JC, Pellecchia M. Rational design and realtime in-cell detection of the proapoptotic activity of a novel compound targeting Bcl-X(L) Chem Biol. 2004;11:389–395. doi: 10.1016/j.chembiol.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 29.Prime SS, Eveson JW, Stone AM, Huntley SP, Davies M, Paterson IC, Robinson CM. Metastatic dissemination of human malignant oral keratinocyte cell lines following orthotopic transplantation reflects response to TGF-beta 1. J Pathol. 2004;203:927–932. doi: 10.1002/path.1603. [DOI] [PubMed] [Google Scholar]
- 30.Osaki M, Kase S, Adachi K, Takeda A, Hashimoto K, Ito H. Inhibition of the PI3K-Akt signaling pathway enhances the sensitivity of Fas-mediated apoptosis in human gastric carcinoma cell line, MKN-45. J Cancer Res Clin Oncol. 2004;130:8–14. doi: 10.1007/s00432-003-0505-z. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe M, Dewan MZ, Okamura T, Sasaki M, Itoh K, Higashihara M, Mizoguchi H, Honda M, Sata T, Watanabe T, et al. A novel NF-kappaB inhibitor DHMEQ selectively targets constitutive NF-kappaB activity and induces apoptosis of multiple myeloma cells in vitro and in vivo. Int J Cancer. 2005;114:32–38. doi: 10.1002/ijc.20688. [DOI] [PubMed] [Google Scholar]
- 32.Zhong X, Li X, Wang G, Zhu Y, Hu G, Zhao J, Neace C, Ding H, Reed E, Li QQ. Mechanisms underlying the synergistic effect of SU5416 and cisplatin on cytotoxicity in human ovarian tumor cells. Int J Oncol. 2004;25:445–451. [PubMed] [Google Scholar]
- 33.Hayward RL, Macpherson JS, Cummings J, Monia BP, Smyth JF, Jodrell DI. Antisense Bcl-xL down-regulation switches the response to topoisomerase I inhibition from senescence to apoptosis in colorectal cancer cells, enhancing global cytotoxicity. Clin Cancer Res. 2003;9:2856–2865. [PubMed] [Google Scholar]
- 34.Jarvis WD, Turner AJ, Povirk LF, Traylor RS, Grant S. Induction of apoptotic DNA fragmentation and cell death in HL-60 human promyelocytic leukemia cells by pharmacological inhibitors of protein kinase C. Cancer Res. 1994;54:1707–1714. [PubMed] [Google Scholar]
- 35.Coyle T, Levante S, Shetler M, Winfield J. In vitro and in vivo cytotoxicity of gossypol against central nervous system tumor cell lines. J Neuro-Oncol. 1994;19:25–35. doi: 10.1007/BF01051046. [DOI] [PubMed] [Google Scholar]
- 36.Ligueros M, Jeoung D, Tang B, Hochhauser D, Reidenberg MM, Sonenberg M. Gossypol inhibition of mitosis, cyclin D1 and Rb protein in human mammary cancer cells and cyclin-D1 transfected human fibrosarcoma cells. Br J Cancer. 1997;76:21–28. doi: 10.1038/bjc.1997.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou DX, Uto T, Tong X, Takeshita T, Tanigawa S, Imamura I, Ose T, Fuji M. Involvement of reactive oxygen species-independent mitochondrial pathway in gossypol-induced apoptosis. Arch Biochem Biophys. 2004;428:179–187. doi: 10.1016/j.abb.2004.06.007. [DOI] [PubMed] [Google Scholar]