Abstract
Ultrasound imaging of the prostate is commonly used to assess the size of the gland and for needle placement during systematic biopsy. Ultrasound evaluation of prostate cancer is limited by difficulty in distinguishing benign from malignant tissue. Although Doppler techniques may provide some improvement in the detection of prostate cancer, targeted biopsy based on conventional ultrasound with Doppler is not sufficient to replace systematic biopsy. Contrast-enhanced ultrasound imaging techniques that employ microbubble contrast agents represent an innovative approach to imaging of the neovascularity associated with prostate cancer. This review describes the application of contrast-enhanced ultrasound to improve detection and assessment of prostate cancer.
Key words: Prostate cancer, Ultrasound, Doppler, Microbubble contrast agents, Harmonic imaging
Imaging of the prostate is central to the diagnosis, detection, and treatment of prostate cancer. In 2005, an estimated 230,000 new prostate cancer cases occurred in the United States.1 Over the past few decades, the prostate has been the leading site for a new diagnosis of cancer in American men. Although it is difficult to detect the presence of prostate cancer with conventional ultrasound imaging, ultrasound-guided biopsy of the prostate is the primary method of diagnosis for prostate cancer. This article reviews various imaging strategies for detecting cancer of the prostate, with particular emphasis on contrast-enhanced ultrasound imaging of prostate cancer.
Approach to Imaging of Prostate Cancer
An intelligent approach to the imaging of prostate cancer begins with an understanding of the histologic growth patterns of this disease. The most common location of origin for prostatic adenocarcinoma is along the outer portion of the prostate, within the glandular tissue of the peripheral zone.2,3 In contrast to visceral neoplasms in other organs, prostate cancer does not generally present as a solitary round mass. In 85% of cases, prostate cancer is multifocal,4 and it tends to grow along the capsule of the gland with an oblong shape.5 Conventional imaging techniques that are optimized for well-defined, round tumor masses may be less effective for the prostate.
On a microscopic level, the features that distinguish prostate cancer from benign prostate tissue are loss of the normal glandular architecture, increased cellular density, and altered microvasculature. The loss of normal glandular architecture, most characteristic of high-grade prostate cancer, results in fewer reflective interfaces and reduced echotexture on conventional ultrasound (Figure 1A). Increased cellular density is related to increased firmness of the tissue and a corresponding reduction in elasticity, which may be visualized with real-time elastography (Figure 1B).6 Changes in cellular volume and density are also related to altered tissue relaxation times that may be visible with magnetic resonance imaging (MRI).7 Changes in cellular metabolism of prostate cancer may be detected on MR spectroscopy.8,9 Finally, increased perfusion of prostate cancer is related to increased microvessel density (neovascularity).10,11 Increased local perfusion may be visualized with contrast-enhanced computed tomography12 or MRI.13–15 Changes in prostate perfusion are visible in larger feeding vessels on conventional Doppler ultrasound (Figure 1C). Although the flow within intratumoral neovessels (10–40 µm diameter) is below the resolution of conventional Doppler ultrasound, this flow may be visualized during contrast-enhanced ultrasound imaging of prostate cancer (see Figure 2 to Figure 4).
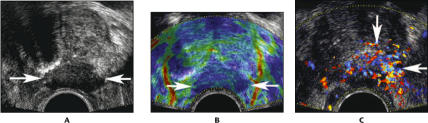
Figure 1.
An 80-year-old man with biopsy cores demonstrating Gleason 9 and Gleason 10 cancer in the left midgland: transverse images through the midgland of the prostate. A. Conventional gray scale image shows a hypoechoic mass extending exophytically from the prostate (arrows). The hypoechoic appearance is the classic description for prostate cancer. B. Real-time elastography shows reduced tissue elasticity (darker blue color) in the region of the mass (arrows). C. Color Doppler shows increased flow within and around the mass (arrows).
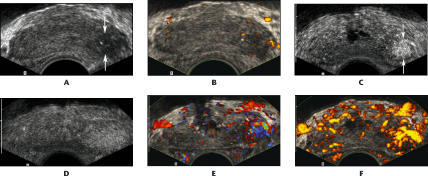
Figure 2.
A 75-year-old man with prostate-specific antigen of 5.1 and Gleason 8 cancer in the left base: transverse images through the base of the prostate. A. Conventional gray scale image shows a hypoechoic area in the left base (arrows). B. Power Doppler image shows no significant increase in flow in this hypoechoic area. C. Harmonic gray scale during contrast infusion shows a clearly defined area of focal enhancement, corresponding to the cancer (arrows). D. Harmonic gray scale with intermittent imaging shows a less well-defined, larger area of parenchymal enhancement around the cancer. E. Contrast-enhanced color Doppler image shows increased flow associated with the cancer. F. Contrast-enhanced power Doppler image also shows increased flow associated with the cancer.
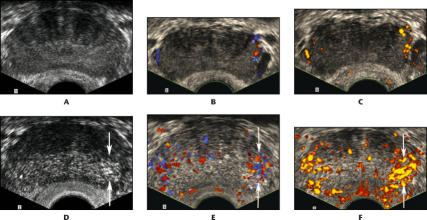
Figure 4.
A 60-year-old man with prostate-specific antigen of 7.4 and Gleason 6 cancer in the left base, found only by targeted biopsy: transverse images through the base of the prostate. A. Conventional gray scale image shows no focal lesion. B. Color Doppler image shows only minimal flow within capsular vessels along the margin of the prostate. C. Power Doppler image shows only minimal flow within capsular vessels along the margin of the prostate. D. Continuous harmonic gray scale during contrast infusion shows a defined area of focal parenchymal enhancement, corresponding to the cancer (arrows). E. Contrast-enhanced color Doppler image shows increased flow, corresponding to the cancer (arrows). F. Contrast-enhanced power Doppler image also shows increased flow corresponding to the cancer (arrows).
Numerous published studies have described the sensitivity and specificity of various imaging techniques for detection of prostate cancer. Unfortunately, it is impossible to determine the true sensitivity or specificity of an imaging technique unless a study includes an evaluation of thin-section, whole-mount prostatectomy specimens from all study participants. Without a complete pathologic evaluation, one does not know how many cancers have been overlooked. Furthermore, studies of prostate imaging rarely include long-term follow-up to demonstrate which lesions were clinically significant. Thus, it is likely that most published studies overestimate the sensitivity of imaging methods for the detection of prostate cancer.
The process of screening for prostate cancer is complicated by the definition of “significant disease” that requires therapy. Conservative management may be reasonable for lower-grade, localized prostate cancer.16 Tumor volume (stage), tumor grade (Gleason score), and microvessel density17,18 are all predictors of significant disease. An ideal approach to the diagnosis of prostate cancer should limit the number of patients subjected to needle biopsy and should detect significant disease with a limited number of targeted biopsy cores. As described in more detail below, a tailored approach to prostate biopsy based on contrast-enhanced ultrasound represents an innovative approach to detecting significant disease with fewer biopsy cores.
Conventional Ultrasound Imaging
The classic gray scale ultrasound description for cancer of the prostate is a hypoechoic lesion.19 However, prostate cancer may appear echogenic or isoechoic.20 Approximately half of prostate cancer lesions are invisible by gray scale imaging.21 Furthermore, 2 common forms of prostate pathology-prostatitis and benign prostatic hyperplasia-mimic the gray scale appearance of prostate cancer. Prostatitis may result in a heterogeneous appearance in the prostate peripheral zone and can present with hypoechoic lesions that are indistinguishable from cancer. Although most hyperplastic prostatic nodules develop in the transition zone (inner gland), benign prostatic hyperplasia may also occur in the peripheral zone of the prostate.22–26 One study suggests that benign prostatic hyperplasia is present in the outer portion of the gland in up to 18.5% of prostate specimens.27 Furthermore, since the peripheral zone wraps around the transition zone, hyperplastic nodules from the transition zone may protrude into the peripheral zone. Given the diverse appearance of prostate cancer and the potential for benign processes to mimic the gray scale appearance of prostate cancer, conventional prostate ultrasound has little advantage over digital rectal examination for detecting malignant areas.28
Ultrasound Doppler techniques demonstrate the presence of blood flow by detecting a frequency/phase shift in the ultrasound radiofrequency signal reflected from moving blood. Since prostate cancer is associated with increased perfusion, the sensitivity of ultrasound for detection of prostate cancer may be increased with color Doppler imaging of blood flow within the prostate.29–31 The color Doppler signal correlates positively with both stage and grade of a prostate tumor, as well as with the risk of recurrence after treatment.32 Power Doppler, a newer Doppler technique for demonstrating the presence of blood flow, reflects the amplitude of the Doppler signal. Although power Doppler does not demonstrate directionality of flow, this technique is more sensitive to small amounts of low-velocity flow. Several small studies have suggested that power Doppler may be even more useful than color Doppler in detecting prostate cancer.33,34 Nonetheless, the sensitivity of gray scale and Doppler ultrasound-guided targeted biopsy is not sufficient to eliminate the need for systematic biopsy.35–38
Contrast-Enhanced Ultrasound
Prostate cancer tissue is associated with increased microvessel density due to the proliferation of neovessels. The microvascular blood supply to prostate tissue is more uniform in malignant than in benign tissue.39 Microvessels in malignant tissue are smaller than those of benign prostate tissue.40 In terms of the prognosis of prostate cancer, there is a clear association between increased microvessel density and metastases,41 stage of disease,42–44 and disease-specific survival.45,46 Quantitative assessment of microvascular density can provide important data to guide therapeutic decisions.47 Unfortunately, the microvessels that proliferate in prostate cancer are below the resolution of conventional Doppler imaging; only the larger feeding vessels are visualized by this type of imaging.
Microbubble contrast agents provide a practical solution to the problem of imaging microvasculature in the prostate.48,49 Microbubble ultrasound contrast agents have intravascular residence times of several minutes, pass through the pulmonary circulation, and may be used for intravascular or parenchymal organ enhancement. Microbubbles show enhanced acoustic reflectivity with a nonlinear frequency response. Specifically, the reflected ultrasound signal from microbubbles contains harmonic frequencies of the original transmitted signal. Microvessels that are below the limits of resolution for a conventional ultrasound Doppler system may be visualized by the intense reflected signal from microbubbles within these vessels. Several clinical studies have demonstrated selective Doppler enhancement of regions with increased microvessel density50,51 and significant improvement in Doppler detection of prostate cancer with microbubble contrast agents.52,53 With contrast-enhanced targeted biopsy, a larger number of prostate cancers may be detected with fewer needle biopsy cores.54,55
Two basic ultrasound technologies are available to image microbubble contrast agents: Doppler and gray scale harmonic imaging (Figure 2–Figure 4). Color and power Doppler imaging, techniques that are available on most modern ultrasound systems, use relatively high energy levels that destroy a large proportion of the microbubbles as they are imaged. An intense Doppler signal is generated as the microbubbles burst during the Doppler imaging. Gray scale harmonic imaging is a technological advance that permits low-energy gray scale imaging of contrast agents.56,57 Higher harmonics of the insonating frequency are produced by resonance of the microbubbles in ultrasound contrast agents. As a result of these harmonic signals, a substantial proportion of the acoustic reverberations created by microbubbles differ from the frequency of insonation.58 Because the preponderance of reflected sound from tissue is at the fundamental frequency of insonation, most harmonic signals received during contrast infusion come from contrast material.
The earliest ultrasound systems for harmonic imaging used a narrow-bandwidth technology to insonate tissue with a narrow range of fundamental frequencies. To achieve an image from reflected harmonic signals, the fundamental frequencies are filtered out of the received signal. A narrow bandwidth is required to permit ample separation between the transmitted fundamental signal frequency and the received harmonic frequency. However, the narrow bandwidth results in a loss of spatial resolution. More recently, broad-bandwidth harmonic imaging has become possible with phase inversion (pulse inversion) technology. Two short ultrasound pulses are transmitted 180 degrees out of phase, resulting in cancellation of ultrasound energy reflected at the fundamental frequency of insonation. Harmonic signals, reflected back at double the transmit frequency, will be back in phase and not subject to cancellation. This type of broad-bandwidth harmonic imaging allows high-resolution, gray scale harmonic imaging of ultrasound microbubble contrast agents.
The intrinsic resolution of gray scale harmonic imaging is greater than that of conventional Doppler imaging. Because harmonic gray scale uses lower energy levels than conventional Doppler, harmonic gray scale imaging results in less bubble destruction and allows microbubble agents to progress further into the microvasculature for imaging.59,60 Contrast-enhanced imaging with color or power Doppler demonstrates flow primarily in larger feeding vessels, as most microbubbles are destroyed by the process of Doppler imaging before they reach the neovasculature. Contrast-enhanced gray scale harmonic imaging demonstrates parenchymal enhancement based on imaging of microbubbles that travel into the microcirculation. To further improve the survival of microbubbles in the circulation, harmonic gray scale imaging can be used with a reduced mechanical index or in an intermittent imaging mode. Intermittent imaging employs a reduced frame rate to lower the energy deposition into tissue, improve the survival time of microbubble contrast, and increase the parenchymal enhancement provided by ultrasound contrast agents.61–63
When used during contrast-enhanced targeted biopsy of the prostate, harmonic gray scale has several additional advantages over color and power Doppler (Table 1). There is no blooming of the harmonic gray scale enhancement around a vessel, in contrast to intense blooming with color or power Doppler imaging. The superior spatial and temporal resolution of harmonic gray scale allows better visualization and placement of the biopsy needle. Finally, there is substantial color flash artifact around the biopsy needle in the color or power Doppler image, which limits the ability to see the needle as it is advanced into the prostate. No such artifact is present with harmonic gray scale imaging.
Table 1.
Comparison of Ultrasound Techniques for Detection of Prostate Cancer
| Spatial | Temporal | Sensitivity | Imaging | Blooming | Flash | |
|---|---|---|---|---|---|---|
| Resolution | Resolution | to Flow | Neovessels | Artifact | Artifact | |
| Conventional gray scale | +++ | +++ | − | − | − | − |
| Color Doppler | + | + | + | − | ++ | ++ |
| Power Doppler | + | + | + | − | ++ | ++ |
| Harmonic gray scale with microbubbles | +++ | +++ | +++ | +++ | − | − |
| Color Doppler with microbubbles | + | + | +++ | ++ | +++ | ++ |
| Power Doppler with microbubbles | + | + | +++ | ++ | +++ | ++ |
Symbols represent a scale where “ −” equals none/poor and “+++” equals greatly increased/optimal.
Clinical Trials with Contrast-Enhanced Ultrasound
Several clinical trials at the Jefferson Prostate Diagnostic Center of Thomas Jefferson University have demonstrated the efficacy of microbubble contrast imaging for the detection of prostate cancer. An early phase II trial of the ultrasound contrast agent Imagent (Alliance Pharmaceutical Corp; San Diego, CA) was performed between June 1998 and January 1999.64 Twenty-six patients with suspected prostate cancer were referred for contrast-enhanced imaging during biopsy. Gray scale and power Doppler enhancement were demonstrated in the prostate gland of all patients after administration of Imagent. Selective gray scale enhancement of malignant foci was also demonstrated. This was the first published study to suggest that gray scale harmonic imaging could be optimized to selectively enhance neovascularity associated with prostate cancer.
A subsequent study included 100 subjects with suspected cancer of the prostate between October 1999 and November 2000. This study correlated harmonic gray scale enhancement with sextant biopsy.65 The microbubble agent used in this study was Definity (DuPont Pharmaceuticals; North Billerica, MA; now part of Bristol Myers Squibb). Based on cancers detected by needle biopsy, this study demonstrated a significant improvement in sensitivity for cancer detection, from 38% at baseline to 65% after infusion of Definity. Furthermore, those lesions detected by contrast enhancement were generally larger and with a higher Gleason sum—that is, they were the more clinically significant cancers. The last 40 subjects in this study were evaluated with additional targeted biopsies directed toward areas of intense contrast enhancement. A statistically significant increase in the positive biopsy rate was found for these targeted biopsy sites.66
A study correlating whole-mount prostatectomy specimens with contrast-enhanced imaging of the microbubble agent Sonazoid (Amersham Health; Oslo, Norway) was performed between January and October of 2000.67 Thirty-one foci of cancer were correlated between contrast-enhanced sonography and whole-mount pathologic inspection. Contrast-enhanced imaging doubled the sensitivity for detection of cancer in the peripheral zone of the prostate, but did not improve the detection of inner gland cancer. The contrast-enhanced detection of cancers in the inner gland was limited by intense enhancement in areas of benign prostatic hyperplasia. False-positive diagnoses of prostate cancer were suggested in both the inner and outer parts of the gland in areas of benign hyperplasia. Interestingly, prostatitis was not a significant cause of false-positive diagnoses with contrast enhancement.
A recently completed clinical trial used microbubble-enhanced imaging with Imagent to direct targeted biopsy of the prostate. This study evaluated 301 subjects with harmonic gray scale, color Doppler, and power Doppler ultrasound.68 Cancer was detected in 363 biopsy cores from 104 of 301 subjects (35%), including 15.5% (175/1133) of cores targeted to areas of contrast enhancement and 10.4% (188/1806) of sextant cores (P < .01). Among subjects with cancer, targeted cores were twice as likely to return a positive biopsy (odds ratio [OR] = 2.0, P < .001). Although targeted biopsy detected 11% (11/104) of cancers not found by the sextant approach, targeted biopsy failed to detect 20% (21/104) of cancers. The majority of cancers missed by the targeted approach were at the apex of the prostate. The low proportion of cores targeted to the apex suggests that contrast enhancement is less efficacious at the apex. Based on these findings, we recommended a contrast-enhanced targeted biopsy strategy with additional cores at the apex of the prostate, in order to maximize cancer detection and minimize the number of biopsy cores.
Given the association of false-positive areas of enhancement with benign prostatic hyperplasia, one might expect to improve the specificity of contrast-enhanced ultrasound by pretreatment with a 5-alpha-reductase inhibitor to suppress perfusion to areas of benign prostatic hyperplasia. In a preliminary trial, 11 subjects scheduled for prostate biopsy were evaluated by gray scale, color, and power Doppler at baseline and weekly for up to 3 weeks while taking the 5-alpha-reductase inhibitor dutasteride (0.5 mg/day).69 Doppler flow suppression occurred in all 11 subjects after 1 week (P < .01). Further suppression was noted after 2 weeks in 8 subjects (P = .04). Suppression of flow was greatest in the peripheral zone and least obvious in the periurethral zone. Cancer was detected in 20% (8/40) of targeted cores and 7.6% (5/66) of sextant cores. Cancer was detected in 4 subjects by targeted biopsy and in 3 of 4 by systematic biopsy. In the 4 men with cancer, targeted cores were 5.9 times more likely to be positive (P = .027). Selective suppression of flow in benign tissue was observed in 2 of the 4 subjects with cancer.
Conclusion
Microbubble contrast-enhanced studies demonstrate a clear association between contrast enhancement in the prostate and the diagnosis of clinically significant cancer. Sensitivity for the diagnosis of prostate cancer is increased by targeted biopsy of the prostate with contrast-enhanced ultrasound. However, false-positive foci of enhancement are frequent and are often related to the presence of benign prostatic hyperplasia. Based on preliminary studies, it seems possible that targeted biopsy of the prostate with a microbubble contrast agent after short-term therapy with a 5-alpha-reductase inhibitor may selectively identify moderate to high-grade, clinically significant cancers in areas of contrast enhancement. As suggested in this review, most published research on microbubble-enhanced imaging of the prostate has focused on developing a costeffective strategy for improving the detection of prostate cancer. If these microbubble-based techniques are successful, future applications of microbubble agents will likely expand to include the staging of prostate cancer and the monitoring of response to therapy.
Main Points.
Given the diverse appearance of prostate cancer and the potential for benign processes to mimic the gray scale appearance of prostate cancer, conventional prostate ultrasound has little advantage over digital rectal examination for detecting malignant areas.
A tailored approach to prostate biopsy based on contrast-enhanced ultrasound represents an innovative approach to detecting significant disease with fewer biopsy cores.
When used during contrast-enhanced targeted biopsy of the prostate, harmonic gray scale has several advantages over color and power Doppler, including no blooming of the harmonic gray scale enhancement around a vessel, superior spatial and temporal resolution, and no color flash artifact around the biopsy needle.
Microbubble contrast-enhanced studies demonstrate a clear association between contrast enhancement in the prostate and the diagnosis of clinically significant cancer.
Targeted biopsy of the prostate with a microbubble contrast agent after short-term therapy with a 5-alpha-reductase inhibitor may selectively identify moderate to high-grade, clinically significant cancers in areas of contrast enhancement.
Figure 3.
A 78-year-old man with prostate-specific antigen of 5.6 and Gleason 8 cancer in the left midgland: transverse images through the midgland of the prostate. A. Conventional gray scale image shows no focal lesion. B. Power Doppler image shows only minimal flow along the left side of the prostate. C. Continuous harmonic gray scale during contrast infusion shows a defined area of focal enhancement, corresponding to the cancer (arrows). D. Harmonic gray scale with intermittent imaging shows the same area of enhancement with better definition. E. Contrast-enhanced color Doppler image shows increased flow on the side of the cancer, but does not localize the lesion as well as the harmonic gray scale. F. Contrast-enhanced power Doppler image also shows increased flow on the side of the cancer.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.McNeal JE. Origin and development of carcinoma in the prostate. Cancer. 1969;23:24–33. doi: 10.1002/1097-0142(196901)23:1<24::aid-cncr2820230103>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.McNeal JE, Price HM, Redwine EA, et al. Stage A versus stage B carcinoma of the prostate: morphologic comparison and biologic significance. J Urol. 1988;139:61–65. doi: 10.1016/s0022-5347(17)42293-2. [DOI] [PubMed] [Google Scholar]
- 4.Byar DP, Mostofi FK. Carcinoma of the prostate: prognostic evaluation of certain pathologic features in 208 radical prostatectomies. Cancer. 1972;30:5–13. doi: 10.1002/1097-0142(197207)30:1<5::aid-cncr2820300103>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.McNeal JE, Redwine EA, Freiha FS, Stamey TRA. Zonal distribution of prostatic adenocarcinoma: correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12:897–906. doi: 10.1097/00000478-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Konig K, Scheipers U, Pesavento A, et al. Initial experiences with real-time elastography guided biopsies of the prostate. J Urol. 2005;174:115–117. doi: 10.1097/01.ju.0000162043.72294.4a. [DOI] [PubMed] [Google Scholar]
- 7.Heenan SD. Magnetic resonance imaging in prostate cancer. Prostate Cancer Prostatic Dis. 2004;7:282–288. doi: 10.1038/sj.pcan.4500767. [DOI] [PubMed] [Google Scholar]
- 8.Claus FG, Hricak H, Hattery RR. Pretreatment evaluation of prostate cancer: role of MR imaging and 1H MR spectroscopy. Radiographics. 2004;24(suppl 1):S167–180. doi: 10.1148/24si045516. [DOI] [PubMed] [Google Scholar]
- 9.Jung JA, Coakley FV, Vigneron DB, et al. Prostate depiction at endorectal MR spectroscopic imaging: investigation of a standardized evaluation system. Radiology. 2004;233:701–708. doi: 10.1148/radiol.2333030672. [DOI] [PubMed] [Google Scholar]
- 10.Bigler SA, Deering RE, Brawer MK. Comparison of microscopic vascularity in benign and malignant prostate tissue. Hum Pathol. 1993;24:220–226. doi: 10.1016/0046-8177(93)90304-y. [DOI] [PubMed] [Google Scholar]
- 11.Weidner N, Carroll PR, Flax J, et al. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]
- 12.Ives EP, Burke MA, Edmonds PR, et al. Quantitative CT perfusion of prostate cancer: correlation with whole mount pathology. Clin Prostate Cancer. 2005;4:109–112. doi: 10.3816/cgc.2005.n.018. [DOI] [PubMed] [Google Scholar]
- 13.Brown G, Macvicar DA, Ayton V, et al. The role of intravenous contrast enhancement in magnetic resonance imaging of prostatic carcinoma. Clin Radiol. 1995;50:601–606. doi: 10.1016/s0009-9260(05)83288-x. [DOI] [PubMed] [Google Scholar]
- 14.Jager GJ, Ruijter E, van de Kaa CA, et al. Dynamic TurboFLASH subtraction technique for contrast-enhanced MR imaging of the prostate: correlation with histopathologic results. Radiology. 1997;203:645–651. doi: 10.1148/radiology.203.3.9169683. [DOI] [PubMed] [Google Scholar]
- 15.Engelbrecht MR, Huisman HJ, Laheij R, et al. Discrimination of prostate cancer from normal peripheral zone and central gland tissue by using dynamic contrast-enhanced MR imaging. Radiology. 2003;229:248–254. doi: 10.1148/radiol.2291020200. [DOI] [PubMed] [Google Scholar]
- 16.Chodak GW, Thisted RA, Gerber GS, et al. Results of conservative management of clinically localized prostate cancer. N Engl J Med. 1994;330:242–248. doi: 10.1056/NEJM199401273300403. [DOI] [PubMed] [Google Scholar]
- 17.Lissbrant IF, Stattin P, Damber JE, Bergh A. Vascular density is a predictor of cancer-specific survival in prostatic carcinoma. Prostate. 1997;33:38–45. doi: 10.1002/(sici)1097-0045(19970915)33:1<38::aid-pros7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Borre M, Offersen BV, Nerstrom B, Overgaard J. Microvessel density predicts survival in prostate cancer patients subjected to watchful waiting. Br J Cancer. 1998;78:940–944. doi: 10.1038/bjc.1998.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rifkin MD, Dahnert W, Kurtz AB. State of the art: endorectal sonography of the prostate gland. AJR Am J Roentgenol. 1990;154:691–700. doi: 10.2214/ajr.154.4.1690499. [DOI] [PubMed] [Google Scholar]
- 20.Dahnert WF, Hamper UM, Eggleston JC, et al. Prostatic evaluation by transverse sonography with histopathologic correlation: echogenic appearance of early carcinoma. Radiology. 1986;158:97–102. doi: 10.1148/radiology.158.1.3510032. [DOI] [PubMed] [Google Scholar]
- 21.Halpern EJ, Strup SE. Using gray scale, color and power Doppler sonography to detect prostatic cancer. AJR Am J Roentgenol. 2000;174:623–627. doi: 10.2214/ajr.174.3.1740623. [DOI] [PubMed] [Google Scholar]
- 22.Hamper UM, Sheth S, Walsh PC, et al. Stage B adenocarcinoma of the prostate: transrectal US and pathologic correlation of nonmalignant hypoechoic peripheral zone lesions. Radiology. 1991;180:101–104. doi: 10.1148/radiology.180.1.2052673. [DOI] [PubMed] [Google Scholar]
- 23.Kerley SW, Corica FA, Qian J, et al. Peripheral zone involvement by prostatic hyperplasia. J Urol Pathol. 1997;6:87–94. [Google Scholar]
- 24.Moore RA. Benign hypertrophy of the prostate: a morphological study. J Urol. 1943;50:680–710. [Google Scholar]
- 25.Ohori M, Egawa S, Wheeler TM. Nodules resembling nodular hyperplasia in the peripheral zone of the prostate gland. J Urol Pathol. 1994;2:222–223. doi: 10.1111/j.1464-410x.1994.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 26.Oyen RH, Van de Voorde WM, Van Poppel, et al. Benign hyperplastic nodules that originate in the peripheral zone of the prostate gland. Radiology. 1993;189:707–711. doi: 10.1148/radiology.189.3.7694310. [DOI] [PubMed] [Google Scholar]
- 27.Van de Voorde WM, Oyen RH, Van Poppel HP, et al. Peripherally localized benign hyperplastic nodules of the prostate. Mod Pathol. 1995;8:46–50. [PubMed] [Google Scholar]
- 28.Aarnink RG, Beerlage HP, De La Rosette JJ, et al. Transrectal ultrasound of the prostate: innovations and future applications. J Urol. 1998;159:1568–1579. doi: 10.1097/00005392-199805000-00045. [DOI] [PubMed] [Google Scholar]
- 29.Rifkin MD, Sudakoff GS, Alexander AA. Prostate: techniques, results and potential applications of color Doppler US scanning. Radiology. 1993;186:509–513. doi: 10.1148/radiology.186.2.7678467. [DOI] [PubMed] [Google Scholar]
- 30.Sudakoff GS, Smith R, Vogelzang NJ, et al. Color Doppler imaging and transrectal sonography of the prostate fossa after radical prostatectomy: early experience. AJR Am J Roentgenol. 1996;167:883–888. doi: 10.2214/ajr.167.4.8819374. [DOI] [PubMed] [Google Scholar]
- 31.Kravchick S, Cytron S, Peled R, et al. Using gray-scale and two different techniques of color Doppler sonography to detect prostate cancer. Urology. 2003;61:977–981. doi: 10.1016/s0090-4295(02)02520-7. [DOI] [PubMed] [Google Scholar]
- 32.Ismail M, Petersen RO, Alexander AA, et al. Color Doppler imaging in predicting the biologic behavior of prostate cancer: correlation with disease-free survival. Urology. 1997;50:906–912. doi: 10.1016/S0090-4295(97)00403-2. [DOI] [PubMed] [Google Scholar]
- 33.Cho JY, Kim SH, Lee SE. Diffuse prostatic lesions: role of color Doppler and power Doppler ultrasonography. J Ultrasound Med. 1998;17:283–287. doi: 10.7863/jum.1998.17.5.283. [DOI] [PubMed] [Google Scholar]
- 34.Okihara K, Kojima M, Naya Y, et al. Ultrasonic power Doppler imaging for prostatic cancer: a preliminary report. Tohoku J Exp Med. 1997;182:277–281. doi: 10.1620/tjem.182.277. [DOI] [PubMed] [Google Scholar]
- 35.Kelly IMG, Lees WR, Rickards D. Prostate cancer and the role of color Doppler US. Radiology. 1993;189:153–156. doi: 10.1148/radiology.189.1.7690489. [DOI] [PubMed] [Google Scholar]
- 36.Newman JS, Bree RL, Rubin JM. Prostate cancer: diagnosis with color Doppler sonography with histologic correlation of each biopsy site. Radiology. 1995;195:86–90. doi: 10.1148/radiology.195.1.7534429. [DOI] [PubMed] [Google Scholar]
- 37.Cornud F, Belin X, Piron D, et al. Color Dopplerguided prostate biopsies in 591 patients with an elevated serum PSA level: impact on Gleason score for non-palpable lesions. Urology. 1997;49:709–715. doi: 10.1016/S0090-4295(96)00632-2. [DOI] [PubMed] [Google Scholar]
- 38.Halpern EJ, Frauscher F, Strup SE, et al. Prostate: high frequency Doppler US imaging for cancer detection. Radiology. 2002;225:71–77. doi: 10.1148/radiol.2251011938. [DOI] [PubMed] [Google Scholar]
- 39.Kay PA, Robb RA, Bostwick DG. Prostate cancer microvessels: a novel method for three-dimensional reconstruction and analysis. Prostate. 1998;37:270–277. doi: 10.1002/(sici)1097-0045(19981201)37:4<270::aid-pros9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 40.Louvar E, Littrup PJ, Goldstein A, et al. Correlation of color flow in the prostate with tissue microvascularity. Cancer. 1998;83:135–140. [PubMed] [Google Scholar]
- 41.Weidner N, Carroll PR, Flax J, et al. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]
- 42.Fregene TA, Khanuja PS, Noto AC, et al. Tumorassociated angiogenesis in prostate cancer. Anticancer Res. 1993;13:2377–2382. [PubMed] [Google Scholar]
- 43.Brawer MK, Deering RE, Brown M, et al. Predictors of pathologic stage in prostate carcinoma, the role of neovascularity. Cancer. 1994;73:678–687. doi: 10.1002/1097-0142(19940201)73:3<678::aid-cncr2820730329>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 44.Bostwick DG, Wheeler TM, Blute M, et al. Optimized microvessel density analysis improves prediction of cancer stage from prostate needle biopsies. Urology. 1996;48:47–57. doi: 10.1016/s0090-4295(96)00149-5. [DOI] [PubMed] [Google Scholar]
- 45.Lissbrant IF, Stattin P, Damber JE, Bergh A. Vascular density is a predictor of cancer-specific survival in prostatic carcinoma. Prostate. 1997;33:38–45. doi: 10.1002/(sici)1097-0045(19970915)33:1<38::aid-pros7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 46.Borre M, Offersen BV, Nerstrom B, Overgaard J. Microvessel density predicts survival in prostate cancer patients subjected to watchful waiting. Br J Cancer. 1998;78:940–944. doi: 10.1038/bjc.1998.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brawer MK. Quantitative microvessel density: a staging and prognostic marker for human prostatic carcinoma. Cancer. 1996;78:345–349. doi: 10.1002/(SICI)1097-0142(19960715)78:2<345::AID-CNCR25>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 48.Goldberg BB, Liu JB, Forsberg F. Ultrasound contrast agents: a review. Ultrasound Med Biol. 1994;20:319–333. doi: 10.1016/0301-5629(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg BB, Raichlen JS, Forsberg F. Ultrasound Contrast Agents: Basic Principles and Clinical Applications. 2nd ed. London: Dunitz; 2001. [Google Scholar]
- 50.Sedelaar JP, van Leenders GJ, Hulsbergen-van de Kaa CA, et al. Microvessel density: correlation between contrast ultrasonography and histology of prostate cancer. Eur Urol. 2001;40:285–293. doi: 10.1159/000049788. [DOI] [PubMed] [Google Scholar]
- 51.Strohmeyer D, Frauscher F, Klauser A, et al. Contrast-enhanced transrectal color Doppler ultrasonography (TRCDUS) for assessment of angiogenesis in prostate cancer. Anticancer Res. 2001;21:2907–2913. [PubMed] [Google Scholar]
- 52.Bogers HA, Sedelaar JP, Beerlage HP, et al. Contrast-enhanced three-dimensional power Doppler angiography of the human prostate: correlation with biopsy outcome. Urology. 1999;54:97–104. doi: 10.1016/s0090-4295(99)00040-0. [DOI] [PubMed] [Google Scholar]
- 53.Roy C, Buy X, Lang H, et al. Contrast enhanced color Doppler endorectal sonography of the prostate: efficiency for detecting peripheral zone tumors and role for biopsy procedure. J Urol. 2003;170:69–72. doi: 10.1097/01.ju.0000072342.01573.8d. [DOI] [PubMed] [Google Scholar]
- 54.Frauscher F, Klauser A, Halpern EJ, et al. Detection of prostate cancer with a microbubble ultrasound contrast agent. Lancet. 2001;357:1849–1850. doi: 10.1016/s0140-6736(00)04970-9. [DOI] [PubMed] [Google Scholar]
- 55.Frauscher F, Klauser A, Volgger H, et al. Comparison of contrast-enhanced color Doppler targeted biopsy to conventional systematic biopsy: impact on prostate cancer detection. J Urol. 2002;167:1648–1652. [PubMed] [Google Scholar]
- 56.de Jong N, Cornet R, Lancee CT. Higher harmonics of vibrating gas-filled microspheres. Part one: simulations. 1994;32:447–453. [Google Scholar]
- 57.de Jong N, Cornet R, Lancee CT. Higher harmonics of vibrating gas-filled microspheres. Part two: measurements. 1994;32:455–459. [Google Scholar]
- 58.Forsberg F, Goldberg BB, Liu JB, et al. On the feasibility of real-time, in vivo harmonic imaging with proteinaceous microspheres. J Ultrasound Med. 1996;15:853–860. doi: 10.7863/jum.1996.15.12.853. [DOI] [PubMed] [Google Scholar]
- 59.Schrope BA, Newhouse VL, Uhlendorf V. Simulated capillary blood flow measurement using a nonlinear ultrasonic contrast agent. Ultrason Imaging. 1992;14:134–158. doi: 10.1177/016173469201400204. [DOI] [PubMed] [Google Scholar]
- 60.Schrope BA, Newhouse VL. Second harmonic ultrasound blood perfusion measurement. Ultrasound Med Biol. 1993;19:567–579. doi: 10.1016/0301-5629(93)90080-8. [DOI] [PubMed] [Google Scholar]
- 61.Porter TR, Xie F. Transient myocardial contrast after initial exposure to diagnostic ultrasound pressures with minute doses of intravenously injected microbubbles. Circulation. 1995;92:2391–2395. doi: 10.1161/01.cir.92.9.2391. [DOI] [PubMed] [Google Scholar]
- 62.Colon PJ, Richards DR, Moreno CA, et al. Benefits of reducing the cardiac cycle-triggering frequency of ultrasound imaging to increase myocardial opacification with FS069 during fundamental and second harmonic imaging. JAm Soc Echocardiogr. 1997;10:602–607. doi: 10.1016/s0894-7317(97)70022-1. [DOI] [PubMed] [Google Scholar]
- 63.Broillet A, Puginier J, Ventrone R, Schneider M. Assessment of myocardial perfusion by intermittent harmonic power Doppler using SonoVue, a new ultrasound contrast agent. Invest Radiol. 1998;33:209. doi: 10.1097/00004424-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Halpern EJ, Verkh L, Forsberg F, et al. Initial experience with contrast-enhanced sonography of the prostate. AJR Am J Roentgenol. 2000;174:1575–1580. doi: 10.2214/ajr.174.6.1741575. [DOI] [PubMed] [Google Scholar]
- 65.Halpern EJ, Rosenberg M, Gomella LG. Prostate Cancer: contrast-enhanced US for detection. Radiology. 2001;219:219–225. doi: 10.1148/radiology.219.1.r01ap21219. [DOI] [PubMed] [Google Scholar]
- 66.Halpern EJ, Frauscher F, Rosenberg M, Gomella LG. Directed biopsy during contrast enhanced sonography of the prostate. AJR Am J Roentgenol. 2001;178:915–919. doi: 10.2214/ajr.178.4.1780915. [DOI] [PubMed] [Google Scholar]
- 67.Halpern EJ, McCue PA, Aksnes AK, et al. Contrast enhanced sonography of the prostate with Sonazoid: comparison with prostatectomy specimens in twelve patients. Radiology. 2002;222:361–366. doi: 10.1148/radiol.2222010582. [DOI] [PubMed] [Google Scholar]
- 68.Halpern EJ, Ramey JR, Strup SE, et al. Detection of prostate cancer with contrast enhanced sonography using intermittent harmonic imaging. Cancer. 2005;104:2372–2383. doi: 10.1002/cncr.21440. [DOI] [PubMed] [Google Scholar]
- 69.Ives EP, Gomella LG, Halpern EJ. Effect of dutasteride therapy on Doppler evaluation of the prostate: preliminary results. Radiology. 2005;237:197–201. doi: 10.1148/radiol.2371041543. [DOI] [PubMed] [Google Scholar]