Abstract
Magnetic resonance imaging may improve the staging of prostate cancer compared with clinical evaluation alone, computerized tomography, or transrectal ultrasound, and it allows simultaneous and detailed evaluation of prostatic, periprostatic, and pelvic anatomy. Endorectal magnetic resonance imaging and magnetic resonance spectroscopic imaging (endoMRI/MRSI) allow better visualization of the zonal anatomy of the prostate and better delineation of tumor location, volume, and extent (stage). Metabolic criteria used to identify and localize prostate cancer with endoMRI/MRSI have been standardized, thus improving the accuracy of the examination and limiting interobserver variations in interpretation. Evidence is now emerging that endoMRI/MRSI may also be helpful in assessing response to prostate cancer treatment, most commonly with radiation and/or androgen-deprivation therapy.
Key words: Prostate cancer, Magnetic resonance imaging, Magnetic resonance spectroscopy, Endorectal MRI
Prostate cancer is the most common cancer detected in men in the United States. Approximately 230,000 American men were diagnosed with prostate cancer in 2005.1 Prostate cancer is the second leading cause of cancer death for men, but mortality rates have been declining since the mid-1990s. The widespread and repeated use of serum prostate-specific antigen (PSA) tests and extended-pattern transrectal ultrasound (TRUS)-guided biopsy has resulted in considerable stage migration.2,3 Few men now present with locoregional or metastatic disease identified by standard imaging (ie, bone scan or cross-sectional pelvic imaging with magnetic resonance imaging [MRI] or computerized to mography [CT]). Therefore, most clinicians have shifted from the use of imaging-based staging paradigms to a combination of clinical variables (serum PSA, T stage, Gleason score, and extent of disease on biopsy) as a more efficient means to assess the likely extent of disease and determine the best initial treatment. However, despite the efficiency of clinical staging, it remains imperfect for precise local cancer staging and intraprostatic tumor localization, both of which play important roles in initial assessment, treatment, and, in many cases, follow-up. Understaging, which was reported to be in the range of 30% to 60%, has become less frequent but is still at least 22%. Thus a selective use of imaging complements clinical staging.4
The ideal imaging technique should be affordable and minimally invasive, with little interobserver variability in interpretation. In addition, the test should be able to predict tumor stage, volume, and location with high specificity and sensitivity. Although such an ideal imaging method does not yet exist, many imaging modalities now available show considerable clinical value.
Cross-Sectional Imaging of the Pelvis for Lymph Node Metastases
Cross-sectional imaging of the pelvis with CT or MRI in cases of prostate cancer is often performed to exclude lymph node metastases in patients who are thought to be candidates for definitive local therapy. CT and MRI have similar sensitivities for this purpose. However, the incidence of lymph node metastases is currently low (< 5%), and imaging is costly with limited sensitivity. A review of the literature that encompassed 15 series and 1354 patients, with an incidence of lymph node metastases of 22%, revealed a sensitivity of CT and MRI of approximately 36% and a specificity of 97%.5 Only those patients with a very high risk of lymph node metastases would benefit from cross-sectional imaging. Such patients would include those with a normal bone scan, a Gleason score greater than 6, a palpable abnormality on digital rectal examination (DRE) (ie, T2–T4 disease), and a serum PSA above 25 ng/mL.6 However, intravenous administration of super-paramagnetic nanoparticles (which enter lymph nodes by means of interstitial-lymphatic fluid transport) at the time of high-resolution MRI seems to improve visualization of small nodal metastases.7 Validation of this technique in larger series of patients is needed, as the improved sensitivity suggests that more patients would benefit from such imaging.
Prostate Imaging
Prostate Anatomy as Assessed by Endorectal MRI
Endorectal MRI utilizes a magnetic coil placed in the rectum to better visualize the zonal anatomy of the prostate and better delineate tumor location, volume, and extent (stage).4 Patients are imaged in a whole-body scanner, using a pelvic phased array coil combined with an inflatable, balloon-covered, endorectal surface coil positioned in the rectum. Both T1- and T2-weighted spin-echo MRI images are required to evaluate prostate cancer. Thin-section axial images (Figure 1) are used for tumor localization and assessment of the extent (stage) of the tumor. Specifically, the presence of extracapsular extension (ECE) and/or seminal vesicle invasion (SVI) is noted. Coronal imaging (Figure 2) may be helpful for tumor localization and in assessing SVI.
Figure 1.
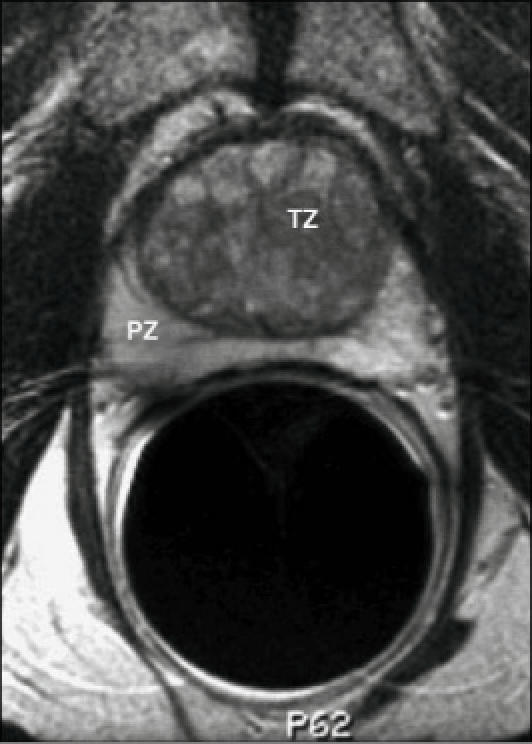
Normal prostate gland, endorectal MRI, axial image. PZ, peripheral zone; TZ, transition zone.
Figure 2.
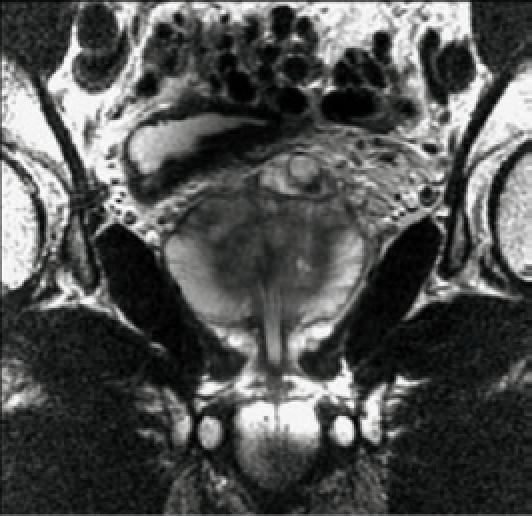
Normal prostate gland, endorectal MRI, coronal image.
Prostate zonal anatomy cannot be fully appreciated on T1-weighted images, as the gland is of intermediate signal intensity on such images. However, on T2-weighted images, zonal anatomy is evident: the peripheral zone is of high-signal intensity and is surrounded by a thin rim of low-signal intensity, representing the capsule of the gland. The central and transition zones are both of lower T2 signal intensity than the peripheral zone, likely due to its smooth muscle elements. With aging, the transition zone increases in size, compressing the peripheral zone and producing an increase in T2 signal intensity of the peripheral zone. Prostate cancer is characterized by low T2 signal intensity in the normally high-signal-intensity peripheral zone (Figure 3).
Figure 3.

Left peripheral zone cancer (Ca) depicted on endorectal MRI, axial image.
Unlike in other forms of imaging, the surrounding structures, including the neurovascular bundles, seminal vesicle, vas deferens, and crura of the penis, are very well visualized by MRI. Some investigators have suggested that prostate cancer may be better demonstrated using gadolinium, but in general, most agree that gadolinium enhancement is not helpful for either prostate cancer localization or staging. However, the development and use of macromolecular contrast media may prove useful in the future.
Magnetic Resonance Spectroscopy
Prostate cancer is associated with proportionately lower levels of citrate and higher levels of choline and creatine than are seen in benign prostatic hyperplasia (BPH) or in normal prostate tissue. This difference can be detected by magnetic resonance spectroscopic imaging (MRSI).8 MRSI uses a strong magnetic field to obtain metabolic information (spectra) that identifies the relative concentrations of various metabolites in the cell cytoplasm and the extracellular space. This technique can identify metabolic differences in prostate tissue (BPH, prostate cancer, and normal prostate tissue) (Figure 4). The anatomic and metabolic criteria used to identify and localize prostate cancer through the use of endorectal MRI/MRSI (endoMRI/MRSI) have been standardized,9 and these criteria improve the accuracy of the examination and limit interobserver variations in interpretation.
Figure 4.
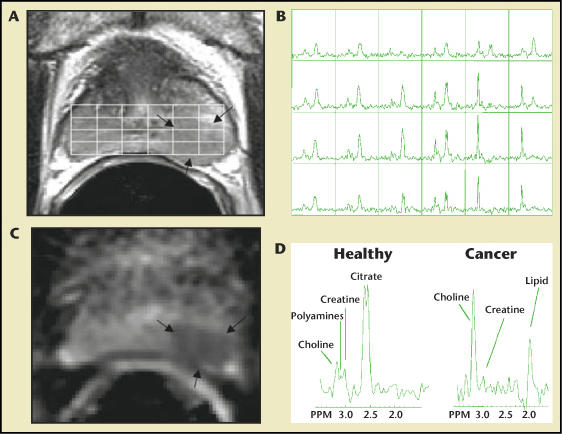
Combined magnetic resonance imaging, diffusion tensor imaging, and 3-dimensional magnetic resonance spectroscopic imaging (MRSI) of the prostate at 1.5 tesla. A. Axial T2-weighted image and 3-dimensional MRSI spectral grid. The arrows indicate a region of prostate cancer. B. Corresponding 3-dimensional MRSI spectral array, showing the presence of an aggressive-appearing tumor (very elevated choline and reduced citrate) on the left side of the gland (right side of the image). C. Image of the mean diffusional coefficient of water demonstrates a region of prostate cancer (arrows) in the same location as the T2-weighted image and MRSI. D. Representative spectra taken from the region of healthy prostate tissue and prostate cancer. PPM, parts per million.
Prostate Cancer Detection and Staging with EndoMRI/MRSI
Magnetic resonance imaging may improve the staging of prostate cancer compared with clinical evaluation alone, CT, or TRUS, and it allows simultaneous and detailed evaluation of prostatic, periprostatic, and pelvic anatomy. Previous reports suggesting a limited applicability of MRI did not incorporate MRSI or the use of endorectal coils, and they used sequences and equipment that were not up to current standards.
Distinctions between normal and pathologic tissue may be hampered by postbiopsy hemorrhage, which can appear as a high-signal-intensity area on T1-weighted imaging.10 Although the ability to detect ECE may not be hampered by recent biopsy, such biopsies can significantly degrade spectral images. Qayyum and colleagues10 noted that the mean percentage of degraded peripheral-zone voxels was 18.5% within 8 weeks of biopsy, compared with 7% after 8 weeks. Therefore, endoMRI/MRSI evaluation is best performed 8 weeks after prostate biopsy.
Accurate localization of cancer within the prostate gland is of increasing value, given the widespread interest in more focal forms of therapy, whether with radiation or percutaneous sources (ie, cryotherapy or high-intensity focused ultrasound). The ability of endoMRI/MRSI to identify cancer is dependent on tumor size and grade. Cancers less than 0.5 cm in diameter may be missed with endoMRI/MRSI, whereas those larger than 0.5 cm are identified with reasonable accuracy, although volumetric measurements may be imprecise.11 Similarly, cancers of higher grade (Gleason score 8–10) are better imaged than those of lower grade.12 Although the inability of endoMRI/MRSI to consistently identify low-volume or low-grade cancers may be seen as a limitation of the technique, such cancers are likely to have a long natural history even without treatment, so failure to detect and treat them may have little impact on cancer-specific morbidity and/or mortality.13 Clinicians routinely use both TRUS and DRE to localize cancers within the prostate gland. Both methods can be imprecise, although use of color and power Doppler techniques and use of microbubble contrast agents with TRUS have shown promise in improving the ability to better detect and/or localize prostate cancer.14,15 Wefer and colleagues16 demonstrated that endoMRI/MRSI could localize prostate cancer to a sextant of the prostate with an overall accuracy similar to that of sextant TRUS- guided biopsy. However, endoMRI/MRSI was more accurate for cancers in the apical portion of the prostate. In a more recent study, Mullerad and colleagues17 compared the abilities of endoMRI/MRSI, TRUS, and DRE to localize prostate cancer to the seminal vesicles and 12 locations in the prostate gland itself. The criteria for the presence of cancer were any cancer greater than 5 mm and/or any cancer with a Gleason pattern score of 4 or 5. The area under receiver operator curves for cancer localization was higher for endoMRI/MRSI than for DRE at all locations. Interestingly, endoMRI/MRSI was superior to TRUS in the midgland and base but not in the apex. This latter finding may be a result of more contemporary biopsy techniques, which sample the apex better. A mixed model demonstrated that endoMRI/MRSI added significant incremental value to TRUS-guided biopsy for cancer localization (P < .001).
Some investigators have used endoMRI/MRSI to detect cancer in patients with previously negative results from TRUS-guided biopsies but persistently abnormal PSA values suggestive of malignancy. Prando and colleagues18 evaluated 42 men with endoMRI/MRSI who had persistently abnormal PSA levels, despite at least 2 negative prostate biopsies. Thirty-one of the 42 patients demonstrated metabolic abnormalities consistent with cancer. For the 11 with no metabolic activity consistent with cancer, biopsy results were negative; however, cancer was detected in 17 (55%) of the 31 men with positive metabolic findings. In this study, endoMRI/MRSI had a sensitivity of 100%, specificity of 44%, positive predictive value of 55%, negative predictive value of 100%, and accuracy of 67%. Whether endoMRI/MRSI should be used routinely in such men is as yet unknown, as extended-pattern biopsy schemes have been developed that are more sensitive than previous, sextant biopsy techniques.
Identifying the presence or absence of ECE and SVI before treatment is of considerable value not only in selecting but also in applying the treatment. For instance, patients with suspected ECE who select radical prostatectomy may require a wide surgical excision. Similarly, those who select radiation may benefit from a combined modality treatment (eg, androgen deprivation and radiation, combined external beam radiation and brachytherapy). ECE is seen as a focal irregular capsular bulge, asymmetry, or invasion of the neurovascular bundles and/or obliteration of the rectoprostatic angle. Obviously, the extent of ECE affects the sensitivity of MRI. Some investigators have shown that the sensitivity of MRI for ECE of less than 1 mm is lower (14%) than that for ECE exceeding 1 mm (71%)19 (Figure 5). SVI is seen as a low-signal- intensity mass or diffuse enlargement with low-signal intensity and loss of the bladder wall on both T1- and T2-weighted images.
Figure 5.
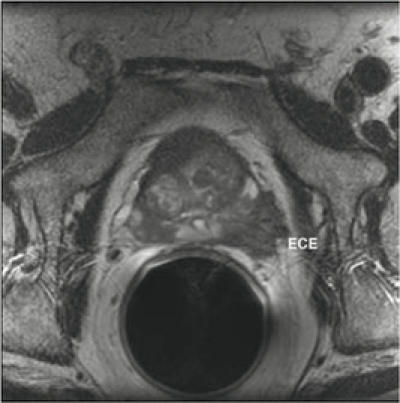
Gross extracapsular extension (ECE) of prostate cancer, depicted on endorectal MRI, axial image.
Endorectal MRI has been reported to have a sensitivity of 13% to 91% and specificity of 57% to 97% for the assessment of ECE.4 The sensitivity and specificity for assessing SVI vary between 20% and 80% and between 92% and 98%, respectively, in several previously reported series.4 More contemporary series show a greater value for endoMRI/MRSI in assessing cancer stage. Two recent reports from Memorial Sloan-Kettering Cancer Center assessed the incremental value of endoMRI/MRSI over the use of clinical variables alone and/or clinical variables incorporated into a pretreatment nomogram.20 The results of endoMRI/MRSI added incremental value to the use of clinical variables alone. Importantly, endoMRI/MRSI contributed significant incremental value to the use of the nomogram alone. Although endoMRI/MRSI added value over the range of risk categories, the incremental value was greatest in intermediate- and high-risk groups. Others have suggested, using decision analysis, that imaging may be most cost effective in these groups.21
In a practical application of endoMRI/MRSI for treatment planning, Hricak22 examined the role of preoperative endoMRI/MRSI in the decision to preserve or resect the neurovascular bundles during radical prostatectomy. EndoMRI/MRSI findings suggested that the surgical plan should be altered for 39% of the neurovascular bundles at risk. However, the real impact of such imaging was seen in patients with high-risk disease: endoMRI/MRSI changed surgical planning in the majority of such patients. Specifically, some patients who may have undergone wide surgical excision of the neurovascular bundle if one relied on clinical criteria only (ie, PSA, tumor volume, and/or grade) underwent a nerve-sparing approach. In intermediate- and low-risk patients, the benefit of endoMRI/MRSI lies largely in its negative predictive value, providing reassurance to the surgeon and patient that nerve-sparing techniques are appropriate.
Assessing Response to Therapy
Although there has been widespread interest in and publication on the use of endoMRI/MRSI for prostate cancer detection and staging, evidence is now emerging that it may also be helpful in assessing response to treatment, most commonly with radiation and/or androgen deprivation.23–26 Both treatment modalities are widely used for the management of clinically localized prostate cancer. Response to therapy with both techniques is usually assessed by periodic serum PSA measurements. However, what constitutes an appropriate PSA response after radiation remains a matter of debate, the time of PSA nadir may be quite prolonged, and PSA elevations in the long term may be due to a variety of causes (local recurrence, distant recurrence, or a false-positive laboratory result termed “PSA bounce”). More quantitative and informative estimates of the effects of therapy on the primary cancer soon after initiating treatment or at the time of PSA elevation are needed. To date, CT, MRI, TRUS, and DRE cannot reliably distinguish normal from malignant tissue after either androgen deprivation or radiation therapy.
Patients receiving androgen-deprivation treatment experience a significant time-dependent loss of the prostatic metabolites choline, creatine, citrate, and polyamines, resulting in total metabolic atrophy in 25% of patients on long-term therapy.24,27 Some investigators have used MRI to assess the effects of chemotherapy in addition to androgen deprivation.28 Investigators also have used endoMRI/MRSI to assess the effects of radiation therapy (either external beam radiation or brachytherapy) on the prostate. Pickett and colleagues25 studied 55 patients who underwent endoMRI/MRSI before and at varying time points after external beam radiation therapy. The presence of and time to resolution of malignant tissue were determined and correlated with serum PSA levels. Thirty percent of patients had evidence of malignant metabolic activity. A strong correlation between endoMRI/MRSI and biopsy findings was noted, whereas there was a very weak correlation with PSA levels. The mean time to disease resolution, as assessed by MRSI, was 40.3 months. This same group of authors26 used endoMRI/MRSI to assess the response of the prostate to brachytherapy either alone or combined with external beam radiation or androgen deprivation. Complete metabolic activity increased from 48% to 100% between 6 and 48 months. Metabolic atrophy seemed to precede PSA nadir in most patients, but in those treated with all 3 methods it occurred almost simultaneously.
Future Directions
The use of endoMRI/MRSI for the detection and staging of prostate cancer will likely increase, given the widespread interest in this form of imaging and the availability of the necessary instrumentation and software, and further refinements are ongoing. Areas of active research include imaging at high field strength (≥3 tesla); novel spectroscopic markers of malignancy, such as polyamines and spermine; and MRI-guided biopsy and treatment.29–31
Main Points.
Endorectal magnetic resonance imaging (endoMRI) utilizes a magnetic coil placed in the rectum to better visualize the zonal anatomy of the prostate and better delineate tumor location, volume, and extent (stage).
Magnetic resonance spectroscopic imaging (MRSI) uses a strong magnetic field to obtain metabolic information that can identify metabolic differences among benign prostatic hyperplasia, prostate cancer, and normal prostate tissue.
MRI may improve the staging of prostate cancer compared with clinical evaluation alone, computerized tomography, or transrectal ultrasound, and it allows simultaneous and detailed evaluation of prostatic, periprostatic, and pelvic anatomy.
Recent reports from Memorial Sloan-Kettering Cancer Center found that endoMRI/MRSI added incremental value to the use of clinical variables alone for assessing prostate cancer. The incremental value was greatest for intermediate- and high-risk groups of patients.
In a study of the role of preoperative endoMRI/MRSI in the decision to preserve or resect the neurovascular bundles during radical prostatectomy, endoMRI/MRSI changed surgical planning in the majority of high-risk patients.
Evidence is now emerging that endoMRI/MRSI may be helpful in assessing response to treatment, most commonly with radiation and/or androgen deprivation.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Lubeck DP, Mehta SS, et al. Time trends in clinical risk stratification for prostate cancer: implications for outcomes (data from CaPSURE) J Urol. 2003;170:S21–25. doi: 10.1097/01.ju.0000095025.03331.c6. [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23:8146–8151. doi: 10.1200/JCO.2005.02.9751. [DOI] [PubMed] [Google Scholar]
- 4.Purohit RS, Shinohara K, Meng MV, et al. Imaging clinically localized prostate cancer. Urol Clin North Am. 2003;30:279–293. doi: 10.1016/s0094-0143(02)00184-2. [DOI] [PubMed] [Google Scholar]
- 5.Wolf JS , Jr, Cher M, Dall’era M, et al. The use and accuracy of cross-sectional imaging and fine needle aspiration cytology for detection of pelvic lymph node metastases before radical prostatectomy. J Urol. 1995;153:993–999. [PubMed] [Google Scholar]
- 6.Rees MA, Resnick MI, Oesterling JE. Use of prostate- specific antigen, Gleason score, and digital rectal examination in staging patients with newly diagnosed prostate cancer. Urol Clin North Am. 1997;24:379–388. doi: 10.1016/s0094-0143(05)70384-0. [DOI] [PubMed] [Google Scholar]
- 7.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 8.Kurhanewicz J, Vigneron DB, Hricak H, et al. Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24–0.7 cm3) spatial resolution. Radiology. 1996;198:795–805. doi: 10.1148/radiology.198.3.8628874. [DOI] [PubMed] [Google Scholar]
- 9.Jung JA, Coakley FV, Vigneron DB, et al. Prostate depiction at endorectal MR spectroscopic imaging: investigation of a standardized evaluation system. Radiology. 2004;233:701–708. doi: 10.1148/radiol.2333030672. [DOI] [PubMed] [Google Scholar]
- 10.Qayyum A, Coakley FV, Lu Y, et al. Organconfined prostate cancer: effect of prior transrectal biopsy on endorectal MRI and MR spectroscopic imaging. AJR Am J Roentgenol. 2004;183:1079–1083. doi: 10.2214/ajr.183.4.1831079. [DOI] [PubMed] [Google Scholar]
- 11.Coakley FV, Qayyum A, Kurhanewicz J. Magnetic resonance imaging and spectroscopic imaging of prostate cancer. J Urol. 2003;170:S69–S75. doi: 10.1097/01.ju.0000094958.23276.c4. [DOI] [PubMed] [Google Scholar]
- 12.Zakian KL, Sircar K, Hricak H, et al. Correlation of proton MR spectroscopic imaging with Gleason score based on step-section pathologic analysis after radical prostatectomy. Radiology. 2005;234:804–814. doi: 10.1148/radiol.2343040363. [DOI] [PubMed] [Google Scholar]
- 13.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 14.Halpern EJ, Frauscher F, Strup SE, et al. Prostate: high-frequency Doppler US imaging for cancer detection. Radiology. 2002;225:71–77. doi: 10.1148/radiol.2251011938. [DOI] [PubMed] [Google Scholar]
- 15.Halpern EJ, Rosenberg M, Gomella LG. Prostate cancer: contrast-enhanced US for detection. Radiology. 2001;219:219–225. doi: 10.1148/radiology.219.1.r01ap21219. [DOI] [PubMed] [Google Scholar]
- 16.Wefer AE, Hricak H, Vigneron DB, et al. Sextant localization of prostate cancer: comparison of sextant biopsy, magnetic resonance imaging and magnetic resonance spectroscopic imaging with step section histology. J Urol. 2000;164:400–404. [PubMed] [Google Scholar]
- 17.Mullerad M, Hricak H, Kuroiwa K, et al. Comparison of endorectal magnetic resonance imaging, guided prostate biopsy and digital rectal examination in the preoperative anatomical localization of prostate cancer. J Urol. 2005;174:2158–2163. doi: 10.1097/01.ju.0000181224.95276.82. [DOI] [PubMed] [Google Scholar]
- 18.Prando A, Kurhanewicz J, Borges AP, et al. Prostatic biopsy directed with endorectal MR spectroscopic imaging findings in patients with elevated prostate specific antigen levels and prior negative biopsy findings: early experience. Radiology. 2005;236:903–910. doi: 10.1148/radiol.2363040615. [DOI] [PubMed] [Google Scholar]
- 19.Jager GJ, Ruijter ET, van de Kaa CA, et al. Local staging of prostate cancer with endorectal MR imaging: correlation with histopathology. AJR Am J Roentgenol. 1996;166:845–852. doi: 10.2214/ajr.166.4.8610561. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Hricak H, Kattan MW, et al. Prediction of organ-confined prostate cancer: incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology. 2005;238:597–603. doi: 10.1148/radiol.2382041905. [DOI] [PubMed] [Google Scholar]
- 21.Jager GJ, Severens JL, Thornbury JR, et al. Prostate cancer staging: should MR imaging be used? A decision analytic approach. Radiology. 2000;215:445–451. doi: 10.1148/radiology.215.2.r00ap09445. [DOI] [PubMed] [Google Scholar]
- 22.Hricak H. MR imaging and MR spectroscopic imaging in the pre-treatment evaluation of prostate cancer. Br J Radiol. 2005;78(spec no 2):S103–S111. doi: 10.1259/bjr/11253478. [DOI] [PubMed] [Google Scholar]
- 23.Kurhanewicz J, Vigneron DB, Hricak H, et al. Prostate cancer: metabolic response to cryosurgery as detected with 3D H-1 MR spectroscopic imaging. Radiology. 1996;200:489–496. doi: 10.1148/radiology.200.2.8685346. [DOI] [PubMed] [Google Scholar]
- 24.Mueller-Lisse UG, Swanson MG, Vigneron DB, et al. Time-dependent effects of hormone-deprivation therapy on prostate metabolism as detected by combined magnetic resonance imaging and 3D magnetic resonance spectroscopic imaging. Magn Reson Med. 2001;46:49–57. doi: 10.1002/mrm.1159. [DOI] [PubMed] [Google Scholar]
- 25.Pickett B, Kurhanewicz J, Coakley F, et al. Use of MRI and spectroscopy in evaluation of external beam radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60:1047–1055. doi: 10.1016/j.ijrobp.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Pickett B, Ten Haken RK, Kurhanewicz J, et al. Time to metabolic atrophy after permanent prostate seed implantation based on magnetic resonance spectroscopic imaging. Int J Radiat Oncol Biol Phys. 2004;59:665–673. doi: 10.1016/j.ijrobp.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Mueller-Lisse UG, Vigneron DB, Hricak H, et al. Localized prostate cancer: effect of hormone deprivation therapy measured by using combined three-dimensional 1H MR spectroscopy and MR imaging: clinicopathologic case-controlled study. Radiology. 2001;221:380–390. doi: 10.1148/radiol.2211001582. [DOI] [PubMed] [Google Scholar]
- 28.Febbo PG, Richie JP, George DJ, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005;11:5233–5240. doi: 10.1158/1078-0432.CCR-05-0299. [DOI] [PubMed] [Google Scholar]
- 29.Atalar E, Menard C. MR-guided interventions for prostate cancer. Magn Reson Imaging Clin N Am. 2005;13:491–504. doi: 10.1016/j.mric.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Futterer JJ, Heijmink SW, Scheenen TW, et al. Prostate cancer: local staging at 3-T endorectal MR imaging—early experience. Radiology. 2006;238:184–191. doi: 10.1148/radiol.2381041832. [DOI] [PubMed] [Google Scholar]
- 31.D’Amico AV, Cormack RA, Tempany CM. MRI-guided diagnosis and treatment of prostate cancer. N Engl J Med. 2001;344:776–777. doi: 10.1056/NEJM200103083441017. [DOI] [PubMed] [Google Scholar]


