Abstract
Despite advances in noninvasive staging, pelvic lymph node dissection (PLND) remains the most accurate means of detecting lymph node metastases in men with clinically localized prostate cancer. Nomograms exist that can identify patients at low risk for lymphatic metastases according to preoperative information. In general, it seems reasonable to omit PLND in men with a biopsy Gleason sum of 6 or less and a prostate-specific antigen level of 10 ng/mL or less. Ultimately, however, this decision should be made according to physician and patient preference, considering the low contemporary morbidity associated with PLND. When PLND is performed, studies suggest that an extended dissection maximizes the detection rate of nodal involvement. Retrospective data indicate that an extended dissection might play a therapeutic role in a subset of patients with a limited lymph node burden. However, this might be an artifact of stage migration, and prospective studies are needed to evaluate this further.
Key words: Prostate cancer, Lymphadenectomy, Clinical staging, Magnetic resonance imaging, Radioimmunoscintigraphy, Nomograms, Algorithms
The presence of lymph node metastasis in men diagnosed with clinically localized prostate cancer portends a poor prognosis.1–3 Accurate identification of these men allows more precise prognostication and might have important implications regarding the initiation of adjuvant therapy. Although in unique situations imaging modalities might assist in the detection of lymph node metastases, in the vast majority of cases these tests are not yet reliable.4,5 Early enthusiasm for radioimmunoscintigraphy and molecular staging techniques has been tempered by their limited accuracy in clinical studies.6–8 Pelvic lymph node dissection (PLND) remains the most accurate staging procedure for the detection of occult nodal involvement.9
The advent of prostate-specific antigen (PSA) screening has resulted in a steady decline in the incidence of pelvic lymph node metastasis, from rates of 20% to 40% in the 1970s and 1980s to less than 6% today.10,11 As a result of this stage shift, PLND is often omitted before various curative treatment approaches (radical retropubic prostatectomy, laparoscopic radical prostatectomy, perineal prostatectomy, radiation therapy, and cryotherapy) or is performed within a more restricted anatomic template. Nomograms and other algorithms have been developed to predict the likelihood of lymph node metastasis and identify patients suitable for PLND omission.10,12,13
Given the individual variation in prostatic lymphatic drainage patterns and the fact that some investigators performing extended PLND have reported higher rates of lymph node metastases than those predicted by popular nomograms, some investigators favor performing extended PLND on the majority of patients with clinically localized prostate cancer. Recent studies have suggested that an extended PLND not only maximizes the detection of lymph node-positive disease but might also play a therapeutic role in a subset of patients.14–16
Thus, the importance of PLND in men with clinically localized prostate cancer remains a matter of debate. This articles aims to systematically review this controversial topic and clarify the current state of knowledge on the basis of the contemporary literature.
Goals of PLND
Historically, PLND was performed before radical prostatectomy, and lymph nodes were sent for frozen section analysis. In this context, the goal of PLND was to determine whether one should proceed with radical surgery, the philosophy being that if metastatic disease was discovered, the patient was spared the morbidity of a prostatectomy. Interestingly, the false-negative rate of frozen section for micrometastatic disease can be as high as 30%.17 Today some surgeons will perform a laparoscopic lymphadenectomy before proceeding with definitive surgical treatment on patients with locally advanced disease or Gleason sum greater than 7 because it is those patients who are less likely to benefit from radical surgery in the setting of lymphatic spread.18
Another and more common reason to perform PLND is to present patients with the most accurate assessment of their disease burden, thus providing them with relevant treatment options and prognostic information. Some patients insist on undergoing PLND, regardless of their risk of metastasis, for peace of mind. Finally, some investigators contend that there might be a therapeutic benefit associated with PLND.14–16
Clinical Staging
The goal of clinical staging is to use pretreatment parameters to predict the true extent of disease. This allows assessment of prognosis and facilitates educated decision making regarding treatment options. Ultimately, pathologic outcome is the most reliable means of predicting outcome of therapy in men with clinically localized prostate cancer.3
Cross-Sectional Imaging
Cross-sectional imaging by computed tomography or magnetic resonance imaging (MRI) to evaluate lymph node involvement is not routinely recommended, owing to the low sensitivity (0–30%) of these modalities in imaging microscopic disease.19,20 Specialized techniques, such as high-resolution MRI used in tandem with the intravenous administration of lymphotropic superpara-magnetic nanoparticles, might allow the detection of small and otherwise undetectable lymph node disease. In a recent study involving 80 patients with clinical T1, T2, or T3 disease, MRI with lymphotropic superpara-magnetic nanoparticles outperformed conventional MRI and nomograms in the detection of lymph node-positive disease.21 Such techniques, however, require further clinical evaluation and validation before widespread use.
Radioimmunoscintigraphy and Molecular Staging Techniques
Monoclonal antibody radioimmunoscintigraphy (ie, ProstaScint Scan; Cytogen Corporation, Princeton, NJ) has had limited accuracy in the detection of lymph node metastases because the antibody targets an intracellular epitope that is only exposed in dying or dead cells.6,7 Although initially promising, molecular techniques using reverse transcription polymerase chain reaction (RT-PCR) have had varying sensitivities in detecting circulating cancer cells. In addition, a significant proportion of men with organ-confined disease in one study were found to have a positive PSA PCR assay.8 Thus, the significance of a positive assay remains unknown, and positive assays might lead to men being overstaged and denied curative treatment.
Combined Use of Treatment Parameters: Nomograms and Algorithms
Although numerous studies have integrated various clinical parameters to predict the pathologic stage of clinically localized prostate cancer, only a few have specifically focused on lymphatic metastases. These studies have resulted in nomograms, algorithms, and proprietary artificial neural networks that can predict the likelihood of lymph node metastases for a given set of input parameters. Most investigators have focused on Gleason sum, PSA, and clinical stage, but some have included details of the preoperative biopsy in their models. Table 1 summarizes a few pertinent studies focusing on this topic. Most studies conclude that one can designate a significant percentage of patients (20%–80%) as “low risk” for lymph node metastases and avoid a PLND in those patients. All published models seem to be able to achieve this with an impressive false-negative rate (<10%). This is especially significant given the increasing use of treatment modalities that do not permit lymph node staging, such as brachytherapy and external beam radiation therapy.
Table 1.
Algorithms and Nomograms Predicting Lymph Node Metastases
| No. of | Statistical | Definition of Low-Risk | % Patients | % False | ||
|---|---|---|---|---|---|---|
| Authors | Year | Patients | Tool | Patient or Input Variables | Spared PLND | Negative |
| Bluestein DL et al12 | 1994 | 1632 | LR | Gleason sum, PSA, cStage | 61% of patients | ≤ 3 |
| with cStage | ||||||
| T1a-T2b | ||||||
| Narayan P et al39 | 1994 | 932 | LR | PSA ≤ 10 ng/mL, Gleason sum ≤ 6 | 42 | 1 |
| Bishoff JT et al40 | 1995 | 481 | LR | Gleason sum, PSA, cStage | 20–63 | 2–10 |
| Parra RO et al41 | 1996 | 155 | None | PSA < 10.0 ng/mL, Gleason sum < 7 | 47 | 0 |
| Conrad S et al22 | 1998 | 344 | CART | ≤ 3 cores with any Gleason 4 or 5 | 80 | 2.2 |
| and no core with predominant | ||||||
| Gleason 4 or 5 | ||||||
| Tewari A and | 1998 | 1200 | ANN | Age, race, perineural invasion, Gleason | 63 | ≤ 2 |
| Narayan P42 | sum, PSA, cStage, biopsy data (unilateral | |||||
| vs bilateral, no. of cores positive) | ||||||
| Crawford ED et al43 | 2000 | 4133 | ANN | Gleason sum ≤ 6, PSA ≤ 10.6 ng/mL, | 44 | 0.8 |
| or cStage ≤ T2a | ||||||
| Batuello JT et al44 | 2001 | 6135 | ANN | Gleason sum, PSA, cStage | 80 | ≤ 2 |
| Naya Y and | 2003 | 695 | CART | All Gleason components < 4 or | 70 | 0.4 |
| Babaian RJ45 | < 4 positive cores Gleason 4 or 5 and | |||||
| PSA < 15 ng/mL and no core with | ||||||
| predominant Gleason 4 or 5 | ||||||
| Cagiannos I et al13 | 2003 | 5510 | LR | Gleason sum, PSA, cStage | 67* | 1.5* |
PLND, pelvic lymph node dissection; LR, logistic regression; PSA, prostate-specific antigen; cStage, clinical stage; CART, classification and regression tree; ANN, artificial neural network.
Numbers shown correspond to when model predicts a probability of 3% or less for nodal involvement.
Cagiannos and colleagues13 recently constructed a nomogram to predict lymph node metastases, based on preoperative Gleason sum, PSA level, and clinical stage of 7014 patients from 7 institutions. Their conclusion was that it is appropriate to omit PLND when the nomogram predicts a probability of metastasis between 1.5% and 3.0% or less. For a 3.0% nomogram probability, 66.8% of patients would have been spared PLND, with a false-negative rate of 1.5%. Interestingly, the rate of lymph node metastases between participating institutions in this study ranged from 1.5% to 7.0%. Including institution as an additional variable in the analysis changed the probability of lymph node metastasis generated by the nomogram, independent of Gleason sum, PSA level, and clinical stage. The extent of lymph node dissection at each institution is not provided and might account for this phenomenon.
Only a few nomograms predicting lymphatic spread have been validated. Bluestein and colleagues12 randomized half of their patients into a training set and exposed the other half in a validation set. Conrad and coworkers22 used classification and regression trees analysis and derived an algorithm (the Hamburg algorithm) based on Gleason sum and sextant biopsy information. This algorithm was internally validated, with consistent results on a cohort of 239 patients from the same institution. Haese and colleaguesB23 verified the performance of the Hamburg algorithm in a cohort of 443 men treated for clinically localized prostate cancer at the Johns Hopkins Hospital.
The Partin tables represent a more comprehensive example of these algorithms because they reveal a percentage probability of having a final pathologic stage (including nodal involvement) based on logistic regression analysis of Gleason sum, PSA level, and clinical stage.10,24,25 This nomogram has been updated and validated in a number of multicenter studies, including a 1997 study with 4133 patients.10 In this validation cohort, the Partin nomogram accurately predicted nodal metastases in 83% of patients. For patients with a Gleason sum of 6 or less and a PSA level of 10 ng/mL or less, the likelihood of metastatic disease according to the Partin nomogram is 0 to 3%. For this reason, and consistent with most other nomogram predictions, some surgeons reserve PLND for men with a PSA level greater than 10 ng/mL and Gleason sum greater than 6.
Complications and Financial Implications
The decline in lymph node-positive disease among PSA-screened men and the availability of algorithms that can identify “low-risk” patients has led some urologists to abandon the routine use of PLND. The added operating room time (in the case of concomitant radical prostatectomy), the additional costs, and morbidity are all cited as additional reasons to omit PLND from the routine care of men with clinically localized disease.
It has been estimated that PLND adds approximately $935 to $3120 to the total cost of a radical retropubic prostatectomy.23,26,27 The additional expense includes pathologic consultation and operating room time (15–30 minutes). The significance of this additional financial burden can be appreciated by multiplying this premium by the number of radical retropubic prostatectomies performed annually (> 20,000 in the year 2000).
The complication rate associated with PLND ranges between 4% and 53%; however, these rates are derived from studies spanning several decades, including open and laparoscopic series, and involving different dissection templates.Table 2 summarizes select recent series reporting on the morbidity of PLND. Clark and colleagues28 randomized 123 patients undergoing radical prostatectomy to an extended PLND on the right or left side, with the other side having a limited PLND. They reported a 10.6% overall complication rate, with 75% of these occurring on the extended side. Heidenreich and coworkers15 compared patients who underwent extended or limited PLND and found no difference in terms of complications between the 2 groups. The postoperative complication rate of both groups was approximately 9%. Kavoussi and colleagues29 assessed the complications in 372 patients undergoing laparoscopic PLND at 8 medical centers. The overall complication rate was 15% (requiring open conversion in 13 cases) and included vascular injury in 11 patients, deep vein thrombosis in 5, obturator nerve injury in 2, and lymphocele/lymphedema in 5. This study represented the initial laparoscopic experience, and subsequent reports have documented improvement in the number and scope of these complications. It is generally agreed, though, that the morbidity of PLND today is minimal.
Table 2.
Select Recent Series Reporting on the Morbidity of Pelvic Lymph Node Dissection
| Complication (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurovas- | |||||||||||
| No. of | Laparoscopic | Extended (E)/ | Lympho- | LE | Pelvic | Ureteral | cular | ||||
| Authors | Year | Patients | (L)/Open (O) | Limited (L) | cele | Edema | DVT/PE | Abscess | Injury | Injury | Ileus |
| Kavoussi LR et al29 | 1993 | 372 | L | L | 1.3 | 0* | 1.3 | 0.5 | 0.5 | 3.5 | 1.3 |
| Campbell SC et al27 | 1995 | 245 | O | L | 1.6 | - | 1.6 | - | - | 0.8 | - |
| Raboy A et al46 | 1997 | 125 | L | L | 2.4 | - | 0.8 | - | - | - | 0 |
| Stone NN et al33 | 1997 | 150 | L | L | - | 0 | - | 0 | - | 0.7 | - |
| Stone NN et al33 | 1997 | 39 | L | E | - | 10 | - | 5.1 | - | 5.1 | - |
| Herrell SD et al47 | 1997 | 38 | O | L | 2.6 | 0 | 2.6 | 2.6 | 0 | 0 | 7.9 |
| Heidenreich A et al15 | 2002 | 103 | O | E | 10.6 | - | 6.3 | 0 | 0.9 | 7.1 | - |
| Heidenreich A et al15 | 2002 | 100 | O | L | 9.0 | - | 8.0 | 0 | 0 | 6.0 | - |
| Bader P et al16 | 2003 | 365 | O | E | 1.9 | - | 2.7 | - | - | - | - |
| Clark T et al28 | 2003 | 123 | O | L/E† | 3.3 | 4.1 | 1.6 | 0.8 | 0.8 | - | - |
| Allaf ME et al14 | 2004 | 2135 | O | E | 0.1 | - | - | - | - | - | - |
LE, lower extremity; DVT, deep vein thrombosis; PE, pulmonary embolus.
In this study, LE edema and lymphocele were reported as one category.
75% of complications occurred on side receiving extended pelvic lymph node dissection.
PLND Templates
The extent of PLND has varied by era and individual surgeon. It is important to note the general boundaries of a typical limited and extended dissection. Extended PLND consists of excising the fibrofatty and lymphatic tissues in an area bordered superiorly by the bifurcation of the common iliac artery, inferiorly by the femoral canal, and laterally by the pelvic sidewall. Posteriorly, all tissues surrounding the obturator nerve, obturator vessels, and internal iliac artery are removed. Care is taken to preserve the tissues overlaying and surrounding the external iliac artery. These tissues contain the lymphatics that drain the lower extremities, and their disruption might result in lower extremity edema and lymphocele formation. A limited (or modified) PLND differs in that the posterior extent of the dissection is the obturator fossa, and the internal iliac nodes are not removed. Closed suction drainage and meticulous ligation of lymphatic channels minimize the complications of PLND.
The Case for Extended PLND
Lymphatic drainage of the prostate is variable and involves regions not sampled during “routine” PLND. Earlier surgical studies have confirmed that significant rates of nodal metastases (15%–30%) might be detected exclusively in areas outside of the boundaries of a limited dissection.30,31 Recently, Bader and colleagues16 evaluated 365 patients with clinically localized prostate cancer who underwent extended pelvic lymphadenectomy at the time of radical prostatectomy. One fourth of these patients were found to have nodal involvement, of whom approximately 20% had metastases solely along the internal iliac vessels. In the study by Heidenreich and coworkers,15 42% of the patients undergoing extended lymph node dissection were found to have nodal involvement outside of the external iliac and obturator lymph nodes. Furthermore, recent lymphoscintigraphy studies have confirmed the individual variation in prostatic lymphatic drainage patterns. 32 In addition to retrieving more lymph nodes, an extended PLND seems to detect a greater proportion of patients with lymph node metastases when compared with a limited lymphadenectomy (Table 3).14,15,33
Table 3.
Studies Demonstrating That Extended Pelvic Lymph Node Dissection Detects a Greater Proportion of Patients With Lymph Node Metastases When Compared With Limited Lymphadenectomy
Certain patients with node-positive disease who undergo radical prostatectomy have good long-term PSA-free survival. Han and colleagues34 reported a 10% actuarial biochemical recurrence-free rate after radical prostatectomy at 10 years for patients found to have lymph node micrometastases. Several studies have documented an association between quantity of lymph nodes involved and survival. In 1987, Golimbu and coworkers35 retrospectively analyzed 42 patients with occult nodal disease who underwent pelvic lymphadenectomy and radical prostatectomy. In this series, patients with low tumor bulk and 1 positive lymph node had survival rates comparable to those of matched controls after a mean follow-up of 5 years. More recently, Bader and colleagues16 reported delayed progression in patients with minimal lymph node involvement detected by extended lymphadenectomy and treated with radical prostatectomy. The investigators hypothesized that this might represent cure in some patients. In 2004, Allaf and coworkers14 reported that among men with lymph node-positive disease involving less than 15% of extracted nodes, the 5-year PSA progression-free rate for extended lymph node dissection was 43%, compared with 10% for the more limited lymph node dissection (Figure 1).14 These investigators concluded that a significant benefit in biochemical recurrence-free survival might exist for certain subgroups undergoing the extended dissection, although this has not been proven. An alternative explanation for the finding of a benefit to extended dissection is that it might be an artifact of stage migration (the Will Rogers phenomenon).36 Other evidence to support the hypothesis that a lymph node dissection can contribute to biochemical cancer control comes from a case report in which a patient with a rising PSA level after a radical prostatectomy experienced a fall in PSA to undetectable levels after excision of a positive lymph node at the time of a colonic resection.37
Figure 1.
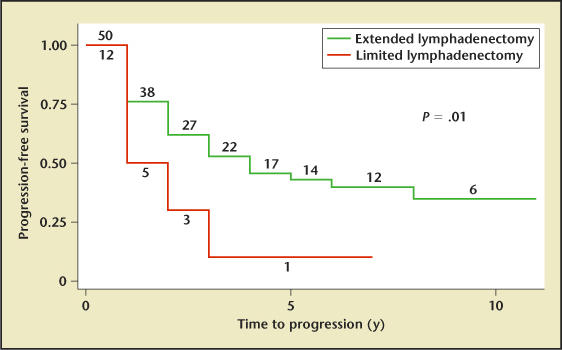
Kaplan-Meier survival analysis of men with clinically localized prostate cancer found to have <15% positive lymph nodes. The analysis is stratified by extent of lymph node dissection (extended lymphadenectomy n = 50, limited lymphadenectomy n = 12; log-rank P = .01). The number of patients at risk at the beginning of each time interval is displayed. Reprinted from Allaf ME et al,14 with permission from the American Urological Association.
Some investigators believe that nomograms underestimate the true rates of lymph node metastases. They argue that algorithms are rarely based on an extended PLND and thus understage most patients.38
Conclusions
Despite advances in noninvasive staging, PLND remains the most accurate means of detecting lymph node metastases in men with clinically localized prostate cancer. Nomograms exist that can identify patients at low risk for lymphatic metastases, on the basis of preoperative information. In general, it seems reasonable to omit PLND in men with a biopsy Gleason sum of 6 or less and a PSA level of 10 ng/mL or less. Ultimately, however, this decision should be made according to physician and patient preference, considering the low contemporary morbidity associated with PLND. When PLND is performed, studies suggest that an extended dissection maximizes the detection rate of nodal involvement. Retrospective data indicate that an extended dissection might play a therapeutic role in a subset of patients with a limited lymph node burden. This, however, might be an artifact of stage migration, and prospective studies are needed to evaluate this further.
Main Points.
Pelvic lymph node dissection (PLND) remains the most accurate staging procedure for the detection of occult nodal involvement in men diagnosed with clinically localized prostate cancer.
Cross-sectional imaging by computed tomography or magnetic resonance imaging to evaluate lymph node involvement is not routinely recommended, owing to the low sensitivity of these modalities in imaging microscopic disease.
Monoclonal antibody radioimmunoscintigraphy has had limited accuracy in the detection of lymph node metastases.
Numerous studies have integrated various clinical parameters to predict the pathologic stage of clinically localized prostate cancer; most studies conclude that one can designate a significant percentage of patients (20%–80%) as “low risk” for lymph node metastases and avoid a PLND in those patients.
The complication rate associated with PLND ranges between 4% and 53%; however, these rates are derived from studies spanning several decades, including open and laparoscopic series, and involving different dissection templates; today, it is generally agreed that the morbidity of PLND is minimal.
In addition to retrieving more lymph nodes, an extended PLND seems to detect a greater proportion of patients with lymph node metastases when compared with a limited lymphadenectomy.
References
- 1.Gervasi LA, Mata J, Easley JD, et al. Prognostic significance of lymph nodal metastases in prostate cancer. J Urol. 1989;142:332–336. doi: 10.1016/s0022-5347(17)38748-7. [DOI] [PubMed] [Google Scholar]
- 2.Partin AW, Pound CR, Clemens JQ, et al. Serum PSA after anatomic radical prostatectomy. The Johns Hopkins experience after 10 years. Urol Clin North Am. 1993;20:713–725. [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395–406. doi: 10.1016/s0094-0143(05)70386-4. [DOI] [PubMed] [Google Scholar]
- 4.Mukamel E, Hannah J, Barbaric Z, deKernion JB. The value of computerized tomography scan and magnetic resonance imaging in staging prostatic carcinoma: comparison with the clinical and histological staging. J Urol. 1986;136:1231–1233. doi: 10.1016/s0022-5347(17)45294-3. [DOI] [PubMed] [Google Scholar]
- 5.Wolf JS , Jr, Cher M, Dall’era M, et al. The use and accuracy of cross-sectional imaging and fine needle aspiration cytology for detection of pelvic lymph node metastases before radical prostatectomy. J Urol. 1995;153:993–999. [PubMed] [Google Scholar]
- 6.Troyer JK, Beckett ML, Wright GL., Jr Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate. 1997;30:232–242. doi: 10.1002/(sici)1097-0045(19970301)30:4<232::aid-pros2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 7.Chang SS, Reuter VE, Heston WD, et al. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–3198. [PubMed] [Google Scholar]
- 8.Katz AE, Olsson CA, Raffo AJ, et al. Molecular staging of prostate cancer with the use of an enhanced reverse transcriptase-PCR assay. Urology. 1994;43:765–775. doi: 10.1016/0090-4295(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 9.Parker CC, Husband J, Dearnaley DP. Lymph node staging in clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 1999;2:191–199. doi: 10.1038/sj.pcan.4500311. [DOI] [PubMed] [Google Scholar]
- 10.Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277:1445–1451. [PubMed] [Google Scholar]
- 11.Fowler JE , Jr, Whitmore WF., Jr The incidence and extent of pelvic lymph node metastases in apparently localized prostatic cancer. Cancer. 1981;47:2941–2945. doi: 10.1002/1097-0142(19810615)47:12<2941::aid-cncr2820471235>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Bluestein DL, Bostwick DG, Bergstralh EJ, Oesterling JE. Eliminating the need for bilateral pelvic lymphadenectomy in select patients with prostate cancer. J Urol. 1994;151:1315–1320. doi: 10.1016/s0022-5347(17)35239-4. [DOI] [PubMed] [Google Scholar]
- 13.Cagiannos I, Karakiewicz P, Eastham JA, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003;170:1798– 1803. doi: 10.1097/01.ju.0000091805.98960.13. [DOI] [PubMed] [Google Scholar]
- 14.Allaf ME, Palapattu GS, Trock BJ, et al. Anatomical extent of lymph node dissection: impact on men with clinically localized prostate cancer. J Urol. 2004;172:1840–1844. doi: 10.1097/01.ju.0000140912.45821.1d. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich A, Varga Z, Von Knobloch R. Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: high incidence of lymph node metastasis. J Urol. 2002;167:1681–1686. [PubMed] [Google Scholar]
- 16.Bader P, Burkhard FC, Markwalder R, Studer UE. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J Urol. 2003;169:849–854. doi: 10.1097/01.ju.0000049032.38743.c7. [DOI] [PubMed] [Google Scholar]
- 17.Epstein JI, Oesterling JE, Eggleston JC, Walsh PC. Frozen section detection of lymph node metastases in prostatic carcinoma: accuracy in grossly uninvolved pelvic lymphadenectomy specimens. J Urol. 1986;136:1234–1237. doi: 10.1016/s0022-5347(17)45295-5. [DOI] [PubMed] [Google Scholar]
- 18.Sgrignoli AR, Walsh PC, Steinberg GD, et al. Prognostic factors in men with stage D1 prostate cancer: identification of patients less likely to have prolonged survival after radical prostatectomy. J Urol. 1994;152:1077–1081. doi: 10.1016/s0022-5347(17)32507-7. [DOI] [PubMed] [Google Scholar]
- 19.Borley N, Fabrin K, Sriprasad S, et al. Laparoscopic pelvic lymph node dissection allows significantly more accurate staging in “high-risk” prostate cancer compared to MRI or CT. Scand J Urol Nephrol. 2003;37:382–386. doi: 10.1080/00365590310006309. [DOI] [PubMed] [Google Scholar]
- 20.Engeler CE, Wasserman NF, Zhang G. Preoperative assessment of prostatic carcinoma by computerized tomography. Weaknesses and new perspectives. Urology. 1992;40:346–350. doi: 10.1016/0090-4295(92)90386-b. [DOI] [PubMed] [Google Scholar]
- 21.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 22.Conrad S, Graefen M, Pichlmeier U, et al. Systematic sextant biopsies improve preoperative prediction of pelvic lymph node metastases in patients with clinically localized prostatic carcinoma. J Urol. 1998;159:2023–2029. doi: 10.1016/S0022-5347(01)63234-8. [DOI] [PubMed] [Google Scholar]
- 23.Haese A, Epstein JI, Huland H, Partin AW. Validation of a biopsy-based pathologic algorithm for predicting lymph node metastases in patients with clinically localized prostate carcinoma. Cancer. 2002;95:1016–1021. doi: 10.1002/cncr.10811. [DOI] [PubMed] [Google Scholar]
- 24.Partin AW, Yoo J, Carter HB, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993;150:110–114. doi: 10.1016/s0022-5347(17)35410-1. [DOI] [PubMed] [Google Scholar]
- 25.Partin AW, Mangold LA, Lamm DM, et al. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58:843–848. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 26.Link RE, Morton RA. Indications for pelvic lymphadenectomy in prostate cancer. Urol Clin North Am. 2001;28:491–498. doi: 10.1016/s0094-0143(05)70157-9. [DOI] [PubMed] [Google Scholar]
- 27.Campbell SC, Klein EA, Levin HS, Piedmonte MR. Open pelvic lymph node dissection for prostate cancer: a reassessment. Urology. 1995;46:352–355. doi: 10.1016/S0090-4295(99)80219-2. [DOI] [PubMed] [Google Scholar]
- 28.Clark T, Parekh DH, Cookson MS, et al. Randomized prospective evaluation of extended versus limited lymph node dissection in patients with clinically localized prostate cancer. J Urol. 2003;169:145–147. doi: 10.1016/S0022-5347(05)64055-4. [DOI] [PubMed] [Google Scholar]
- 29.Kavoussi LR, Sosa E, Chandhoke P, et al. Complications of laparoscopic pelvic lymph node dissection. J Urol. 1993;149:322–325. doi: 10.1016/s0022-5347(17)36069-x. [DOI] [PubMed] [Google Scholar]
- 30.Golimbu M, Morales P, Al-Askari S, Brown J. Extended pelvic lymphadenectomy for prostatic cancer. J Urol. 1979;121:617–620. doi: 10.1016/s0022-5347(17)56906-2. [DOI] [PubMed] [Google Scholar]
- 31.McDowell GC, Johnson JW, Tenney DM, Johnson. Pelvic lymphadenectomy for staging clinically localized prostate cancer. Indications, complications, and results in 217 cases. Urology. 1990;35:476–482. doi: 10.1016/0090-4295(90)80098-8. [DOI] [PubMed] [Google Scholar]
- 32.Wawroschek F, Vogt H, Weckermann D, et al. Radioisotope guided pelvic lymph node dissection for prostate cancer. J Urol. 2001;166:1715–1719. [PubMed] [Google Scholar]
- 33.Stone NN, Stock RG, Unger P. Laparoscopic pelvic lymph node dissection for prostate cancer: comparison of the extended and modified techniques. J Urol. 1997;158:1891–1894. doi: 10.1016/s0022-5347(01)64161-2. [DOI] [PubMed] [Google Scholar]
- 34.Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 35.Golimbu M, Provet J, Al-Askari S, Morales P. Radical prostatectomy for stage D1 prostate cancer. Prognostic variables and results of treatment. Urology. 1987;30:427–435. doi: 10.1016/0090-4295(87)90373-6. [DOI] [PubMed] [Google Scholar]
- 36.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. 1985;312:1604–1608. doi: 10.1056/NEJM198506203122504. [DOI] [PubMed] [Google Scholar]
- 37.Katz EE, Harding JN, Brendler C. Return of serum prostate specific antigen to undetectable level following removal of solitary lymph node metastasis. J Urol. 2002;168:2546. doi: 10.1016/S0022-5347(05)64196-1. [DOI] [PubMed] [Google Scholar]
- 38.Burkhard FC, Bader P, Schneider E, et al. Reliability of preoperative values to determine the need for lymphadenectomy in patients with prostate cancer and meticulous lymph node dissection. Eur Urol. 2002;42:84–90. doi: 10.1016/s0302-2838(02)00243-9. [DOI] [PubMed] [Google Scholar]
- 39.Narayan P, Fournier G, Gajendran V, et al. Utility of preoperative serum prostate-specific antigen concentration and biopsy Gleason score in predicting risk of pelvic lymph node metastases in prostate cancer. Urology. 1994;44:519–524. doi: 10.1016/s0090-4295(94)80050-2. [DOI] [PubMed] [Google Scholar]
- 40.Bishoff JT, Reyes A, Thompson IM, et al. Pelvic lymphadenectomy can be omitted in selected patients with carcinoma of the prostate: development of a system of patient selection. Urology. 1995;45:270–274. doi: 10.1016/0090-4295(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 41.Parra RO, Isorna S, Perez MG, et al. Radical perineal prostatectomy without pelvic lymphadenectomy: selection criteria and early results. J Urol. 1996;155:612–615. [PubMed] [Google Scholar]
- 42.Tewari A, Narayan P. Novel staging tool for localized prostate cancer: a pilot study using genetic adaptive neural networks. J Urol. 1998;160:430–436. doi: 10.1016/s0022-5347(01)62916-1. [DOI] [PubMed] [Google Scholar]
- 43.Crawford ED, Batuello JT, Snow P, et al. The use of artificial intelligence technology to predict lymph node spread in men with clinically localized prostate carcinoma. Cancer. 2000;88:2105–2109. doi: 10.1002/(sici)1097-0142(20000501)88:9<2105::aid-cncr16>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 44.Batuello JT, Gamito EJ, Crawford ED, et al. Artificial neural network model for the assessment of lymph node spread in patients with clinically localized prostate cancer. Urology. 2001;57:481–485. doi: 10.1016/s0090-4295(00)01039-6. [DOI] [PubMed] [Google Scholar]
- 45.Naya Y, Babaian RJ. The predictors of pelvic lymph node metastasis at radical retropubic prostatectomy. J Urol. 2003;170(6 pt 1):2306–2310. doi: 10.1097/01.ju.0000097180.98966.06. [DOI] [PubMed] [Google Scholar]
- 46.Raboy A, Adler H, Albert P. Extraperitoneal endoscopic pelvic lymph node dissection: a review of 125 patients. J Urol. 1997;158:2202–2204. doi: 10.1016/s0022-5347(01)68195-3. [DOI] [PubMed] [Google Scholar]
- 47.Herrell SD, Trachtenberg J, Theodorescu D. Staging pelvic lymphadenectomy for localized carcinoma of the prostate: a comparison of 3 surgical techniques. J Urol. 1997;157:1337–1339. [PubMed] [Google Scholar]


